Viral and Bacterial Epibionts in Thermally-Stressed Corals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Enumeration of Symbiodinium, Bacteria, and Viruses
2.3. Bacterial Physiological State
2.4. Viral Lytic Production
2.5. DGGE Analysis of Bacteria Community Structure
2.6. Statistical Analysis
3. Results
3.1. Abundances of Symbiodinium, Bacteria and Viruses
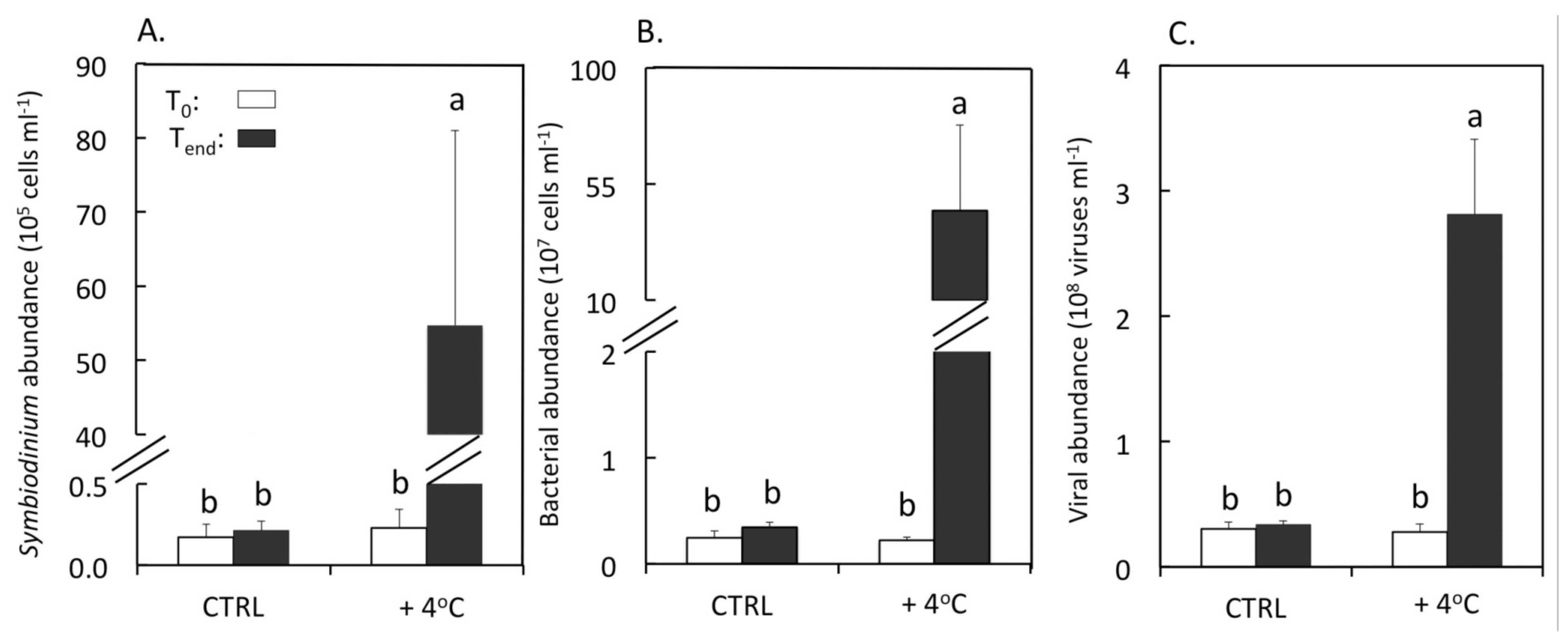
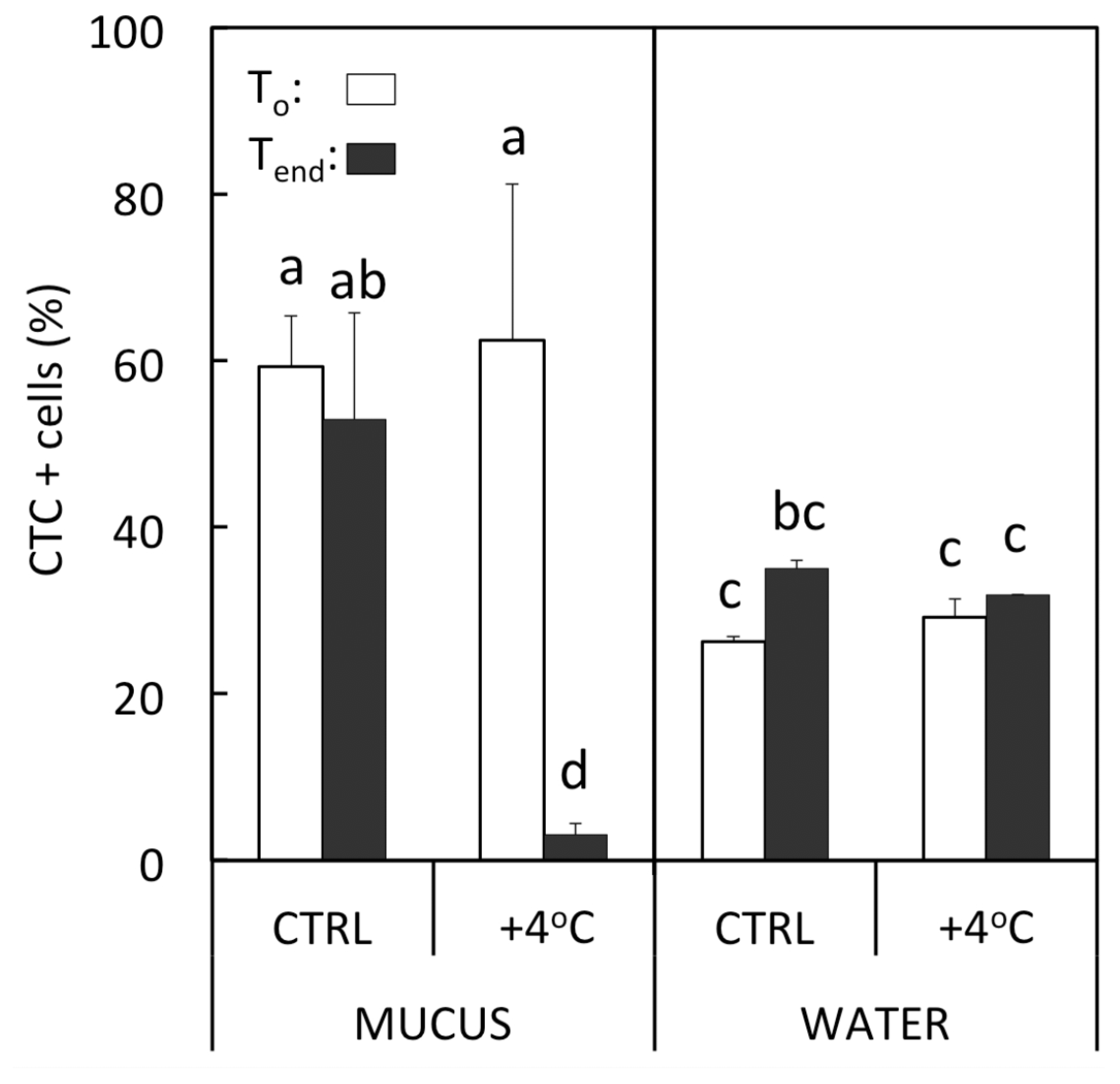
3.2. Physiological State of Bacteria
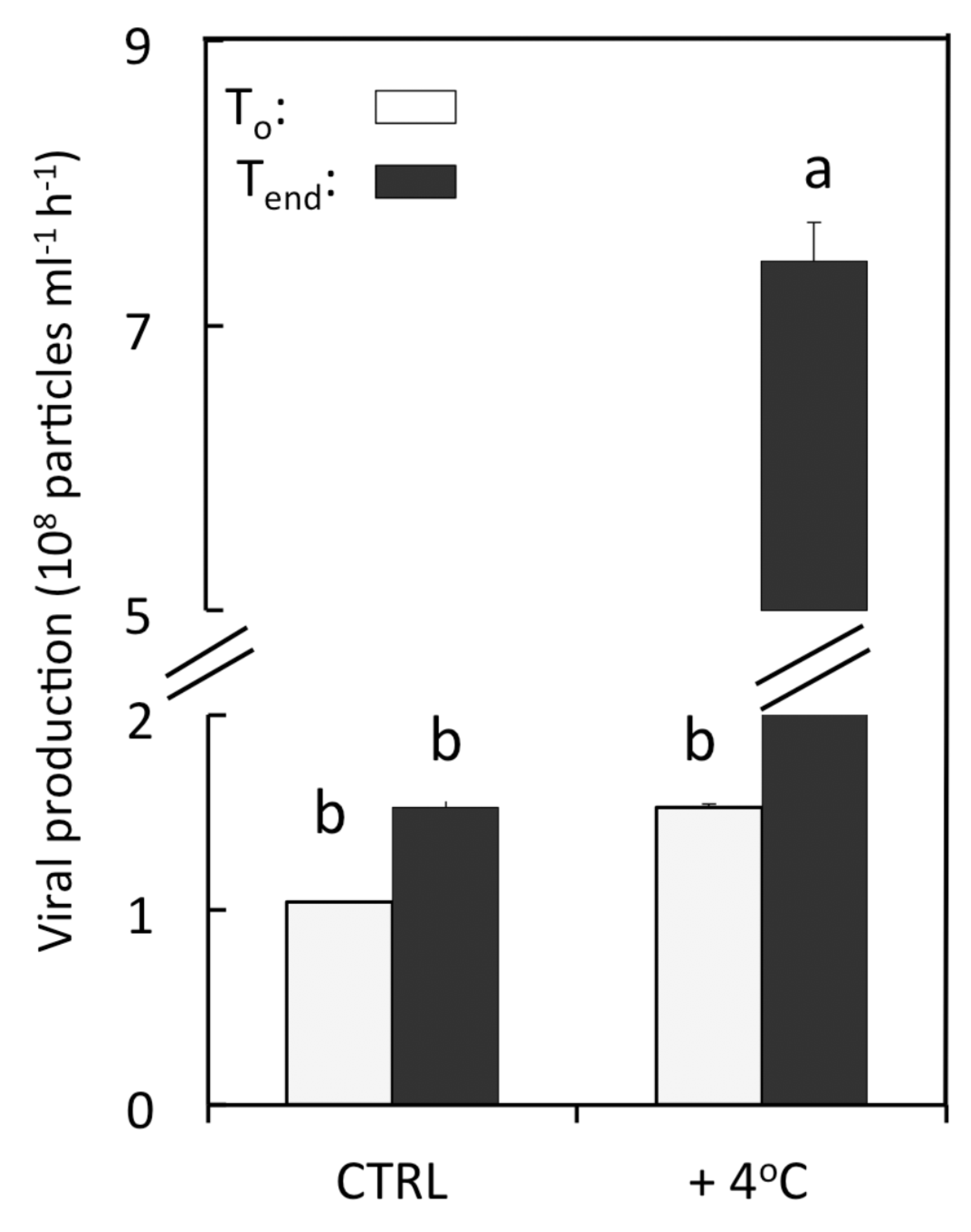
3.3. Viral Lytic Production
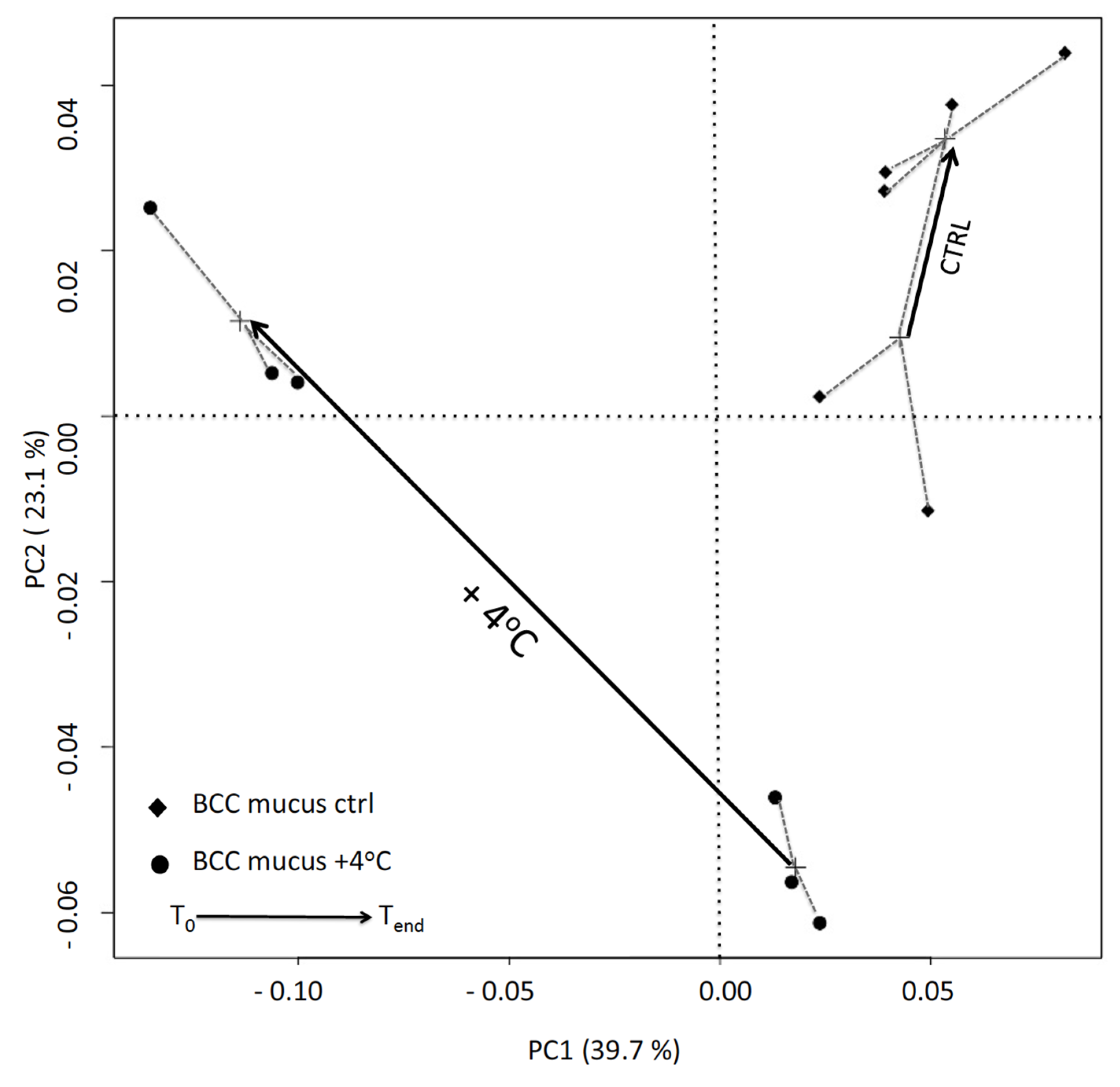
3.4. DGGE Analysis
4. Discussion
4.1. High Abundance but Low Metabolic Activity of Bleached-Coral-Associated Bacteria
4.2. High Viral Production and Abundance in the Mucus of Bleached F. repanda
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoegh-Guldberg, O. Coral reef ecosystems and anthropogenic climate change. Reg. Environ. Chang. 2011, 11, S215–S227. [Google Scholar] [CrossRef]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reefs 1997, 16, S129–S138. [Google Scholar] [CrossRef]
- Glynn, P.W.; Dcroz, L. Experimental evidence for high temperature stress as the cause of El Nino-coincident coral mortality. Coral Reefs 1990, 8, 181–191. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Brown, E.K. Global warming, regional trends and inshore environmental conditions influence coral bleaching in hawaii. Glob. Chang. Biol. 2004, 10, 1627–1641. [Google Scholar] [CrossRef]
- Lesser, M.P.; Stochaj, W.R.; Tapley, D.W.; Shick, J.M. Bleaching in coral reef anthozoans: Effects of irradiance, ultraviolet radiation and temperature on the activities of protective enzymes against active oxygen. Coral Reefs 1990, 8, 225–232. [Google Scholar] [CrossRef]
