Growth-Prediction Model for Blue Mussels (Mytilus edulis) on Future Optimally Thinned Farm-Ropes in Great Belt (Denmark)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model for Calculating Mussel-Growth History
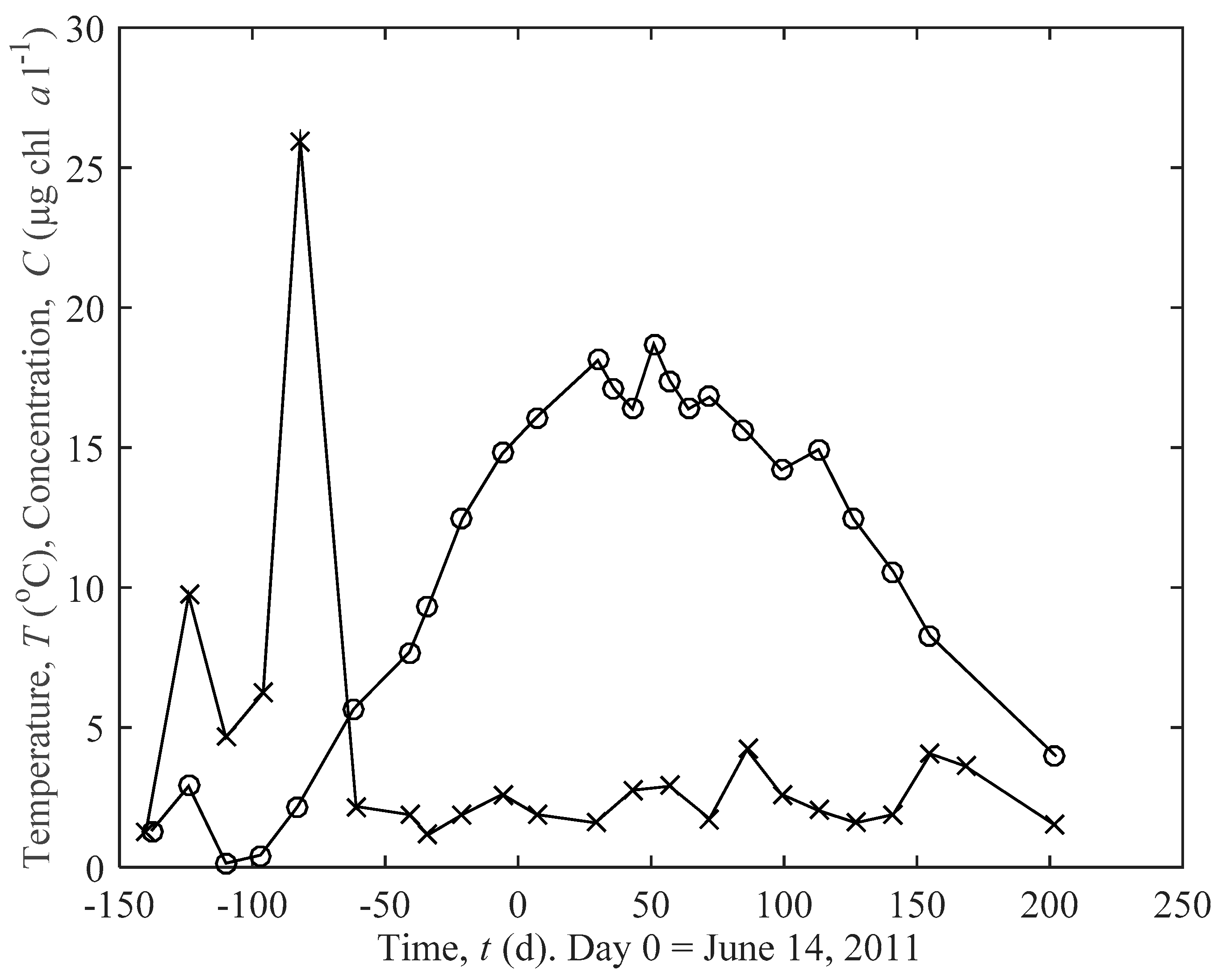
2.2. Field Data and Data Analysis
3. Results
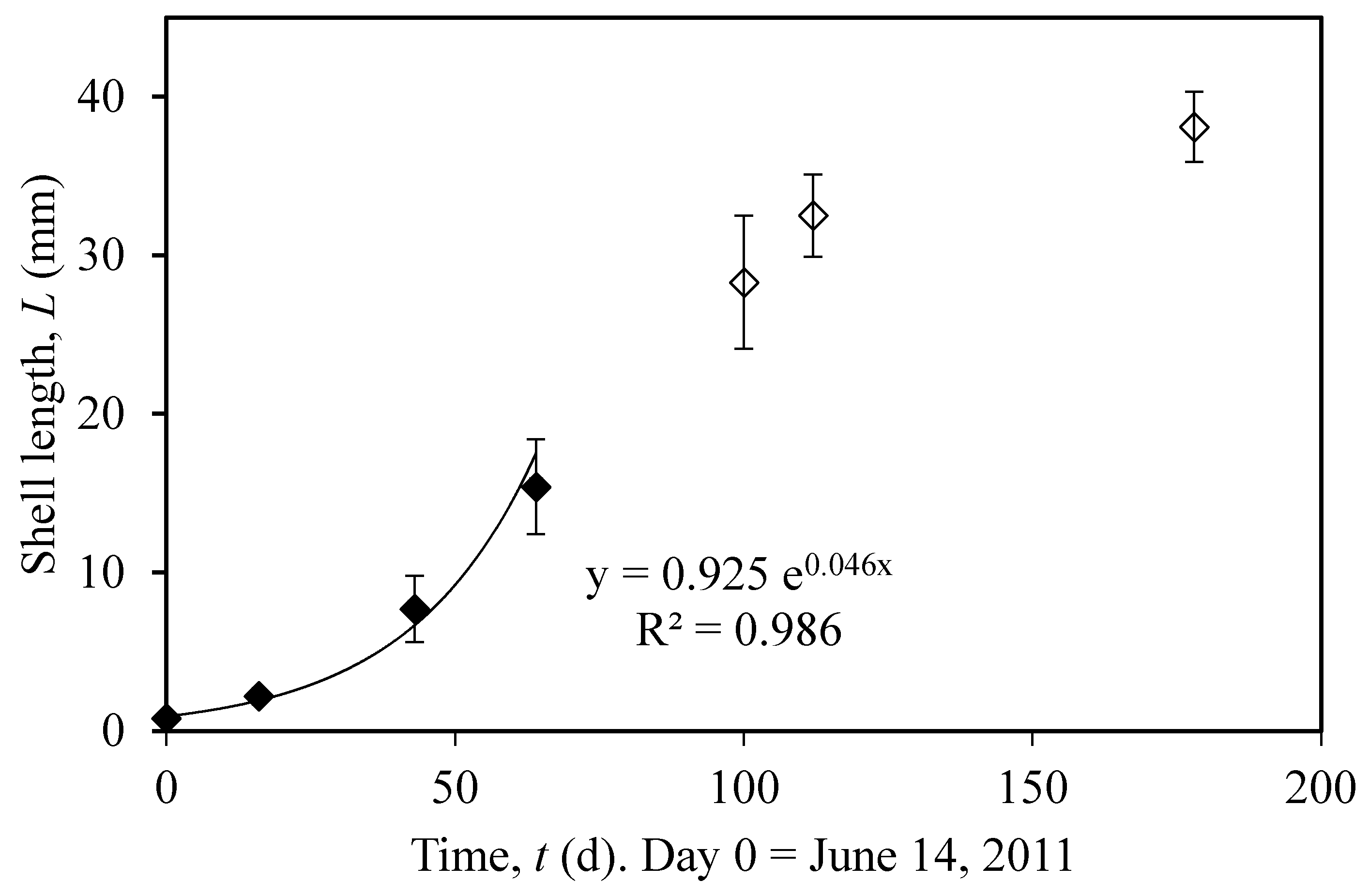
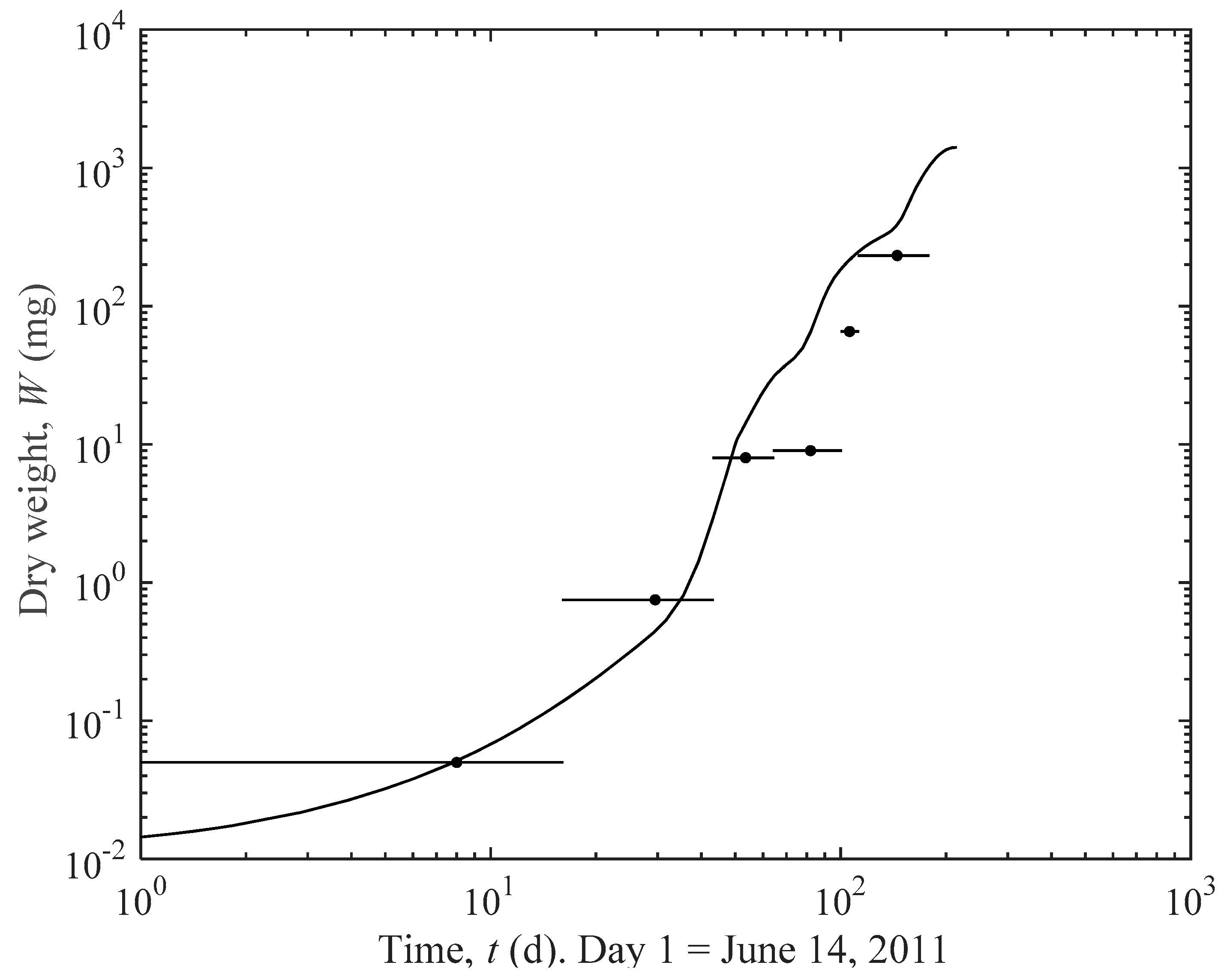
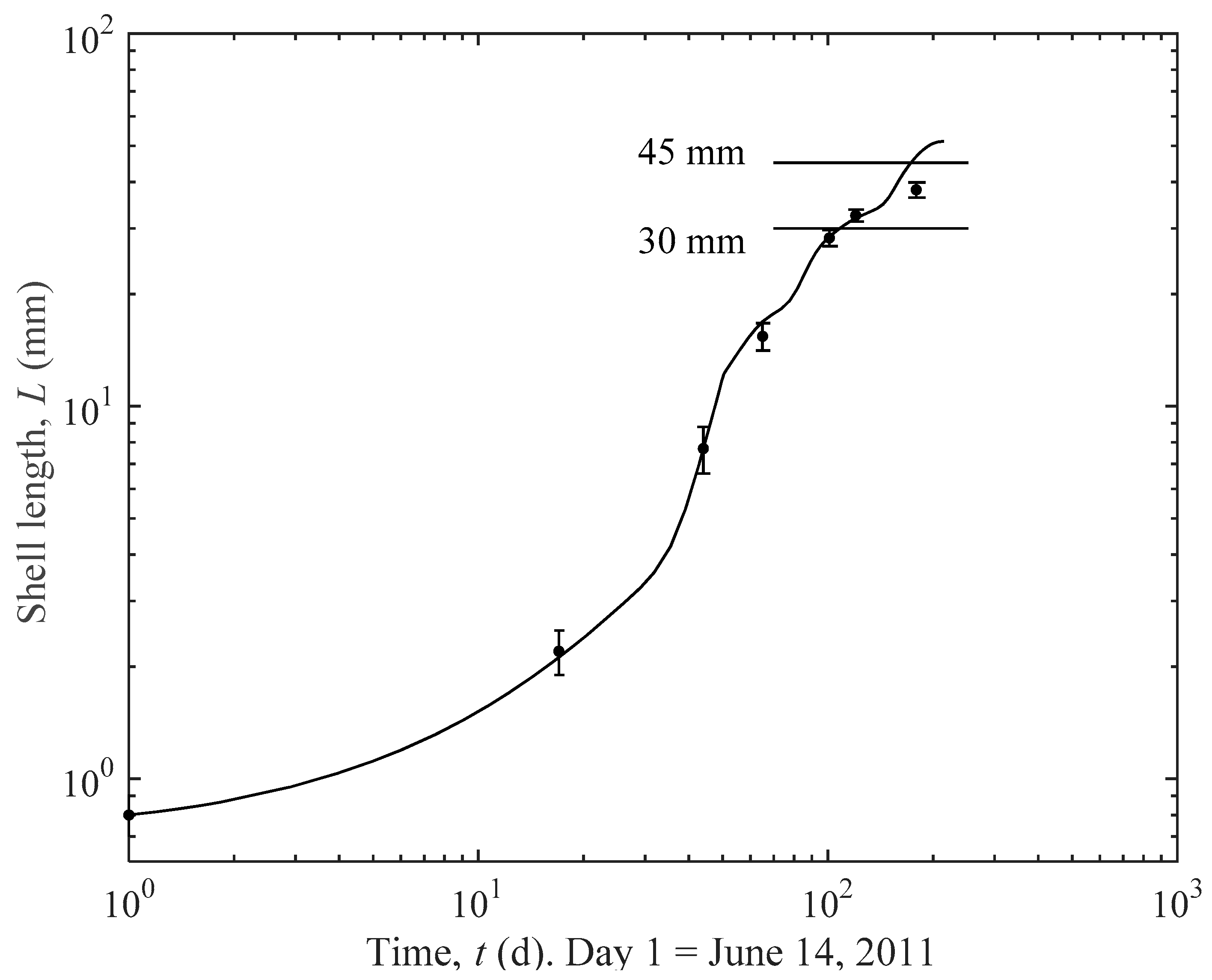
3.1. Actual and Predicted Growth of Mussels, from Newly Settled to Mini-Mussel
3.2. Predicted Influence of chl a and Temperature on Growth
3.3. Sensitivity to Change of Model Exponents
4. Discussion
4.1. Prediction Estimates
4.2. Importance of Thinning for Optimal Growth
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BEG | BioEnergetic Growth model |
Appendix A
- Veliger larvae
- Young post-metamorphic mussels
- Juvenile and adult mussels
- Allometry, W(L)
- Filtration rate, F(W)
- Respiration, R(W)
- F = filtration rate (clearance)
- L = shell length
- Rm = maintenance respiration
- W = dry weight of soft parts
- a0 = 1.12 (factor accounting for cost of growth, assumed = 0.12 × G)
- AE = assimilation efficiency
- C = algal concentration expressed as chlorophyll (chl a)
- G = dW/dt = growth rate
- NGE = net growth efficiency
- t = time
- µ = W−1dW/dt = specific growth rate.
