Comparison of Five TRIzol-Based Protein Preparation Methods for 2-DE Production From Challenging Marine Dinoflagellate Samples: A Case Study on Two Benthic Prorocentrum Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Cultures and Cultivation Condition
2.2. Cell Harvesting
2.3. Protein Extraction
2.3.1. TRIzol Method
2.3.2. Aliquot TRIzol Method
2.3.3. Re-TRIzol Method
2.3.4. TRIzol Method with a Commercial Clean-Up Kit
2.3.5. TRIzol Method with TCA/Acetone Precipitation
2.4. Protein Quantification, Two-Dimensional Gel Electrophoresis and Imaging Analysis
2.5. Statistical Analysis
3. Results and Discussion
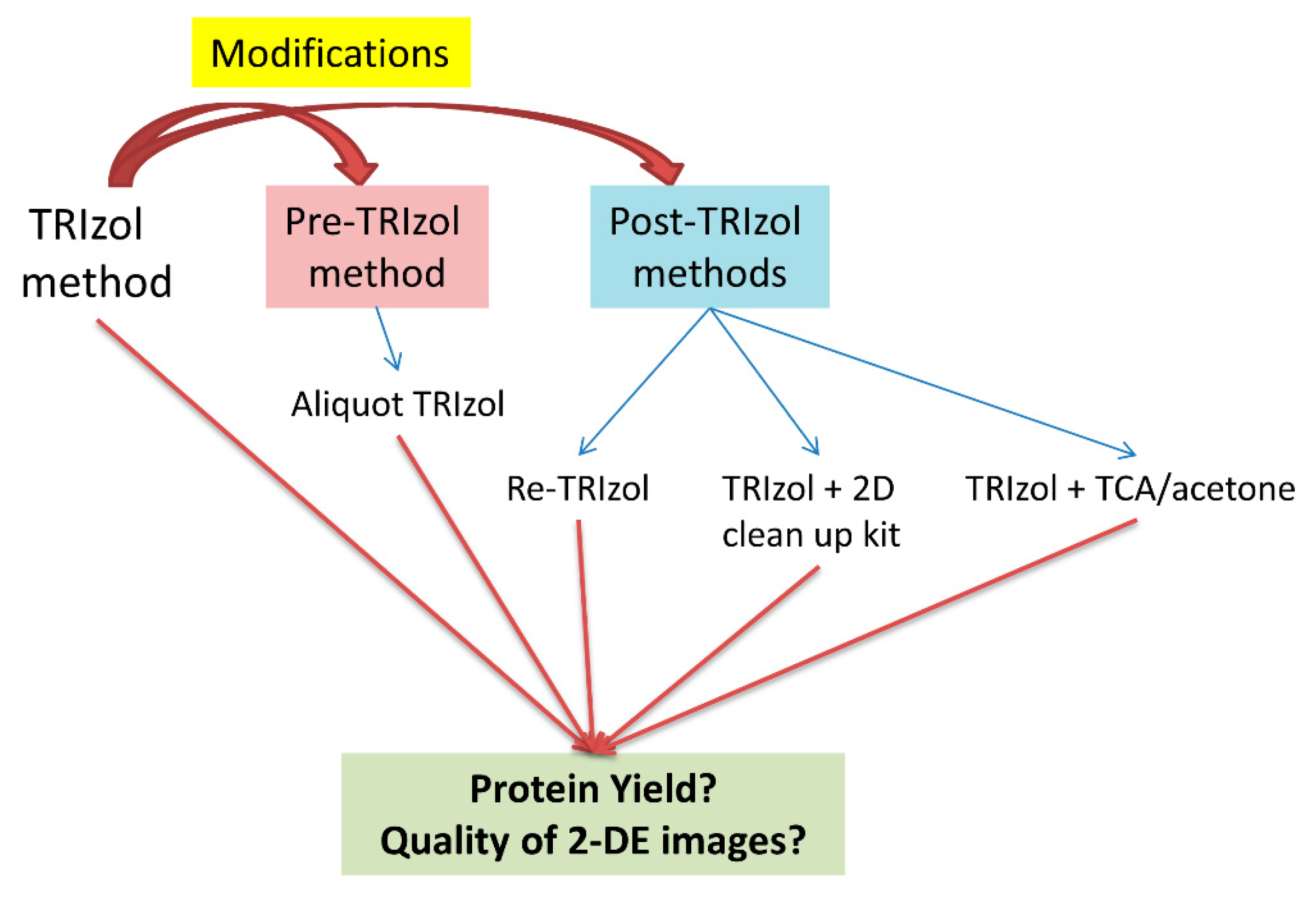
3.1. Characteristics of the Five TRIzol-Based Methods
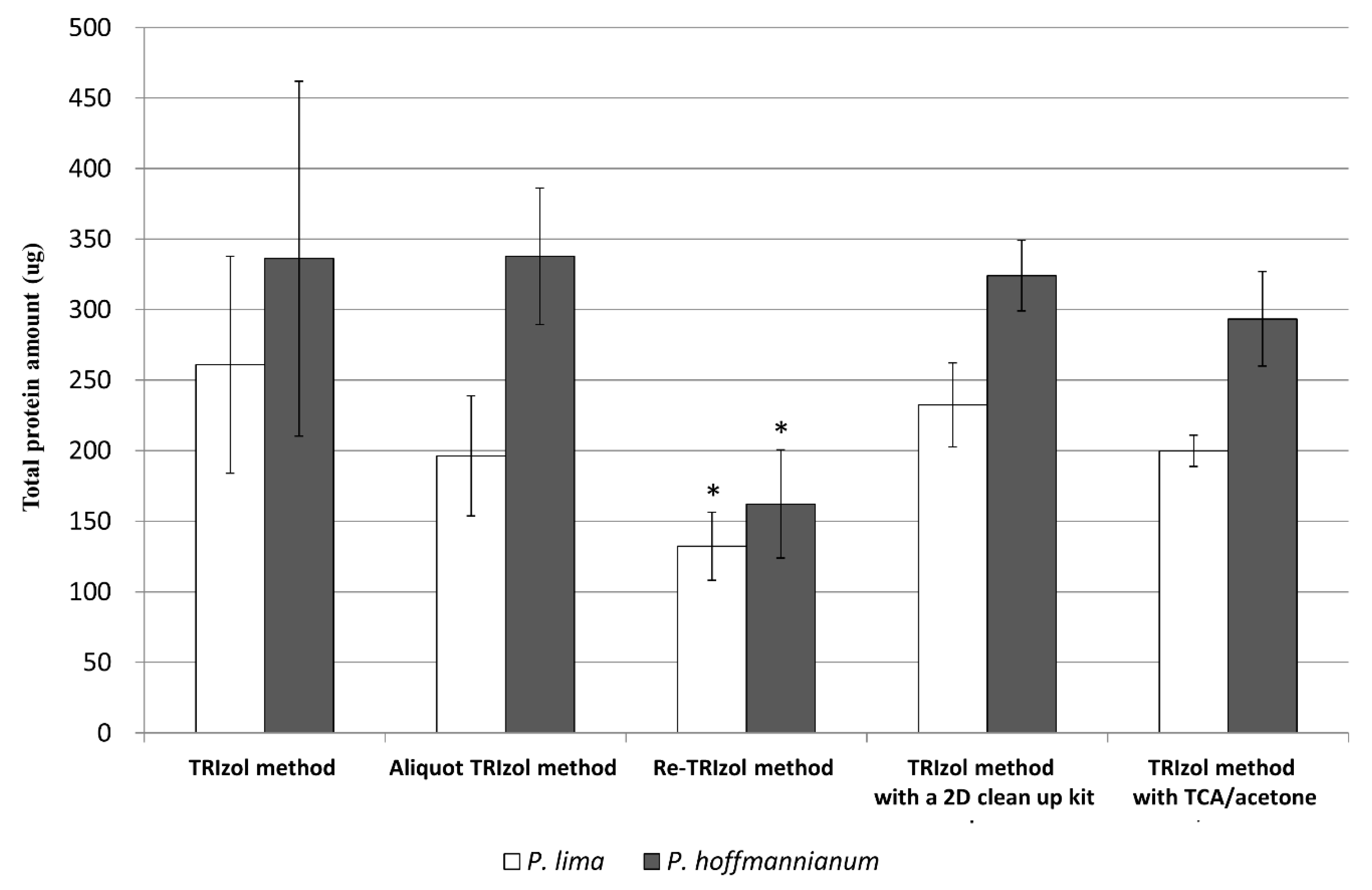
3.2. Yield of Extracted Proteins
3.3. Quality of 2-DE Images
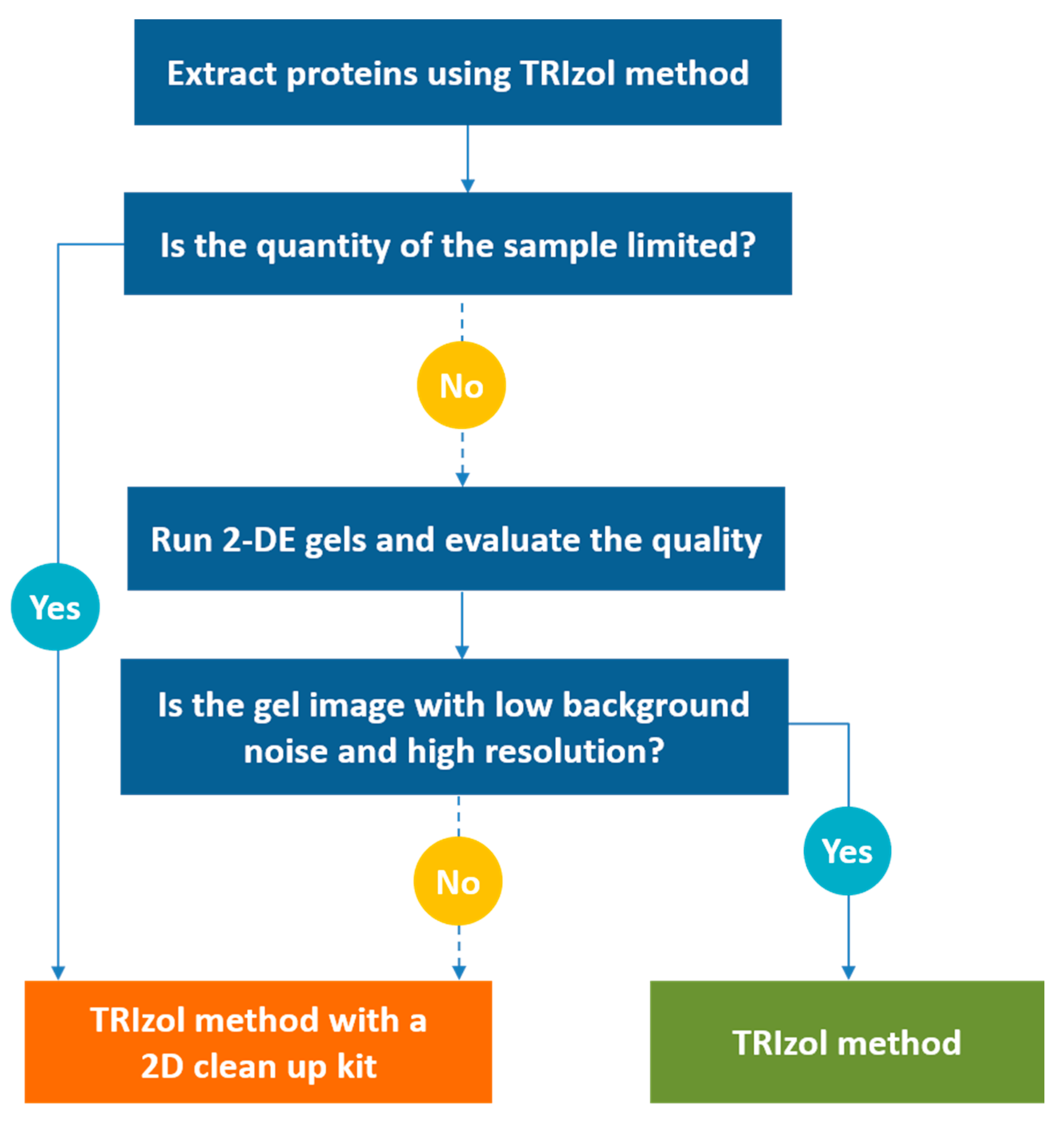
3.4. Evaluation of the Four Modified TRIzol-based Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- O’Farrell, P.H. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 1975, 250, 4007–4021. [Google Scholar] [PubMed]
- Marshall, T.; Williams, K.M. High resolution two-dimensional electrophoresis of the proteins and macromolecular constituents of beer and wine. Electrophoresis 1987, 8, 493–495. [Google Scholar] [CrossRef]
- Lee, F.W.F.; Morse, D.; Lo, S.C.L. Identification of two plastid proteins in the dinoflagellate Alexandrium affine that are substantially down-regulated by nitrogen-depletion. J. Proteome Res. 2009, 8, 5080–5092. [Google Scholar] [CrossRef]
- Chan, L.L.; Hodgkiss, I.J.; Wan, J.M.F.; Lum, J.H.K.; Mak, A.S.C.; Sit, W.H.; Lo, S.C.L. Proteomic study of a model causative agent of harmful algal blooms, Prorocentrum triestinum II: The use of differentially expressed protein profiles under different growth phases and growth conditions for bloom prediction. Proteomics 2004, 4, 3214–3226. [Google Scholar] [CrossRef]