- Coles, S.L.; Jokiel, P.L. Synergistic effects of temperature, salinity and light on hermatypic coral Montipora verrucosa. Mar. Biol. 1978, 49, 187–195. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef] [PubMed]
- Guzman, H.M.; Jackson, J.B.C.; Weil, E. Short term ecological consequences of a major oil spill on panamanian subtidal reef corals. Coral Reefs 1991, 10, 1–12. [Google Scholar] [CrossRef]
- Vega Thurber, R.L.; Burkepile, D.E.; Fuchs, C.; Shantz, A.A.; McMinds, R.; Zaneveld, J.R. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Chang. Biol. 2014, 20, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Kushmaro, A.; Kramarsky-Winter, E. Bacteria as a Source of Coral Nutrition. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer-Verlag: New York, NY, USA, 2004; pp. 231–241. [Google Scholar]
- Rosenberg, E.; Kushmaro, A.; Kramarsky-Winter, E.; Banin, E.; Yossi, L. The role of microorganisms in coral bleaching. ISME J. 2009, 3, 139–146. [Google Scholar] [CrossRef] [PubMed]
- de Lima, L.A.; Migliolo, L.; Barreiro e Castro, C.; Pires, D.D.; Lopez-Abarrategui, C.; Goncalves, E.F.; Vasconcelos, I.M.; de Oliveira, J.T.A.; Otero-Gonzalez, A.D.J.; Franco, O.L.; et al. Identification of a novel antimicrobial peptide from brazilian coast coral Phyllogorgia dilatata. Protein Pept. Lett. 2013, 20, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Kvennefors, E.C.E.; Sampayo, E.; Kerr, C.; Vieira, G.; Roff, G.; Barnes, A.C. Regulation of bacterial communities through antimicrobial activity by the coral holobiont. Microb. Ecol. 2012, 63, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Rypien, K.L.; Ward, J.R.; Azam, F. Antagonistic interactions among coral-associated bacteria. Environ. Microbiol. 2010, 12, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Shnit-Orland, M.; Sivan, A.; Kushmaro, A. Antibacterial activity of Pseudoalteromonas in the coral holobiont. Microb. Ecol. 2012, 64, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Reshef, L.; Koren, O.; Loya, Y.; Zilber-Rosenberg, I.; Rosenberg, E. The coral probiotic hypothesis. Environ. Microb. 