Biosynthesis, Characterization, and Biological Activities of Procyanidin Capped Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Phytochemicals and Formation of SILVER Nanoparticles
2.2.1. Extraction and Purification of Chemical Constituents
2.2.2. Biosynthesis of Silver Nanoparticles
2.3. Stability Evaluation of AgNPs
2.4. Dilution Study
2.5. In-Vitro Enzymatic Assay
2.5.1. Alpha-Amylase Inhibitory Activity
2.5.2. Alpha-Glucosidase Inhibitory Activity
2.6. Antibacterial Activity
2.7. Antioxidant Activity
2.7.1. Ferric Reducing Antioxidant Power (FRAP) Assay
2.7.2. Folin–Ciocalteu (FC) Assay
2.7.3. 2,2′-Azino-bis-3-Ethylbenzotiazolin-6-Sulfonic Acid (ABTS) Assay
2.8. Statistical Analysis
3. Results and Discussion
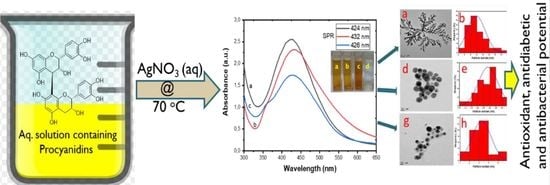
3.1. Identification and Mechanism of Procyanidin-AgNPs Formation
3.2. UV-Visibleible Analysis
3.3. HRTEM Analysis
3.4. XRD Analysis
3.5. DLS Measurement
3.6. In Vitro Stability Study
3.7. Dilution Study
3.8. In-Vitro Enzyme Inhibition
3.9. Antioxidant Activity
3.10. The Antibacterial Assay of AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Das, N.; Satija, N.K.; Mandrah, K.; Roy, S.K.; Rayavarapu, R.G. A Novel Approach towards Synthesis and Characterization of Non-Cytotoxic Gold Nanoparticles Using Taurine as Capping Agent. Nanomaterials 2019, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Janas, D.; Koziol, K.K. Carbon nanotube fibers and films: Synthesis, applications and perspectives of the direct-spinning method. Nanoscale 2016, 8, 19475–19490. [Google Scholar] [CrossRef] [PubMed]
- Muneer, I.; Farrukh, M.A.; Javaid, S.; Shahid, M.; Khaleeq-Ur-Rahman, M. Synthesis of Gd2O3/Sm2O3 nanocomposite via sonication and hydrothermal methods and its optical properties. Superlattices Microstruct. 2015, 77, 256–266. [Google Scholar] [CrossRef]
- Babu, S.; Kumar, B.; Kumar, K. Environment friendly approach for size controllable synthesis of biocompatible Silver nanoparticles using diastase. Environ. Toxicol. Pharmacol. 2017, 49, 131–136. [Google Scholar]
- Lee, J.; Park, E.Y.; Lee, J. Non-toxic nanoparticles from phytochemicals: Preparation and biomedical application. Bioprocess. Biosyst. Eng. 2013, 37, 983–989. [Google Scholar] [CrossRef]
- Xia, D.-L.; Wang, Y.-F.; Bao, N.; He, H.; Li, X.-D.; Chen, Y.-P.; Gu, H.-Y. Influence of reducing agents on biosafety and biocompatibility of gold nanoparticles. Appl. Biochem. Biotechnol. 2014, 174, 2458–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okaiyeto, K.; Hoppe, H.; Okoh, A.I. Plant-Based Synthesis of Silver Nanoparticles Using Aqueous Leaf Extract of Salvia officinalis: Characterization and its Antiplasmodial Activity. J. Clust. Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2017, 25, 1–20. [Google Scholar] [CrossRef]
- Benelli, G.; Kadaikunnan, S.; Alharbi, N.S.; Govindarajan, M. Biophysical characterization of Acacia caesia-fabricated silver nanoparticles: Effectiveness on mosquito vectors of public health relevance and impact on non-target aquatic biocontrol agents. Environ. Sci. Pollut. Res. 2017, 25, 10228–10242. [Google Scholar] [CrossRef]
- Nath, D.; Banerjee, P. Green nanotechnology—A new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Chahardoli, A.; Karimi, N.; Fattahi, A. Biosynthesis, Characterization, Antimicrobial and Cytotoxic Effects of Silver Nanoparticles Using Nigella arvensis Seed Extract. Iran. J. Pharm. Res. 2017, 16, 1167–1175. [Google Scholar] [PubMed]