- Lee, F.W.F.; Lo, S.C.L. The use of Trizol reagent (phenol/guanidine isothiocyanate) for producing high quality two-dimensional gel electrophoretograms (2-DE) of dinoflagellates. J. Microbiol. Methods 2008, 73, 26–32. [Google Scholar] [CrossRef]
- Lyon, B.R.; Lee, P.A.; Bennett, J.M.; Ditullio, G.R.; Janech, M.G. Proteomic analysis of a sea-ice diatom: Salinity acclimation provides new insight into the dimethylsulfoniopropionate production pathway. Plant Physiol. 2011, 157, 1926–1941. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.A.; Azman, S.N.; Abdul Rani, N.R.; Kua, B.C.; Sasidharan, S.; Kiew, L.V.; Othman, N.; Noordin, R.; Chen, Y. Optimal protein extraction methods from diverse sample types for protein profiling by using two-dimensional electrophoresis (2DE). Trop Biomed 2011, 28, 620–629. [Google Scholar]
- Wang, D.Z.; Zhang, Y.J.; Zhang, S.-F.; Lin, L.; Hong, H.S. Quantitative proteomic analysis of cell cycle of the dinoflagellate Prorocentrum donghaiense (Dinophyceae). PLoS ONE 2013, 8, e63659. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.Z.; Li, C.; Xie, Z.X.; Dong, H.P.; Lin, L.; Hong, H.S. Homology-driven proteomics of dinoflagellates with unsequenced genomes using MALDI-TOF/TOF and automated de novo sequencing. Evid. Based Complement. Alternat. Med. Alt. 2011, 2011, 1–16. [Google Scholar]
- Li, C.; Wang, D.; Dong, H.; Xie, Z.; Hong, H. Proteomics of a toxic dinoflagellate Alexandrium catenella dh01: Detection and identification of cell surface proteins using fluorescent labeling. Chin. Sci. Bull. 2012, 57, 3320–3327. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.Z.; Li, C.; Zhang, Y.; Wang, Y.Y.; He, Z.-P.; Lin, L.; Hong, H.-S. Quantitative proteomic analysis of differentially expressed proteins in the toxicity-lost mutant of Alexandrium catenella (Dinophyceae) in the exponential phase. J. Proteom. 2012, 75, 5564–5577. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Xie, Z.X.; He, Z.P.; Lin, L.; Wang, D.Z. Quantitative proteomic analysis reveals evolutionary divergence and species-specific peptides in the Alexandrium tamarense complex (Dinophyceae). J. Proteom. 2013, 86, 85–96. [Google Scholar] [CrossRef]