2006, 8, 2068–2073. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Koren, O.; Reshef, L.; Efrony, R.; Zilber-Rosenberg, I. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microb. 2007, 5, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haim, Y.; Rosenberg, E. A novel Vibrio sp. pathogen of the coral pocillopora damicornis. Mar. Biol. 2002, 141, 47–55. [Google Scholar]
- Kushmaro, A.; Banin, E.; Loya, Y.; Stackebrandt, E.; Rosenberg, E. Vibrio shiloi sp. nov., the causative agent of bleaching of the coral oculina patagonica. Int. J. Syst. Evolut. Microbiol. 2001, 51, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Kushmaro, A.; Rosenberg, E.; Fine, M.; Loya, Y. Bleaching of the coral oculina patagonica by vibrio ak-1. Mar. Ecol. Prog. Ser. 1997, 147, 159–165. [Google Scholar] [CrossRef]
- Toren, A.; Landau, L.; Kushmaro, A.; Loya, Y.; Rosenberg, E. Effect of temperature on adhesion of vibrio strain ak-1 to oculina patagonica and on coral bleaching. Appl. Environ. Microbiol. 1998, 64, 1379–1384. [Google Scholar] [PubMed]
- Ainsworth, T.; Fine, M.; Roff, G.; Hoegh-Guldberg, O. Bacteria are not the primary cause of bleaching in the mediterranean coral Oculina patagonica. ISME J. 2008, 2, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Leggat, W.; Hoegh-Guldberg, O.; Dove, S.; Yellowlees, D. Analysis of an est library from the dinoflagellate (Symbiodinium sp.) symbiont of reef-building corals. J. Phycol. 2007, 43, 1010–1021. [Google Scholar] [CrossRef]