| Eq. | Correlation | Correlation (Alternative Units) | °C | Reference |
|---|---|---|---|---|
| I. Veliger larvae (10−8–10−6 g) | ||||
| 1 | W(µg) = 2.53 × 10−9 L(µm)3.49 | W(mg) = 0.0747 × L(mm)3.49 | 12 | [23] |
| 2 | F(µL·h−1) = 1.25 × 10−5 L(µm)2.85 | F(µL h−1) = 132 × W(µg)0.817 | 17–19 | [23] |
| 3 | F(L h−1) = 10.5 × W(g)0.817 | |||
| 4 | Rm(nL O2 h−1) = 3.10 × W(µg)0.902 | Rm(mL O2 h−1) = 0.801 × W(g)0.903 | 15 | Figure 3 of [8] |
| Potential BEG-model based on Equations (3) and (4) | ||||
| 5 | G = dW/dt = (C × AE × F − Rm)/1.12 | |||
| 6 | G(%g·d−1) = 1.228 × C × (AE/0.8) × W(g)0.817 − 1.664 × W(g)0.902 | |||
| 7 | µ(% d−1) = 1.228 × C × (AE/0.8) × W(g)−0.183 − 1.664 × W(g)−0.098 | |||
| Estimate based on NGE = 2/3 and Equation (4); (NGE ~ 43%–73%) | ||||
| 8 | µ = NGE × R/[(1-NGE) × W] | [7] | ||
| 9 | µ(d−1) = 0.0388 × W(g)−0.097 G(d−1) = 0.0388 × W(g)0.903 | cf. [24] | ||
| Estimate based on measured shell length L versus time t and Equation (1) | 15 | Figure 7 of [23] | ||
| 10 | L(mm) = 0.0634 + 0.0087 × t(d) G(g·d−1) = 0.01254 × W(g)0.713 | |||
| 11 | µL(d−1) = 0.0087 × L(mm)−1 µ(d−1) = 0.01254 × W(g)−0.287 | |||
| Other relations: | ||||
| F(µL·h−1) = 220 × W(µg)0.846 | F(L·h−1) = 26.2 × W(g)0.846 | 15 | Figure 2 of [8] | |
| II. Young post-metamorphic mussels (10−6–10−2 g) | ||||
| 12 | W(mg) = 0.0247 × L(mm)2.42 | see also [5] (Figure 8) | 12 | [23] |
| 13 | F(mL·h−1) = 0.025 × W(µg)1.03 | F(L h−1) = 37.84 × W(g)1.03 | 12–14 | Figure 4 of [6] |
| 14 | Rm(nL O2 h−1) = 0.287 W(µg)1.14 | Rm(mL O2 h−1) = 0.1986 × W(g)1.14 | 12–14 | Figure 6 of [6] |
| Potential BEG-model based on Equations (13) and (14) | ||||
| 15 | G = dW/dt = (C × AE × F − Rm)/1.12 | |||
| 16 | G(%g·d−1) = 4.424 × C × (AE/0.8) × W(g)1.03 − 0.413 × W(g)1.14 | |||
| 17 | µ(% d−1) = 4.424 × C × (AE/0.8) × W(g)0.03 − 0.413 × W(g)0.14 | |||
| 18 | Rm(µL O2 h−1) = 315 × W(g)0.887 | Rm(mL O2 h−1) = 0.315 × W(g)0.887 | 12 | Figure 1 of [7] |
| Extended BEG-model | Based on field data | Figure 5 of [5] | ||
| 19 | G(mg·d−1) = 4.248 × W(mg)0.866 | µ(% d−1) = 4.248 × W(g)−0.134 | Present | |