- Arassu, R.R.T.; Nambikkairaj, B. Pelargonium graveolens plant leaf essential oil mediated green synthesis of Silver Nano particles and its antifungal activity against human pathogenic fungi. J. Pharm. Phytochem. 2018, 7, 1778–1784. [Google Scholar]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Małek, K.; Celichowski, G.; Orlowski, P.; Krzyzowska, M.; Grobelny, J. The synthesis of monodisperse silver nanoparticles with plant extracts. Colloids Surf. B Biointerfaces 2019, 177, 19–24. [Google Scholar] [CrossRef]
- Nayaka, S.; Bhat, M.P.; Chakraborty, B.; Pallavi, S.S.; Airodagi, D.; Muthuraj, R.; Halaswamy, H.M.; Dhanyakumara, S.B.; Shashiraj, K.N.; Kupaneshi, K.N.S.A.C. Seed Extract-mediated Synthesis of Silver Nanoparticles from Putranjiva roxburghii Wall., Phytochemical Characterization, Antibacterial Activity and Anticancer Activity Against MCF-7 Cell Line. Indian J. Pharm. Sci. 2020, 82, 260–269. [Google Scholar] [CrossRef]
- Jemilugba, O.T.; Sakho, E.H.M.; Parani, S.; Mavumengwana, V.; Oluwafemi, O.S. Green synthesis of silver nanoparticles using Combretum erythrophyllum leaves and its antibacterial activities. Colloid Interface Sci. Commun. 2019, 31, 100191. [Google Scholar] [CrossRef]
- Amin, M.; Kouhbanani, J.; Beheshtkhoo, N.; Nasirmoghadas, P. Green synthesis of spherical silver nanoparticles using Ducrosia anethifolia aqueous extract and its antibacterial activity. J. Environ. Treat. Tech. 2019, 7, 461–466. [Google Scholar]
- Chahardoli, A.; Karimi, N.; Fattahi, A. Nigella arvensis leaf extract mediated green synthesis of silver nanoparticles: Their characteristic properties and biological efficacy. Adv. Powder Technol. 2018, 29, 202–210. [Google Scholar] [CrossRef]
- Srinivasan, R.; Vigneshwari, L.; Rajavel, T.; Durgadevi, R.; Kannappan, A.; Balamurugan, K.; Devi, K.P.; Ravi, A.V. Biogenic synthesis of silver nanoparticles using Piper betle aqueous extract and evaluation of its anti-quorum sensing and antibiofilm potential against uropathogens with cytotoxic effects: An in vitro and in vivo approach. Environ. Sci. Pollut. Res. 2017, 25, 10538–10554. [Google Scholar] [CrossRef]
- S Khaleel, K.; Govindaraju, R.; Manikandan, J.; Seog, E.Y.; Singaravelu, G. Phytochemical mediated gold nanoparticles and their PTP 1B inhibitory activity. Colloids Surf. B Biointerfaces 2010, 75, 405–409. [Google Scholar] [CrossRef]
- Shah, M.; Nawaz, S.; Jan, H.; Uddin, N.; Ali, A.; Anjum, S.; Giglioli-Guivarc’H, N.; Hano, C.; Abbasi, B.H. Synthesis of bio-mediated silver nanoparticles from Silybum marianum and their biological and clinical activities. Mater. Sci. Eng. C 2020, 112, 110889. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.M. Preparation of antimicrobial metallic nanoparticles with bioactive compounds. Mater. Sci. Eng. C 2019, 103, 109809. [Google Scholar] [CrossRef] [PubMed]
- Perelshtein, I.; Ruderman, Y.; Francesko, A.; Fernandes, M.M.; Tzanov, T.; Gedanken, A. Tannic acid NPs—Synthesis and immobilization onto a solid surface in a one-step process and their antibacterial and anti-inflammatory properties. Ultrason. Sonochemistry 2014, 21, 1916–1920. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Kumar, S.; Banerjee, U.C. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 2014, 431, 194–199. [Google Scholar] [CrossRef]
- Stephen, A.; Seethalakshmi, S. Phytochemical Synthesis and Preliminary Characterization of Silver Nanoparticles Using Hesperidin. J. Nanosci. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Safaepour, M.; Shahverdi, A.R.; Shahverdi, H.R.; Khorramizadeh, M.R.; Gohari, A.R. Green Synthesis of Small Silver Nanoparticles Using Geraniol and Its Cytotoxicity against Fibrosarcoma-Wehi 164. Avicenna J. Med. Biotechnol. 2009, 1, 111–115. [Google Scholar] [PubMed]
- Satsangi, N. Synthesis and Characterization of Biocompatible Silver Nanoparticles for Anticancer Application. J. Inorg. Organomet. Polym. Mater. 2019, 30, 1907–1914. [Google Scholar] [CrossRef]