- Bourne, D.; Iida, Y.; Uthicke, S.; Smith-Keune, C. Changes in coral-associated microbial communities during a bleaching event. ISME J. 2008, 2, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Lins-de-Barros, M.M.; Cardoso, A.M.; Silveira, C.B.; Lima, J.L.; Clementino, M.M.; Martins, O.B.; Albano, R.M.; Vieira, R.P. Microbial community compositional shifts in bleached colonies of the brazilian reef-building coral siderastrea stellata. Microb. Ecol. 2013, 65, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Littman, R.; Willis, B.L.; Bourne, D.G. Metagenomic analysis of the coral holobiont during a natural bleaching event on the great barrier reef. Environ. Microbiol. Rep. 2011, 3, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Vega Thurber, R.; Willner-Hall, D.; Rodriguez-Mueller, B.; Desnues, C.; Edwards, R.A.; Angly, F.; Dinsdale, E.; Kelly, L.; Rohwer, F. Metagenomic analysis of stressed coral holobionts. Environ. Microbiol. 2009, 11, 2148–2163. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A. Marine viruses and their biogeochemical and ecological effects. Nature 1999, 399, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Marine viruses—Major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Davy, J.E.; Patten, N.L. Morphological diversity of virus-like particles within the surface microlayer of scleractinian corals. Aquat. Microb. Ecol. 2007, 47, 37–44. [Google Scholar] [CrossRef]
- Leruste, A.; Bouvier, T.; Bettarel, Y. Enumerating viruses in coral mucus. Appl. Environ. Microbiol. 2012, 78, 6377–6379. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Kim, H.; Bouvier, T.; Bouvier, C.; Doan, N.H.; Nguyen, N.L.; Rochelle-Newall, E.; Desnues, C.; Reynaud, S.; Ferrier-Pages, C.; Bettarel, Y. High occurence of viruses in the mucus layer of scleractinian corals. Environ. Microbiol. Rep. 2014, 6, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Weinbauer, M.G.; Ogier, J.; Maier, C. Microbial abundance in the coelenteron and mucus of the cold-water coral lophelia pertusa and in bottom water of the reef environment. Aquat. Biol. 2012, 16, 209–216. [Google Scholar] [CrossRef]