- Liu, B.; Lo, S.C.L.; Matton, D.P.; Lang, B.F.; Morse, D. Daily changes in the phosphoproteome of the dinoflagellate Lingulodinium. Protist 2012, 163, 746–754. [Google Scholar] [CrossRef]
- Lee, T.C.H.; Kwok, C.S.N.; Ho, K.C.; Xu, J.L.; Wong, Y.K.; Lee, F.W.F. Effectiveness of previous established TRIzol—methodology for the production of 2-DE of DSP toxin producing Prorocentrum species. In Proceedings of the 1st International Conference on Beneficial Uses of Algal Biomass (ICBUAB 2013), Hong Kong, China, 5–8 November 2013; pp. 79–92. [Google Scholar]
- Jiang, X.W.; Wang, J.; Chan, L.L.; Lam, P.K.S.; Gu, J.-D. Comparison of three protein extraction procedures from toxic and non-toxic dinoflagellates for proteomics analysis. Ecotoxicology 2015, 24, 1395–1406. [Google Scholar] [CrossRef]
- Karthikaichamy, A.; Deore, P.; Rai, V.; Bulach, D.; Beardall, J.; Noronha, S.; Srivastava, S. Time for multiple extraction methods in proteomics? a comparison of three protein extraction methods in the eustigmatophyte alga Microchloropsis gaditana CCMP526. OMICS 2017, 21, 678–683. [Google Scholar] [CrossRef]
- Wang, D.Z.; Lin, L.; Chan, L.L.; Hong, H.-S. Comparative studies of four protein preparation methods for proteomic study of the dinoflagellate Alexandrium sp. using two-dimensional electrophoresis. Harmful Algae 2009, 8, 685–691. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Ramagli, L.S.; Rodriguez, L.V. Quantitation of microgram amounts of protein in two-dimensional polyacrylamide gel electrophoresis sample buffer. Electrophoresis 1985, 6, 559–563. [Google Scholar] [CrossRef]
- Wang, W.; Scali, M.; Vignani, R.; Spadafora, A.; Sensi, E.; Mazzuca, S.; Cresti, M. Protein extraction for two-dimensional electrophoresis from olive leaf, a plant tissue containing high levels of interfering compounds. Electrophoresis 2003, 24, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tai, F.; Chen, S. Optimizing