| Ad hoc BEG-model based on Equations (18) and (19) and scaling of Equation (25) | ||||
| 20 | G(%g·d−1) = [4.89 × C × (AE/0.8) − 5.54] × W(g)0.887 | Present | ||
| III. Juvenile and adult mussels (10−2–1 g) | ||||
| 21 | W(mg) = 0.00215 L(mm)3.40 | Figure 8 of [5] | ||
| 22 | F(L h−1) = 7.45 × W(g)0.66 | 10–13 | Equation (8) of [3] | |
| 23 | Rm(µL O2 h−1) = 475 × W(g)0.663 | Rm(mL O2 h−1) = 0.475 × W(g)0.663 | 14 | Figure 1 of [7] |
| Basic BEG-model based on Equations (23) and (24) | ||||
| 24 | G = dW/dt = (C × AE × F − Rm)/1.12 | [25] | ||
| 25 | G(%g·d−1) = 0.871 × C × (AE/0.8) × W(g)0.663 − 0.986 × W(g)0.66 | [3] | ||
| 26 | µ(% d−1) = 0.871 × C × (AE/0.8) × W(g)−0.34 − 0.986 × W(g)−0.34 | |||
| 27 | Modified BEG-model: G(mg·d−1) = C1 × W(mg)0.66 | Equation (5) of [4] | ||
| 28 | C1 = 0.1047 × m1 × (0.871 × m2 × n2 × C × AE/0.80 − 0.986 × m3 × n3) | |||
| 29 | m1 = 1.12/[1 + (a0 − 1)n3 (1 − E)]; m2 = 1 − E; m3 = 1 − 0.9(1 + C/C0)E; | |||
| 30 | E = exp(−C/C0); C0 = 0.4 µg chl a l−1; n2 = [1 + 0.0251(T − TF)]; | |||
| 31 | n3 = 1.54(T−TQ)/10; TF = 11.5 °C; TQ = 14 °C | |||
References
- Dolmer, P.; Frandsen, R.P. Evaluation of the Danish mussel fishery: Suggestions for an ecosystem management approach. Helgol. Mar. Res. 2002, 56, 13–20. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Lundgreen, K.; Larsen, P.S. Potential for production of ‘mini-mussels’ in Great Belt (Denmark) evaluated on basis of actual growth of young mussels Mytilus edulis. Aquacult. Int. 2014, 22, 859–885. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Lundgreen, K.; Larsen, P.S. Field data and growth model for mussels Mytilus edulis in Danish waters. Mar. Biol. Res. 2012, 8, 683–700. [Google Scholar] [CrossRef]
- Larsen, P.S.; Filgueira, R.; Riisgård, H.U. Somatic growth of mussels Mytilus edulis in field studies compared to predictions using BEG, DEB, and SFG models. J. Sea Res. 2014, 88, 100–108. [Google Scholar] [CrossRef]