- Barr, J.J.; Auro, R.; Furlan, M.; Whiteson, K.L.; Erb, M.L.; Pogliano, J.; Stotland, A.; Wolkowicz, R.; Cutting, A.S.; Doran, K.S.; et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 10771–10776. [Google Scholar] [CrossRef] [PubMed]
- Van Oppen, M.J.H.; Leong, J.A.; Gates, R.D. Coral-virus interactions: A double-edged sword? Symbiosis 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Vega Thurber, R.L.; Correa, A.M.S. Viruses of reef-building scleractinian corals. J. Exp. Mar. Biol. Ecol. 2011, 408, 102–113. [Google Scholar] [CrossRef]
- Correa, A.M.; Welsh, R.M.; Thurber, R.L.V. Unique nucleocytoplasmic dsdna and +ssrna viruses are associated with the dinoglagellate endosymbionts of corals. ISME J. 2013, 7, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Dale, A.L.; Davy, J.E.; Davy, S.K. An enemy within? Observations of virus-like particles in reef corals. Coral Reefs 2005, 24, 145–148. [Google Scholar] [CrossRef]
- Bettarel, Y.; Bouvier, T.; Nguyen, H.K.; Thu, P.T. The versatile nature of coral associated viruses. Environ. Microbiol. 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.S.; Niggl, W.; Laforsch, C.; Glaser, C.; Wild, C. Coral surface area quantification-evaluation of established techniques by comparison with computer tomography. Coral Reefs 2009, 28, 109–117. [Google Scholar] [CrossRef]
- Williamson, K.E.; Wommack, K.E.; Radosevich, M. Sampling natural viral communities from soil for culture-independent analyses. Appl. Environ. Microbiol. 2003, 69, 6628–6633. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Noble, R.T.; Steele, J.A.; Schwalbach, M.S.; Hewson, I.; Fuhrman, J.A. Virus and prokaryote enumeration from planktonic aquatic environments by epifluorescence microscopy with sybr green i. Nat. Protoc. 2007, 2, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Sherr, B.F.; del Giorgio, P.; Sherr, E.B. Estimating abundance and single-cell characteristics of respiring bacteria via the redox dye ctc. Aquat. Microb. Ecol. 1999, 18, 117–131. [Google Scholar] [CrossRef]