- Saratale, G.D.; Benelli, G.; Kumar, G.; Kim, D.S. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2017, 25, 10392–10406. [Google Scholar] [CrossRef]
- Bharathi, D.; Bhuvaneshwari, V. Evaluation of the Cytotoxic and Antioxidant Activity of Phyto-synthesized Silver Nanoparticles Using Cassia angustifolia Flowers. BioNanoScience 2018, 9, 155–163. [Google Scholar] [CrossRef]
- Jini, D.; Sharmila, S. Green synthesis of silver nanoparticles from Allium cepa and its in vitro antidiabetic activity. Mater. Today: Proc. 2020, 22, 432–438. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Shin, H.-S. Facile green biosynthesis of silver nanoparticles using Pisum sativum L. outer peel aqueous extract and its antidiabetic, cytotoxicity, antioxidant, and antibacterial activity. Int. J. Nanomed. 2019, 14, 6679–6690. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, S.; Vinodhini, S.; Elenchezhiyan, C.; Rajeswari, D. Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin-induced diabetic rats. J. Diabetes 2017, 10, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.; Saratale, G.D. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 0, 211–222. [Google Scholar]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Biology Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J. Photochem. Photobiol. B Biol. 2019, 195, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, E.; Afolayan, A.J. Green synthesis, characterization and biological activities of silver nanoparticles from alkalinized Cymbopogon citratus Stapf. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 015017. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; MubarakAli, D.; Prabakar, D.; Muthukumar, H.; Thajuddin, N.; Kumar, S.S.; Pugazhendhi, A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: Green approach. Environ. Sci. Pollut. Res. 2017, 25, 10362–10370. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kishore, N.; Hussein, A.A.; Lall, N. The potential of Leucosidea sericea against Propionibacterium acnes. Phytochem. Lett. 2014, 7, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Nair, J.; Aremu, A.; Van Staden, J. Anti-inflammatory effects of Leucosidea sericea (Rosaceae) and identification of the active constituents. S. Afr. J. Bot. 2012, 80, 75–76. [Google Scholar] [CrossRef] [Green Version]
- Aremu, A.; Ndhlala, A.; Fawole, O.A.; Light, M.; Finnie, J.; Van Staden, J. In vitro pharmacological evaluation and phenolic content of ten South African medicinal plants used as anthelmintics. S. Afr. J. Bot. 2010, 76, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Bosman, A.; Combrinck, S.; Der Merwe, R.R.-V.; Botha, B.; McCrindle, R.; Houghton, P. Isolation of an anthelmintic compound from Leucosidea sericea. S. Afr. J. Bot. 2004, 70, 509–511. [Google Scholar] [CrossRef] [Green Version]
- Adamu, M.; Mukandiwa, L.; Awouafack, M.; Ahmed, A.; Eloff, J.; Naidoo, V. Ultrastructure changes induced by the phloroglucinol derivative agrimol G isolated from Leucosidea sericea in Haemonchus contortus. Exp. Parasitol. 2019, 207, 107780. [Google Scholar] [CrossRef] [PubMed]
- Badeggi, U.M.; Isamil, E.; Adeloye, A.O.; Botha, S.; Badmus, J.A.; Marnewick, J.L.; Cupido, C.N.; Hussein, A.A. Green Synthesis of Gold Nanoparticles Capped with Procyanidins from Leucosidea sericea as Potential Antidiabetic and Antioxidant Agents. Biomolecules 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romeyer, F.M.; Macheix, J.-J.; Sapis, J.-C. Changes and importance of oligomeric procyanidins during maturation of grape seeds. Phytochemistry 1985, 25, 219–221. [Google Scholar] [CrossRef]