- Larsen, P.S.; Lundgreen, K.; Riisgård, H.U. Bioenergetic model predictions of actual growth and allometric transitions during ontogeny of juvenile blue mussels Mytilus edulis. In Mussels: Ecology, Life Habits and Control; Nowak, J., Kozlowski, M., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 101–122. [Google Scholar]
- Riisgård, H.U.; Randløv, A.; Kristensen, P.S. Rates of water processing, oxygen consumption and efficiency of particle retention in veligers and young post-metamorphic Mytilus edulis. Ophelia 1980, 19, 37–47. [Google Scholar] [CrossRef]
- Hamburger, K.; Møhlenberg, F.; Randløv, A.; Riisgård, H.U. Size, oxygen consumption and growth in the mussel Mytilus edulis. Mar. Biol. 1983, 75, 303–306. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Randløv, A.; Hamburger, K. Oxygen consumption and clearance as a function of size in Mytilus edulis L. veliger larvae. Ophelia 1981, 20, 179–183. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Pleissner, D.; Lundgreen, K.; Larsen, P.S. Growth of mussels Mytilus edulis at algal (Rhodomonas salina) concentrations below and above saturation levels for reducted filtration rate. Mar. Biol. Res. 2013, 9, 1005–1017. [Google Scholar] [CrossRef]
- Pleissner, D.; Lundgreen, K.; Lüskow, F.; Riisgård, H.U. Fluorometer controlled apparatus designed for long-term algal-feeding experiments and environmental effect studies with mussels. J. Mar. Biol. 2013. [Google Scholar] [CrossRef]
- Fréchette, M.; Urquiza, J.M.; Daigle, G.; Rioux-Gagnon, D. Clearance rate regulation in mussels: Adding the effect of seston level to a model of internal state-based regulation. J. Exp. Mar. Biol. Ecol. 2016, 475, 1–10. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Egede, P.P.; Saavedra, I.B. Feeding behaviour of mussels, Mytilus edulis, with a mini-review of current knowledge. Mar. Res. 2011, 13. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Larsen, P.S. Physiologically regulated valve-closure makes mussels long-term starvation survivors: test of hypothesis. J. Mollus. Stud. 2015, 81, 303–307. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Lundgreen, K.; Pleissner, D. Environmental factors and seasonal variation in density of mussel larvae (Mytilus edulis) in Danish waters. Open J. Mar. Sci. 2015, 5, 280–289. [Google Scholar] [CrossRef]