- Bettarel, Y.; Desnues, A.; Rochelle-Newall, E. Lytic failure in cross-inoculation assays between phages and prokaryotes from three aquatic sites of contrasting salinity. FEMS Microbiol. Lett. 2010, 311, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.R.; Velimirov, B. High control of bacterial production by viruses in a eutrophic oxbow lake. Aquat. Microb. Ecol. 2002, 27, 1–12. [Google Scholar] [CrossRef]
- Morrow, K.M.; Moss, A.G.; Chadwick, N.E.; Liles, M.R. Bacterial associates of two caribbean coral species reveal species-specific distribution and geographic variability. Appl. Environ. Microbiol. 2012, 78, 6438–6449. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Kuenen, J.G.; Muyzer, G. Nested pcr-denaturing gradient gel electrophoresis approach to determine the diversity of sulfate-reducing bacteria in complex microbial communities. Appl. Environ. Microbiol. 2005, 71, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Ovreas, L.; Forney, L.; Daae, F.L.; Torsvik, V. Distribution of bacterioplankton in meromictic lake saelenvannet, as determined by denaturing gradient gel electrophoresis of pcr-amplified gene fragments coding for 16s rrna. Appl. Environ. Microbiol. 1997, 63, 3367–3373. [Google Scholar] [PubMed]
- Harvell, D.; Jordan-Dahlgren, E.; Merkel, S.; Rosenberg, E.; Raymundo, L.; Smith, G.; Weil, E.; Willis, B. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography 2007, 20, 172–195. [Google Scholar] [CrossRef]