protein extraction from plant tissues for enhanced proteomics analysis. J. Sep. Sci. 2008, 31, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiong, E.; Wang, W.; Scali, M.; Cresti, M. Universal sample preparation method integrating trichloroacetic acid/acetone precipitation with phenol extraction for crop proteomic analysis. Nat. Protoc. 2014, 9, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vignani, R.; Scali, M.; Cresti, M. A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 2006, 27, 2782–2786. [Google Scholar] [CrossRef]
- Reim, D.F.; Speicher, D.W. N-terminal sequence analysis of proteins and peptides. In Current Protocols in Protein Science; Coligan, J.E., Dunn, B.M., Ploegh, H.L., Speicher, D.W., Wingfield, P.T., Eds.; John Wiley & Sons, Inc.: Bundoora, Australia, 2001; Volume 24, pp. 1–31. [Google Scholar]
- Likhite, N.; Warawdekar, U.M. A unique method for isolation and solubilization of proteins after extraction of RNA from tumor tissue using Trizol. J. Biomol. Tech. 2011, 22, 37–44. [Google Scholar]
- Görg, A.; Obermaier, C.; Boguth, G.; Harder, A.; Scheibe, B.; Wildgruber, R.; Weiss, W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 2000, 21, 1037–1053. [Google Scholar] [CrossRef]
- Damerval, C.; Vienne, D.D.; Zivy, M.; Thiellement, H. Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis 1986, 7, 52–54. [Google Scholar] [CrossRef]
- Isaacson, T.; Damasceno, C.M.B.; Saravanan, R.S.; He, Y.; Catalá, C.; Saladié, M.; Rose, J.K.C. Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat. Protoc. 2006, 1, 769–774. [Google Scholar] [CrossRef]
- Wu, X.; Gong, F.; Wang, W. Protein extraction from plant tissues for 2DE and its application in proteomic analysis. Proteomics 2014, 14, 645–658. [Google Scholar] [CrossRef]
- Wang, S.B.; Hu, Q.; Sommerfeld, M.; Chen, F. An optimized protocol for isolation of soluble proteins from microalgae for two-dimensional gel electrophoresis analysis. J. Appl. Phycol. 2003, 15, 485–496. [Google Scholar] [CrossRef]