- Gonthier, M.-P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free Radic. Boil. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Haslam, E. Symmetry and promiscuity in procyanidin biochemistry. Phytochemistry 1977, 16, 1625–1640. [Google Scholar] [CrossRef]
- Berké, B.; De Freitas, V. Influence of procyanidin structures on their ability to complex with oenin. Food Chem. 2005, 90, 453–460. [Google Scholar] [CrossRef]
- Granja, A.; Pinheiro, M.; Reis, S. Epigallocatechin Gallate Nanodelivery Systems for Cancer Therapy. Nutrients 2016, 8, 307. [Google Scholar] [CrossRef]
- Aiello, P.; Consalvi, S.; Poce, G.; Raguzzini, A.; Toti, E.; Palmery, M.; Biava, M.; Bernardi, M.; Kamal, M.A.; Perry, G.; et al. Dietary flavonoids: Nano delivery and nanoparticles for cancer therapy. Semin. Cancer Boil. 2019, 1–16. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Meyer, M.; Cupido, C.N.; Hussein, A.A. Inhibition of Bacteria Associated with Wound Infection by Biocompatible Green Synthesized Gold Nanoparticles from South African Plant Extracts. Nanomaterials 2017, 7, 417. [Google Scholar] [CrossRef] [Green Version]
- Thilagam, E.; Parimaladevi, B.; Kumarappan, C.; Mandal, S.C. α-Glucosidase and α-Amylase Inhibitory Activity of Senna surattensis. J. Acupunct. Meridian Stud. 2013, 6, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Pu, S.; Li, J.; Sun, L.; Zhong, L.; Ma, Q. An in vitro comparison of the antioxidant activities of chitosan and green synthesized gold nanoparticles. Carbohydr. Polym. 2019, 211, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro Evaluation of Antioxidant and Antibacterial Potential of GreenSynthesized Silver Nanoparticles Using Prosopis farcta Fruit Extract. Iran. J. Pharm. Res. 2019, 18, 430–455. [Google Scholar] [PubMed]
- Gomes, J.F.; Garcia, A.C.; Ferreira, E.B.; Pires, C.; Oliveira, V.L.; Tremiliosi-Filho, G.; Gasparotto, L.H. New insights into the formation mechanism of Ag, Au and AgAu nanoparticles in aqueous alkaline media: Alkoxides from alcohols, aldehydes and ketones as universal reducing agents. Phys. Chem. Chem. Phys. 2015, 17, 21683–21693. [Google Scholar] [CrossRef]
- Shamprasad, B.R.; Keerthana, S.; Megarajan, S.; Lotha, R.; Aravind, S.; Veerappan, A.; Anbazhagan, V. Photosynthesized escin stabilized gold nanoparticles exhibit antidiabetic activity in L6 rat skeletal muscle cells. Mater. Lett. 2019, 241, 198–201. [Google Scholar] [CrossRef]
- Selvakannan, P.; Swami, A.; Srisathiyanarayanan, D.; Shirude, P.S.; Pasricha, R.; Mandale, A.B.; Sastry, M. Synthesis of Aqueous Au Core−Ag Shell Nanoparticles Using Tyrosine as a pH-Dependent Reducing Agent and Assembling Phase-Transferred Silver Nanoparticles at the Air−Water Interface. Langmuir 2004, 20, 7825–7836. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Balandran, R. Effect of Constituent Units, Type of Interflavan Bond, and Conformation on the Antioxidant Properties of Procyanidin Dimers: A Computational Outlook. J. Chem. 2017, 2017, 3535148. [Google Scholar] [CrossRef] [Green Version]
- Ponnanikajamideen, M.; RajeshKumar, S.; Vanaja, M.; Annadurai, G. In Vivo Type 2 Diabetes and Wound-Healing Effects of Antioxidant Gold Nanoparticles Synthesized Using the Insulin Plant Chamaecostus cuspidatus in Albino Rats. Can. J. Diabetes 2019, 43, 82–89.e6. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Sosa, I.O.; Noguez, C.; Barrera, R.G.; Noguez, C. Optical Properties of Metal Nanoparticles with Arbitrary Shapes. J. Phys. Chem. B 2003, 107, 6269–6275. [Google Scholar] [CrossRef] [Green Version]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef] [Green Version]
