Taphonomy of a Mysticete Whale from the Lower Pliocene of the Coast of Cádiz (Spain)
Abstract
:1. Introduction
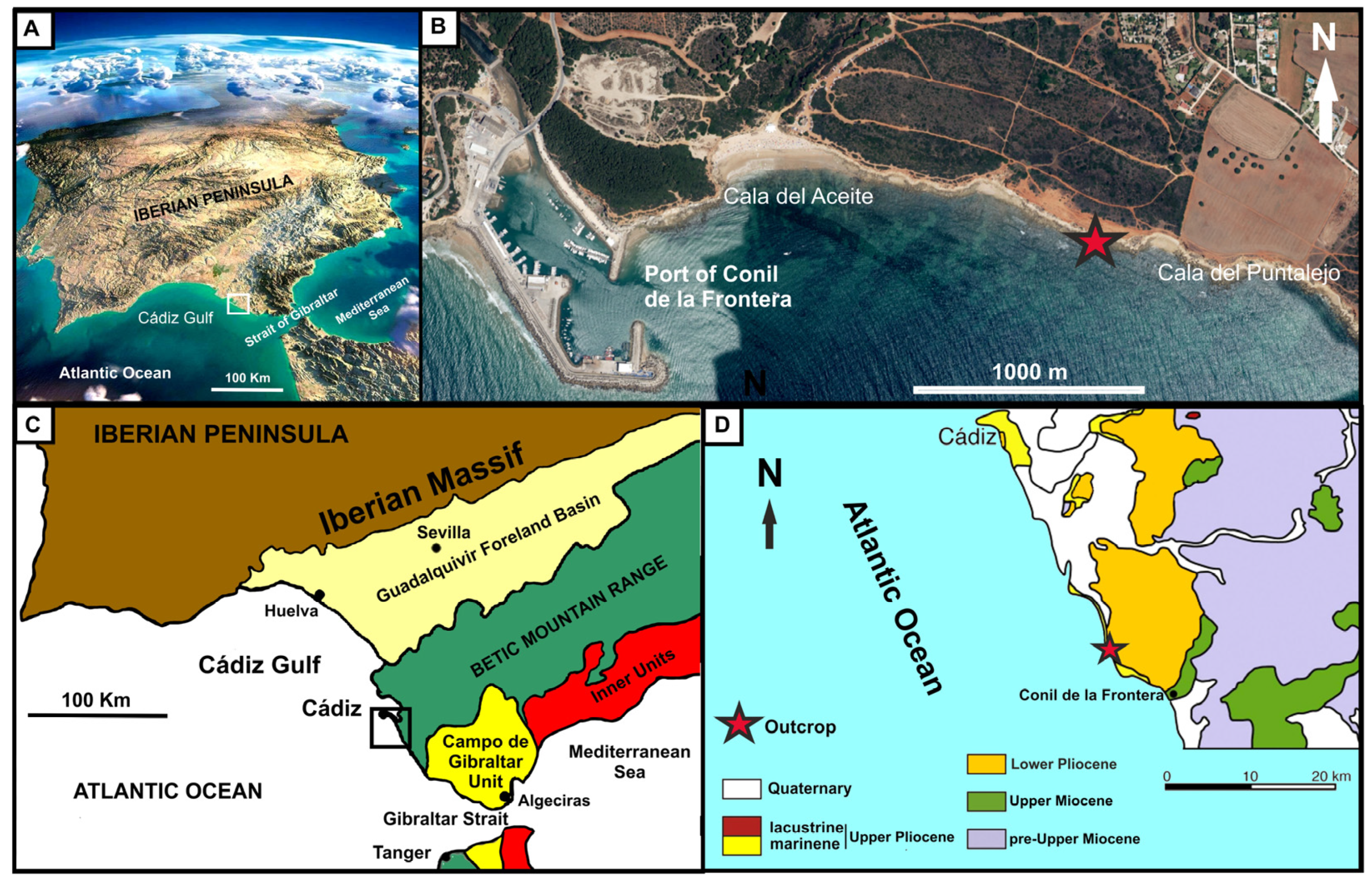
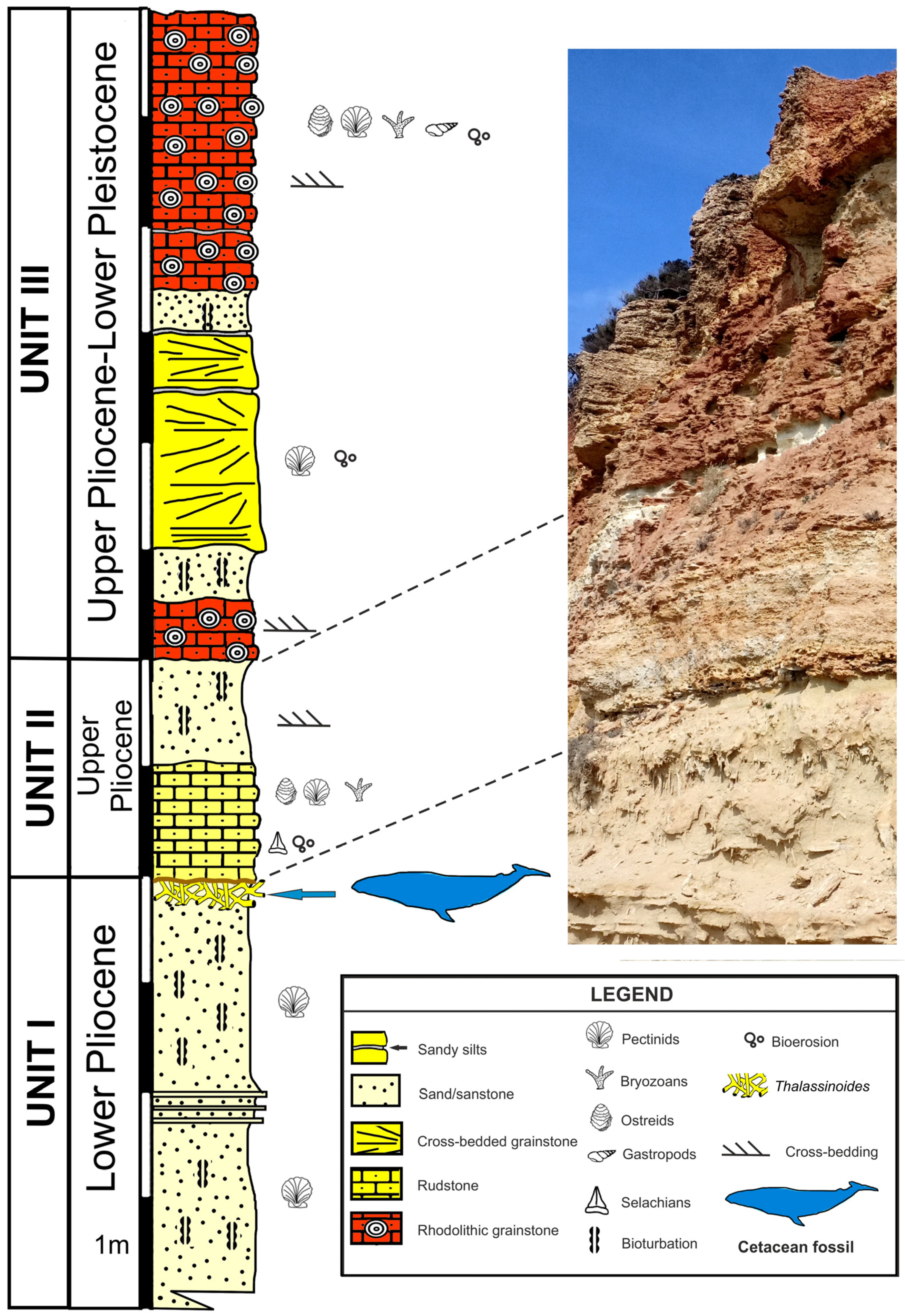
2. Geological and Stratigraphic Setting