- Alunno-Bruscia, M.; Petraitis, P.S.; Bourget, E.; Fréchette, M. Body size-density relationship for Mytilus edulis in an experimental food-regulated situation. Oikos 2000, 90, 28–42. [Google Scholar] [CrossRef]
- Filgueira, R.; Peteiro, L.G.; Labarta, U.; Fernández-Reiriz, M.J. The self-thinning rule applied to cultured populations in aggregate growth matrices. J. Mollus. Stud. 2008, 74, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Lachance-Bernard, M.; Daigle, G.; Himmelman, J.H.; Fréchette, M. Biomass-density relationships and self-thinning of blue mussels (Mytilus spp.) reared on self-regulated longlines. Aquaculture 2010, 308, 34–43. [Google Scholar] [CrossRef]
- Cubillo, A.M.; Peteiro, L.G.; Fernández-Reiriz, M.J.; Labarta, U. Influence of stocking density on growth of mussels (Mytilus galloprovincialis) in suspended culture. Aquaculture 2012, 342–343, 103–111. [Google Scholar] [CrossRef]
- Jacobs, P.; Troost, K.; Riegman, R.; van der Meer, J. Length- and weight-dependent clearance rates of juvenile mussels (Mytilus edulis) on various planktonic prey items. Helgol. Mar. Res. 2015, 69, 101–112. [Google Scholar] [CrossRef]
- Bacher, C.; Gangnery, A. Use of dynamic energy budget and individual based models to simulate the dynamics of cultivated oyster populations. J. Sea Res. 2006, 56, 140–155. [Google Scholar] [CrossRef]
- Brigolin, D.; Maschio, G.D.; Rampazzo, F.; Giani, M.; Pastres, R. An individual-based population dynamic model for estimating biomass yield and nutrient fluxes through an off-shore mussel (Mytilus galloprovincialis) farm. Estuar. Coast. Shelf Sci. 2009, 82, 365–376. [Google Scholar] [CrossRef]
- Theisen, B. Shell cleaning and deposit feeding in Mytilus edulis L. (Bivalvia). Ophelia 1972, 10, 49–55. [Google Scholar] [CrossRef]
- Jespersen, H.; Karin Olsen, K. Bioenergetics in veliger larvae of Mytilus edulis L. Ophelia 1982, 21, 101–113. [Google Scholar] [CrossRef]