- Pires, A.P.F.; Guariento, R.D.; Laque, T.; Esteves, F.A.; Farjalla, V.F. The negative effects of temperature increase on bacterial respiration are independent of changes in community composition. Environ. Microbiol. Rep. 2014, 6, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.B. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Prog. Ser. 2006, 322, 1–14. [Google Scholar] [CrossRef]
- Del Giorgio, P.A.; Bouvier, T.C. Linking the physiologic and phylogenetic successions in free-living bacterial communities along an estuarine salinity gradient. Limnol. Oceanogr. 2002, 47, 471–486. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Hill, R.; Doblin, M.A.; Ralph, P.J. Microbial consortia increase thermal tolerance of corals. Mar. Biol. 2012, 159, 1763–1771. [Google Scholar] [CrossRef]
- Garren, M.; Son, K.; Raina, J.B.; Rusconi, R.; Menolascina, F.; Shapiro, O.H.; Tout, J.; Bourne, D.G.; Seymour, J.R.; Stocker, R. A bacterial pathogen uses dimethylsulfoniopropionate as a cue to target heat-stressed corals. ISME J. 2014, 8, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Lasker, H.R.; Peters, E.C.; Coffroth, M.A. Bleaching of reef coelenterates in san-blas islands, panama. Coral Reefs 1984, 3, 183–190. [Google Scholar] [CrossRef]
- Piggot, A.M.; Fouke, B.W.; Sivaguru, M.; Sanford, R.A.; Gaskins, H.R. Change in zooxanthellae and mucocyte tissue density as an adaptive response to environmental stress by the coral, montastraea annularis. Mar. Biol. 2009, 156, 2379–2389. [Google Scholar] [CrossRef]
- Marhaver, K.L.; Edwards, R.A.; Rohwer, F. Viral communities associated with healthy and bleaching corals. Environ. Microbiol. 2008, 10, 2277–2286. [Google Scholar] [PubMed]
- Rohwer, F.; Seguritan, V.; Azam, F.; Knowlton, N. Diversity and distribution of coral-associated bacteria. Mar. Ecol. Prog. Ser. 2002, 243, 1–10. [Google Scholar] [CrossRef]