- Rajalingam, D.; Loftis, C.; Xu, J.J.; Kumar, T.K.S. Trichloroacetic acid-induced protein precipitation involves the reversible association of a stable partially structured intermediate. Protein Sci. 2009, 18, 980–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fic, E.; Kedracka-Krok, S.; Jankowska, U.; Pirog, A.; Dziedzicka-Wasylewska, M. Comparison of protein precipitation methods for various rat brain structures prior to proteomic analysis. Electrophoresis 2010, 31, 3573–3579. [Google Scholar] [CrossRef] [PubMed]


| Type of Microalgae | Species | Year of Publication | Reference |
|---|---|---|---|
| Dinoflagellate | Alexandrium sp. | 2008 | [7] |
| Scrippsiella sp. | 2008 | [7] | |
| Alexandrium affine | 2009 | [6] | |
| Alexandrium catenella | 2011 | [11] | |
| 2012 | [12,13] | ||
| 2013 | [14] | ||
| Linulodinium polyedrum | 2012 | [15] | |
| Alexandrium tamarense | 2013 | [16] | |
| 2015 | [17] | ||
| Prorocentrum donghaiense | 2013 | [10] | |
| Prorocentrum belizeanum | 2013 | [16] | |
| Prorocentrum lima | 2013 | [16] | |
| Prorocentrum rhathymum | 2013 | [16] | |
| Alexandrium minutum | 2015 | [17] | |
| Gymnodinium catenatum | 2015 | [17] | |
| Gynodinium sp. | 2015 | [17] | |
| Cochlodinium geminatum1 | N/A | N/A | |
| Karenia mikimotoi1 | N/A | N/A | |
| Diatom | Fragilariopsis cylindrus | 2011 | [8] |
| Eustigmatophyte | Microchloropsis gaditana | 2017 | [18] |
| Raphidophyte | Chattonella marina1 | N/A | N/A |
| Methods | Solvents and Kits Required | Total Time Required | Ease of Handling |
|---|---|---|---|
| TRIzol method | TRIzol reagent | ~3 h | Manuals are easy to follow |
| Chloroform | |||
| Ethanol | |||
| Isopropanol | |||
| Aliquot TRIzol method | TRIzol reagent | ~4 h | Handling multiple samples simultaneously |
| Chloroform | |||
| Ethanol | |||
| Isopropanol | |||
| Re-TRIzol method | TRIzol reagent | ~6 h | Manuals are easy to follow |
| Chloroform | |||
| Ethanol | |||
| Isopropanol | |||