- Jørgensen, C.B. Growth efficiencies and factors controlling size in some mytilid bivalves, especially Mytilus edulis L.: Review and interpretation. Ophelia 1976, 15, 175–192. [Google Scholar] [CrossRef]
- Clausen, I.; Riisgård, H.U. Growth, filtration and respiration in the mussel Mytilus edulis: No regulation of the filter-pump to nutritional needs. Mar. Ecol. Prog. Ser. 1996, 141, 37–45. [Google Scholar] [CrossRef]
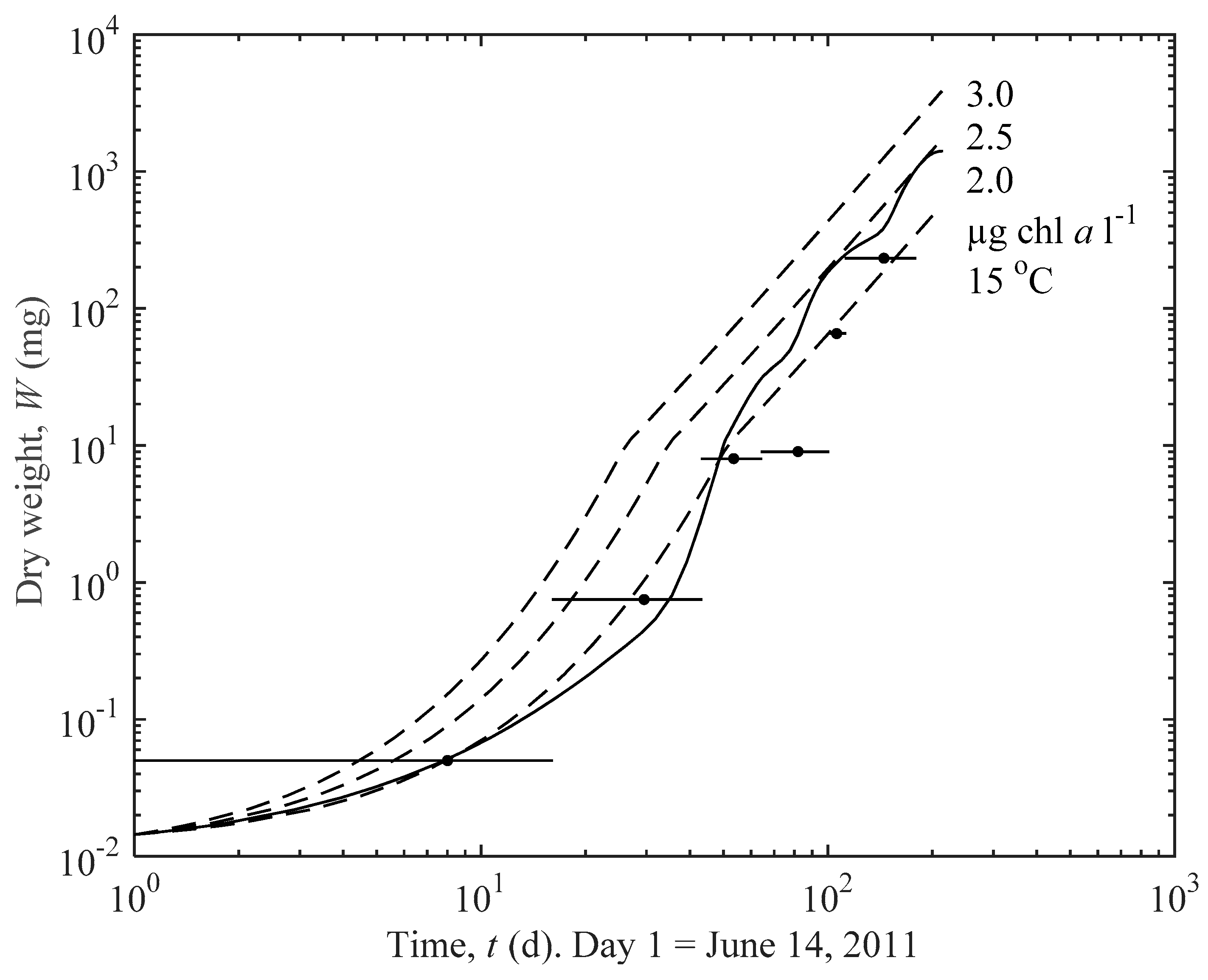
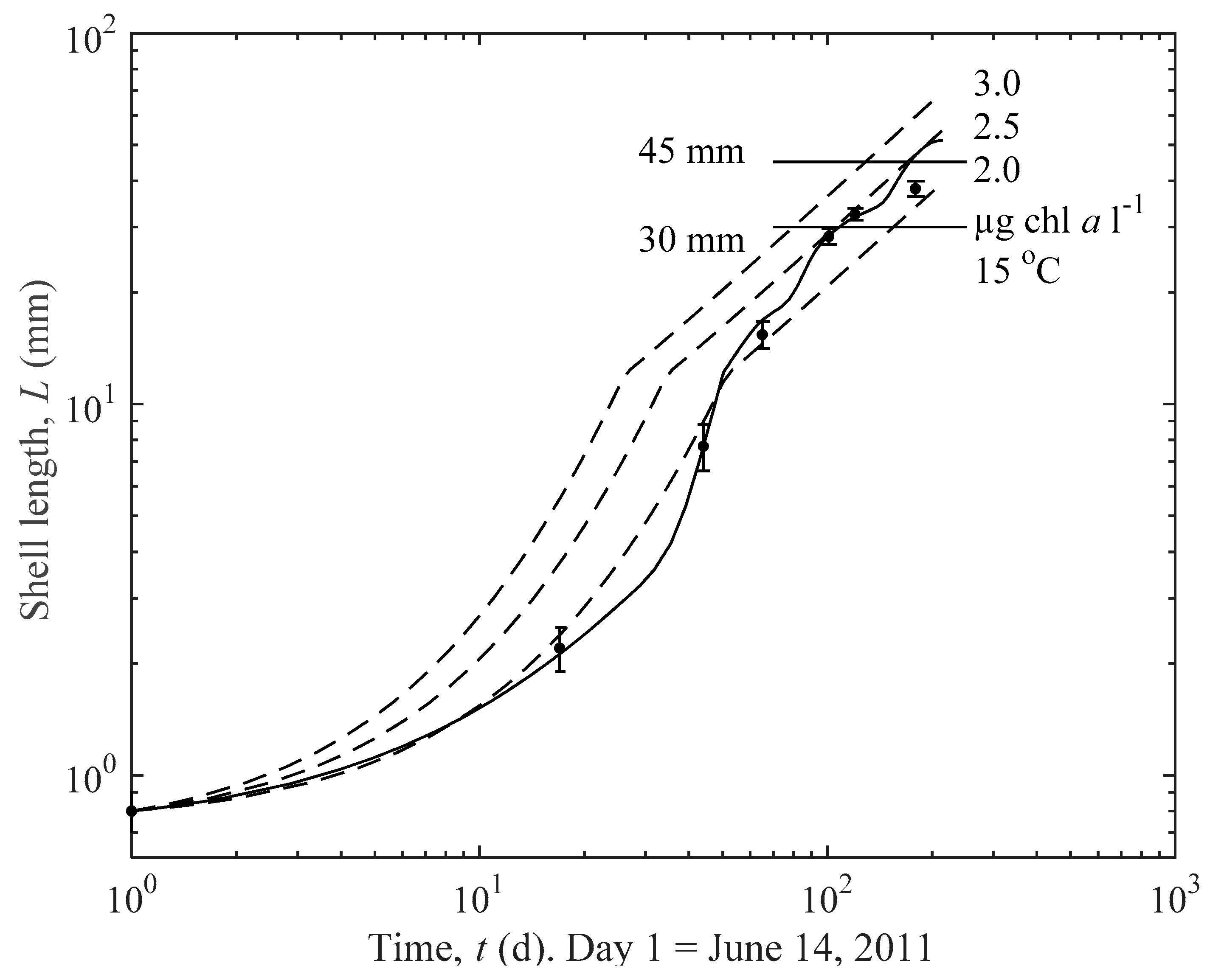
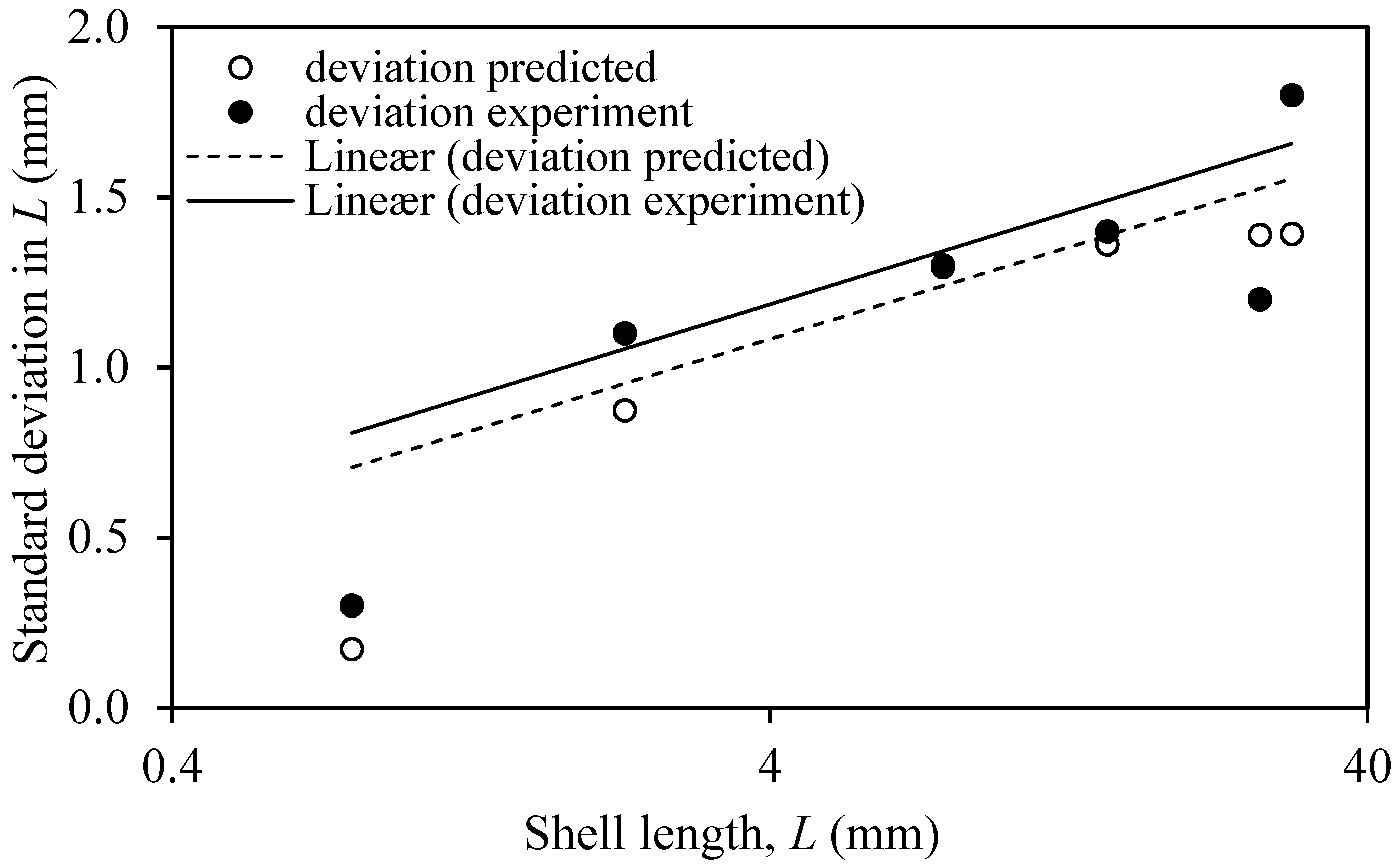
| m1 = 1.12/[1 + (a0 − 1)n3 (1 − E)] |
| m2 = 1 − E |
| m3 = 1 − 0.9(1 + C/C0)E |
| E = exp(−C/C0) |
| C0 = 0.4 µg chl a L−1 |
| n2 = [1 + 0.0251(T − TF)] |
| n3 = 1.54(T−TQ)/10 |
| TF = 11.5 °C; TQ = 14 °C |
| T = 15 °C | |||
| C (µg chl a L−1) | 2 | 2.5 | 3 |
| t30 (d) | 155 | 105 | 80 |
| t45 (d) | 249 | 170 | 129 |
| C = 2.5 µg chl a L−1 | |||
| T (°C) | 5 | 10 | 15 |
| t30 (d) | 115 | 109 | 105 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larsen, P.S.; Riisgård, H.U. Growth-Prediction Model for Blue Mussels (Mytilus edulis) on Future Optimally Thinned Farm-Ropes in Great Belt (Denmark). J. Mar. Sci. Eng. 2016, 4, 42. https://doi.org/10.3390/jmse4030042
Larsen PS, Riisgård HU. Growth-Prediction Model for Blue Mussels (Mytilus edulis) on Future Optimally Thinned Farm-Ropes in Great Belt (Denmark). Journal of Marine Science and Engineering. 2016; 4(3):42. https://doi.org/10.3390/jmse4030042
Chicago/Turabian StyleLarsen, Poul S., and Hans Ulrik Riisgård. 2016. "Growth-Prediction Model for Blue Mussels (Mytilus edulis) on Future Optimally Thinned Farm-Ropes in Great Belt (Denmark)" Journal of Marine Science and Engineering 4, no. 3: 42. https://doi.org/10.3390/jmse4030042
APA StyleLarsen, P. S., & Riisgård, H. U. (2016). Growth-Prediction Model for Blue Mussels (Mytilus edulis) on Future Optimally Thinned Farm-Ropes in Great Belt (Denmark). Journal of Marine Science and Engineering, 4(3), 42. https://doi.org/10.3390/jmse4030042