- Elbagory, A.M.; Cupido, C.N.; Meyer, M.; Hussein, A.A. Large Scale Screening of Southern African Plant Extracts for the Green Synthesis of Gold Nanoparticles Using Microtitre-Plate Method. Molecules 2016, 21, 1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, W.; Zhao, Y.; Cao, L. Fabrication of silver nanoparticles loaded flowerlike CeF3 architectures and their antibacterial activity. J. Phys. Chem. Solids 2018, 120, 154–160. [Google Scholar] [CrossRef]
- Aarthi, C.; Govindarajan, M.; Rajaraman, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Mothana, R.A.; Siddiqui, N.A.; Benelli, G. Eco-friendly and cost-effective Ag nanocrystals fabricated using the leaf extract of Habenaria plantaginea: Toxicity on six mosquito vectors and four non-target species. Environ. Sci. Pollut. Res. 2017, 25, 10317–10327. [Google Scholar] [CrossRef] [PubMed]
- Rajarajeshwari, T.; Shivashri, C.; Rajasekar, P. Synthesis and characterization of biocompatible gymnemic acid—Gold nanoparticles: A study on glucose uptake stimulatory effect in 3T3-L1 adipocytes. RSC Adv. 2014, 4, 63285–63295. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rashid, M.; Rahman, A.U.; Husen, A.; Rehman, S. Tajuddin Biogenic fabrication and characterization of silver nanoparticles using aqueous-ethanolic extract of lichen (Usnea longissima) and their antimicrobial activity. Biomater. Res. 2018, 22, 23. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V. Chitosan Reduced Gold Nanoparticles as Novel Carriers for Transmucosal Delivery of Insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef] [Green Version]
- Rani, R.; Sharma, D.; Chaturvedi, M.; Jp, Y. Green Synthesis, Characterization and Antibacterial Activity of Silver Nanoparticles of Endophytic Fungi Aspergillus terreus. J. Nanomed. Nanotechnol. 2017, 8. [Google Scholar] [CrossRef]
- Sasidharan, J.; Meenakshi, R.V.; Sureshkumar, P. Green Synthesis, Characterization and Evaluation of In-vitro Antioxidant & Anti-diabetic Activity of Nanoparticles from a Polyherbal formulation-Mehani. J. Environ. Nanotechnol. 2018, 7, 51–59. [Google Scholar]
- Patil, M.P.; Seo, Y.B.; Lim, H.K.; Kim, G.-D. Biofabrication of gold nanoparticles using Agrimonia pilosa extract and their antioxidant and cytotoxic activity. Green Chem. Lett. Rev. 2019, 12, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Abusahid, Z.; Kandiah, M. In Vitro Green Synthesis of Phoenix dactylifera Silver Nanoparticles: Assessing Their Antioxidant and Antimicrobial Properties. Int. J. Nanosci. 2019, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Nyoni, S.; Muzenda, E.; Mukaratirwa-muchanyereyi, N. Characterization and Evaluation of Antibacterial Activity of Silver Nanoparticles Prepared from Sclerocarya birrea Stem B ark and Leaf Extracts. Nano Biomed. Eng. 2019, 11, 28–34. [Google Scholar] [CrossRef]
- Sankar, S.; Kumar, L. Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater. Lett. 2018, 226, 47–51. [Google Scholar]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Mani, N. Evaluation of antimicrobial activity of silver nanoparticle using Eichhornia crassipes leaves extract. J. Pharmacogn. Phytochem. 2018, 7, 1308–1311. [Google Scholar]
- Elangovan, M.; Muju, G.; Anantharaman, P. Biosynthesis of Silver Nanoparticles from Platymonas sp. and Its Antibacterial Activity Against Biofouling Causing Bacterial Strains. J. Boil. Act. Prod. Nat. 2019, 9, 269–277. [Google Scholar] [CrossRef]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; SaravanaDevi, S.; Sarangi, B.K.; Pandey, R. Synthesis of silver nanoparticles using flavonoids: Hesperidin, naringin and diosmin, and their antibacterial effects and cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Wu, C.; Wu, T.; Yuan, C.; Chen, S.; Ding, T.; Ye, X.; Hu, Y. Green synthesis of sodium alginate-silver nanoparticles and their antibacterial activity. Int. J. Boil. Macromol. 2018, 111, 1281–1292. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Cho, S.-K.; Ghodake, G.; Bharagava, R.N.; Park, Y.; Mulla, S.I.; Kim, D.S.; Kadam, A.; Nair, S.; et al. Investigation of photocatalytic degradation of reactive textile dyes by Portulaca oleracea-functionalized silver nanocomposites and exploration of their antibacterial and antidiabetic potentials. J. Alloy. Compd. 2020, 833, 155083. [Google Scholar] [CrossRef]
