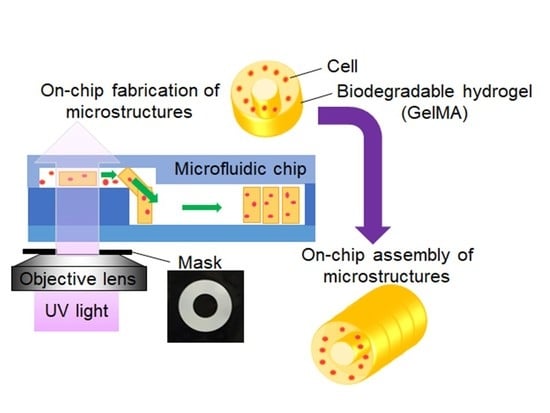
On-Chip Fabrication of Cell-Attached Microstructures using Photo-Cross-Linkable Biodegradable Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Biodegradable Microstructures
2.2. Cell Culture in GelMA Microstructures
2.3. Cell Culture on GelMA Surface
3. Results
3.1. Preparation of Biodegradable Microstructures
3.2. Cell Culture in GelMA Microstructures
3.3. Cell Culture on GelMA Surface
3.4. Movable GelMA Microstructures in a Microfluidic Device
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yue, T.; Nakajima, M.; Takeuchi, M.; Hu, C.; Huang, Q.; Fukuda, T. On-chip self-assembly of cell embedded microstructures to vascular-like microtubes. Lab Chip 2014, 14, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Chumtong, P.; Kojima, M.; Ohara, K.; Mae, Y.; Horade, M.; Akiyama, Y.; Yamato, M.; Arai, T. Design and fabrication of changeable cell culture mold. J. Robot Mechatron. 2013, 25, 657–664. [Google Scholar] [CrossRef]
- Utoa, K.; Tsui, J.H.; De Forest, C.A.; Kim, D.H. Dynamically tunable cell culture platforms for tissueengineering and mechanobiology. Prog. Polym. Sci. 2017, 65, 53–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Vacanti, C.A. The history of tissue engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Bedian, L.; Villalba-Rodríguez, A.M.; Hernández-Vargas, G.; Parra-Saldivar, R.; Iqbal, H.M.N. Bio-based materials with novel characteristics for tissue engineering applications—A review. Int. J. Biol. Macromol. 2017, 98, 837–846. [Google Scholar] [CrossRef]
- Anderson, C.W.; Boardman, N.; Luo, J.; Park, J.; Qyang, Y. Stem cells in cardiovascular medicine: The road to regenerative therapies. Curr. Cardiol. Rep. 2017, 19, 34. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, Y.; Shimizu, T.; Sasagawa, T.; Sekine, H.; Sakaguchi, K.; Kikuchi, T.; Sekine, W.; Sekiya, S.; Yamato, M.; Umezu, M.; et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 2012, 7, 850–858. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Masuda, T.; Matsusaki, M.; Akashi, M.; Yokoyama, U.; Arai, F. Microfluidic perfusion culture system for multilayer artery tissue models. Biomicrofluidics 2014, 8, 064113. [Google Scholar] [CrossRef] [Green Version]
- Sasagawa, T.; Shimizu, T.; Sekiya, S.; Haraguchi, Y.; Yamato, M.; Sawa, Y.; Okano, T. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 2010, 31, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Nakamura, M. Development of a Three-Dimensional Bioprinter: Construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J. Biomech. Eng. 2009, 131, 035001. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for organ regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Jang, J.; Lee, J.S.; Cho, D.W. Three-dimensional printing of tissue/organ analogues containing living cells. Ann. Biomed. Eng. 2017, 45, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Wüst, S.; Müller, R.; Hofmann, S. Controlled positioning of cells in biomaterials—Approaches towards 3D tissue printing. J. Funct. Biomater. 2011, 2, 119–154. [Google Scholar] [CrossRef] [Green Version]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [Green Version]
- Kato-Negishi, M.; Morimoto, Y.; Onoe, H.; Takeuchi, S. Millimeter-Sized Neural Building Blocks for 3D Heterogeneous Neural Network Assembly. Adv. Healthc. Mater. 2013, 2, 1564–1570. [Google Scholar] [CrossRef]
- Gauvin, R.; Ahsan, T.; Larouche, D.; Levesque, P.; Dube, J.; Auger, F.A.; Nerem, R.M.; Germain, L. A Novel Single-Step Self-Assembly Approach for the Fabrication of Tissue-Engineered Vascular Constructs. Tissue Eng. Part A 2010, 16, 1737–1747. [Google Scholar] [CrossRef]
- Khoo, H.S.; Lin, C.; Huang, S.-H.; Tseng, F.-G. Self-Assembly in Micro- and Nanofluidic Devices: A Review of Recent Efforts. Micromachines 2011, 2, 17–48. [Google Scholar] [CrossRef] [Green Version]
- Yue, T.; Liu, N.; Liu, Y.; Peng, Y.; Xie, S.; Luo, J.; Huang, Q.; Takeuchi, M.; Fukuda, T. On-Chip Construction of Multilayered Hydrogel Microtubes for Engineered Vascular-Like Microstructures. Micromachines 2019, 10, 840. [Google Scholar] [CrossRef] [Green Version]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, M.; Nakamura, Y.; Ichikawa, A.; Hasegawa, A.; Hasegawa, Y.; Fukuda, T. On-chip fabrication of movable toroidal cell structures using photo-crosslinkable biodegradable hydrogel. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2017), Vancouver, BC, Canada, 24–28 September 2017. [Google Scholar]
- Ramón-Azcón, J.; Ahadian, S.; Obregón, R.; Camci-Unal, G.; Ostrovidov, S.; Hosseini, V.; Kaji, H.; Ino, K.; Shiku, H.; Khademhosseini, A. Gelatin methacrylate as a promising hydrogel for 3D microscale organization and proliferation of dielectrophoretically patterned cells. Lab Chip 2012, 12, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2011, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, M.; Kozuka, T.; Kim, E.; Ichikawa, A.; Hasegawa, Y.; Huang, Q.; Fukuda, T. On-Chip Fabrication of Cell-Attached Microstructures using Photo-Cross-Linkable Biodegradable Hydrogel. J. Funct. Biomater. 2020, 11, 18. https://doi.org/10.3390/jfb11010018
Takeuchi M, Kozuka T, Kim E, Ichikawa A, Hasegawa Y, Huang Q, Fukuda T. On-Chip Fabrication of Cell-Attached Microstructures using Photo-Cross-Linkable Biodegradable Hydrogel. Journal of Functional Biomaterials. 2020; 11(1):18. https://doi.org/10.3390/jfb11010018
Chicago/Turabian StyleTakeuchi, Masaru, Taro Kozuka, Eunhye Kim, Akihiko Ichikawa, Yasuhisa Hasegawa, Qiang Huang, and Toshio Fukuda. 2020. "On-Chip Fabrication of Cell-Attached Microstructures using Photo-Cross-Linkable Biodegradable Hydrogel" Journal of Functional Biomaterials 11, no. 1: 18. https://doi.org/10.3390/jfb11010018