| TRIzol method with a 2D clean-up kit | TRIzol reagent | ~6 h | Many steps in the clean-up procedure |
| Chloroform | |||
| Ethanol | |||
| Isopropanol | |||
| 2D clean up kit | |||
| Mili-Q water | |||
| TRIzol method with TCA/acetone precipitation | TRIzol reagent | ~20 h 1 | Need for preparation of additional solvents prior to extraction |
| Chloroform | |||
| Ethanol | |||
| Isopropanol | |||
| 10% TCA/acetone | |||
| Cold acetone | |||
| DTT |
| Methods | No. of Spots | Quality of Background | Resolution of Protein Spots | |||
|---|---|---|---|---|---|---|
| PL | PH | PL | PH | PL | PH | |
| TRIzol method | 650 ± 33.95 | 511 ± 98.43 | ++ | - | ++ | + |
| Aliquot TRIzol method | 539 ± 83.43 | N/A | ++ | - | + | - |
| Re-TRIzol method | 434 ± 119.81 * | N/A | ++ | - | - | - |
| TRIzol method with a 2D clean up kit | 650 ± 46 | 551 ± 46.61 | ++ | ++ | ++ | ++ |
| TRIzol method with TCA/acetone precipitation | 329 ± 16.74 * | N/A | ++ | + | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.C.-H.; Lai, K.K.-Y.; Kwok, C.S.-N.; Xu, S.J.-L.; Lee, F.W.-F. Comparison of Five TRIzol-Based Protein Preparation Methods for 2-DE Production From Challenging Marine Dinoflagellate Samples: A Case Study on Two Benthic Prorocentrum Species. J. Mar. Sci. Eng. 2020, 8, 363. https://doi.org/10.3390/jmse8050363
Lee TC-H, Lai KK-Y, Kwok CS-N, Xu SJ-L, Lee FW-F. Comparison of Five TRIzol-Based Protein Preparation Methods for 2-DE Production From Challenging Marine Dinoflagellate Samples: A Case Study on Two Benthic Prorocentrum Species. Journal of Marine Science and Engineering. 2020; 8(5):363. https://doi.org/10.3390/jmse8050363
Chicago/Turabian StyleLee, Thomas Chun-Hung, Kaze King-Yip Lai, Celia Sze-Nga Kwok, Steven Jing-Liang Xu, and Fred Wang-Fat Lee. 2020. "Comparison of Five TRIzol-Based Protein Preparation Methods for 2-DE Production From Challenging Marine Dinoflagellate Samples: A Case Study on Two Benthic Prorocentrum Species" Journal of Marine Science and Engineering 8, no. 5: 363. https://doi.org/10.3390/jmse8050363