- Jin, T.; Wang, M. Antibacterial activity of silver nanoparticles from endophytic fungus Talaromyces purpureogenus. Int. J. Nanomed. 2019, 14, 3427–3438. [Google Scholar]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Banala, R.R.; Nagati, V.B.; Reddy, K.P. Green synthesis and characterization of Carica papaya leaf extract coated silver nanoparticles through X-ray diffraction, electron microscopy and evaluation of bactericidal properties. Saudi J. Boil. Sci. 2015, 22, 637–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Items | Hydrodynamic Size (nm) | Polydisperity Index | Zeta Potential (mV) |
|---|---|---|---|
| LSTE-AgNPs | 87.64 | 0.398 | −25.7 |
| F1-AgNPs | 95.17 | 0.393 | −29.4 |
| F2-AgNPs | 148.80 | 0.472 | −28.8 |
| Items | Alpha-Amylase (IC50) (µg/mL) | Alpha-Glucosidase (IC50) (µg/mL) |
|---|---|---|
| LSTE | 3.50 ± 0.70 a | 8.10 ± 0.60 a |
| LSTE-AgNPs | 14.92 ± 1.0 b | 21.48 ± 0.90 b |
| F1 | NA | 7.30 ± 0.50 a |
| F1-AgNPs | 13.24 ± 0.60 b | 18.76 ± 1.00 c |
| F2 | 18.9 ± 0.20 c | 7.10 ± 0.40 a |
| F2-AgNPs | 19.13 ± 0.80 c | 8.75 ± 0.70 a |
| Acarbose | 10.20 ± 0.40 d | 61.00 ± 1.50 d |
| Items | ABTS (mM TE/g) | FRAP (mM AAE/g) | FC (mM GAE/g) | FC % (AgNPs) |
|---|---|---|---|---|
| LSTE | 814.18 ± 1.80 a | 1113.20 ± 6.70 a | 602.60 ± 6.10 a | 100 |
| LSTE-AgNPs | 499.65 ± 1.50 b | 1438.50 ± 5.60 b | 578.27 ± 7.70 b | 57.8 |
| F1 | 818.20 ± 7.70 a | 1834.00 ± 4.70 c | 889.60 ± 6.00 c | 100 |
| F1-AgNPs | 319.18 ± 1.80 c | 1361.60 ± 6.70 d | 175.25 ± 2.60 d | 17.5 |
| F2 | 861.90 ± 5.30 d | 1166.00 ± 2.10 e | 685.70 ± 6.70 e | 100 |
| F2-AgNPs | 583.22 ± 7.30 e | 326.20 ± 2.20 f | 357.80 ± 5.30 f | 35.7 |
| Bacteria | LSTE | LSTE-AgNPs | F1 | F1-AgNPs | F2 | F2-AgNPs | Control * |
|---|---|---|---|---|---|---|---|
| P. aeruginosa | >2000 | 62.50 | >2000 | 31.25 | >2000 | 62.50 | 31.25 |
| S. aureus | >2000 | 62.50 | >2000 | 15.63 | >2000 | 62.50 | 15.63 |
| B. cereus | >2000 | 62.50 | >2000 | 31.25 | >2000 | 125.00 | 7.81 |
| S. enterica | >2000 | 31.25 | >2000 | 31.25 | >2000 | 62.50 | 7.81 |
| E. coli | >2000 | 62.50 | >2000 | 31.25 | >2000 | 62.50 | 15.63 |
| S. marcescens | >2000 | 125.00 | >2000 | 62.50 | >2000 | 62.50 | 3.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badeggi, U.M.; Badmus, J.A.; Botha, S.S.; Ismail, E.; Marnewick, J.L.; Africa, C.W.J.; Hussein, A.A. Biosynthesis, Characterization, and Biological Activities of Procyanidin Capped Silver Nanoparticles. J. Funct. Biomater. 2020, 11, 66. https://doi.org/10.3390/jfb11030066
Badeggi UM, Badmus JA, Botha SS, Ismail E, Marnewick JL, Africa CWJ, Hussein AA. Biosynthesis, Characterization, and Biological Activities of Procyanidin Capped Silver Nanoparticles. Journal of Functional Biomaterials. 2020; 11(3):66. https://doi.org/10.3390/jfb11030066
Chicago/Turabian StyleBadeggi, Umar M., Jelili A. Badmus, Subelia S. Botha, Enas Ismail, Jeanine L. Marnewick, Charlene W. J. Africa, and Ahmed A. Hussein. 2020. "Biosynthesis, Characterization, and Biological Activities of Procyanidin Capped Silver Nanoparticles" Journal of Functional Biomaterials 11, no. 3: 66. https://doi.org/10.3390/jfb11030066