- Maurice, C.F.; Bouvier, C.; de Wit, R.; Bouvier, T. Linking the lytic and lysogenic bacteriophage cycles to environmental conditions, host physiology and their variability in coastal lagoons. Environ. Microbiol. 2013, 15, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Weinbauer, M.G. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Kim, H.; Bettarel, Y.; Bouvier, T.; Bouvier, C.; Doan-Nhu, H.; Nguyen-Ngoc, L.; Nguyen-Thanh, T.; Tran-Quang, H.; Brune, J. Coral mucus is a hot spot of viral infections. Appl. Environ. Microbiol. 2015, 81. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.W.; Wild, C. Coral mucus release and following particle trapping contribute to rapid nutrient recycling in a northern red sea fringing reef. Mar. Freshw. Res. 2010, 61, 1006–1014. [Google Scholar] [CrossRef]
- Wild, C.; Huettel, M.; Klueter, A.; Kremb, S.G.; Rasheed, M.Y.M.; Jorgensen, B.B. Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 2004, 428, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.; Munn, C.B.; Wilson, W.H. Characterization of a latent virus-like infection of symbiotic zooxanthellae. Appl. Environ. Microbiol. 2007, 73, 2976–2981. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen-Kim, H.; Bouvier, T.; Bouvier, C.; Bui, V.N.; Le-Lan, H.; Bettarel, Y. Viral and Bacterial Epibionts in Thermally-Stressed Corals. J. Mar. Sci. Eng. 2015, 3, 1272-1286. https://doi.org/10.3390/jmse3041272
Nguyen-Kim H, Bouvier T, Bouvier C, Bui VN, Le-Lan H, Bettarel Y. Viral and Bacterial Epibionts in Thermally-Stressed Corals. Journal of Marine Science and Engineering. 2015; 3(4):1272-1286. https://doi.org/10.3390/jmse3041272
Chicago/Turabian StyleNguyen-Kim, Hanh, Thierry Bouvier, Corinne Bouvier, Van Ngoc Bui, Huong Le-Lan, and Yvan Bettarel. 2015. "Viral and Bacterial Epibionts in Thermally-Stressed Corals" Journal of Marine Science and Engineering 3, no. 4: 1272-1286. https://doi.org/10.3390/jmse3041272