3. Data Gathering and Research Methods
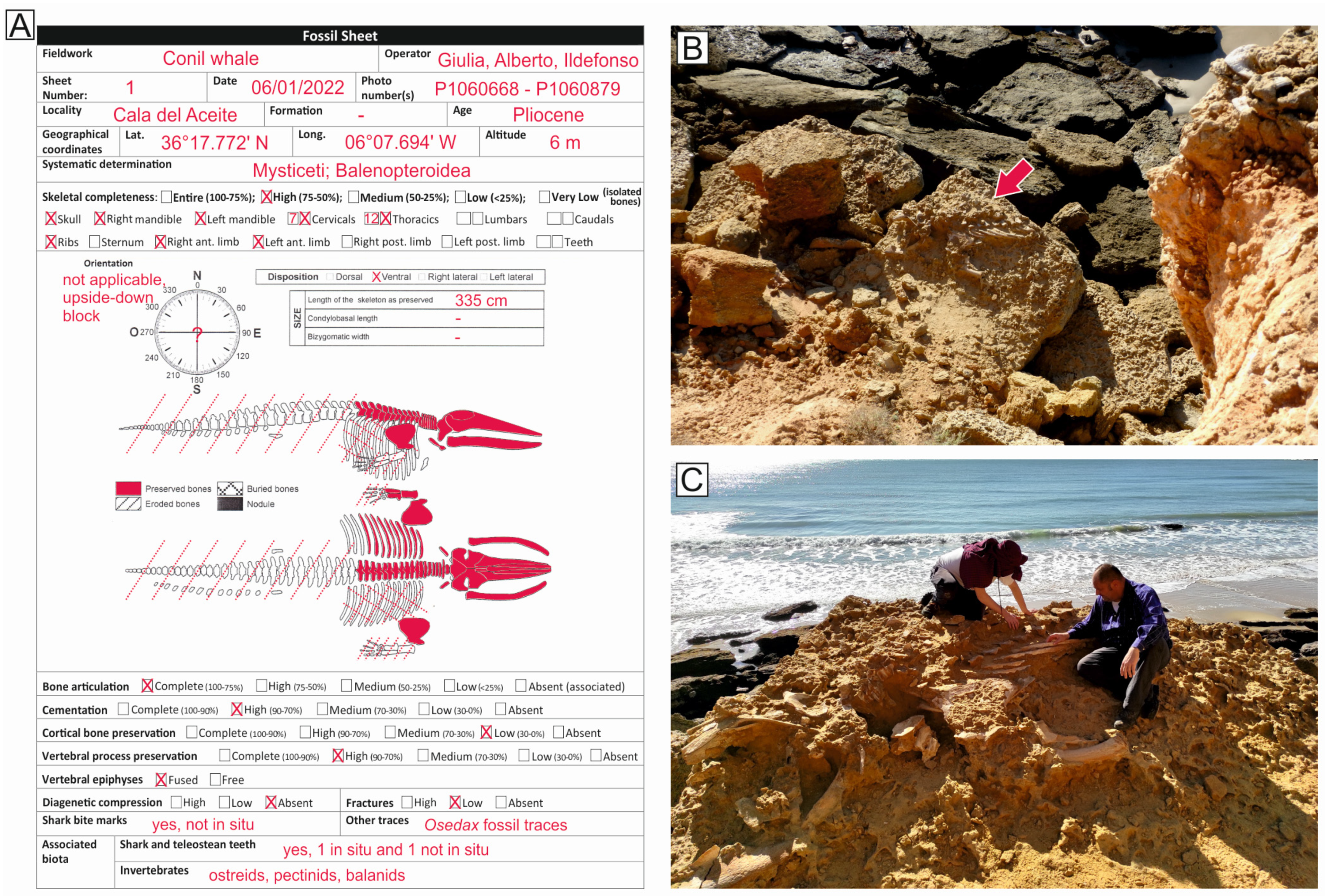
3.1. Field Data Collection
3.2. Analytical Methods
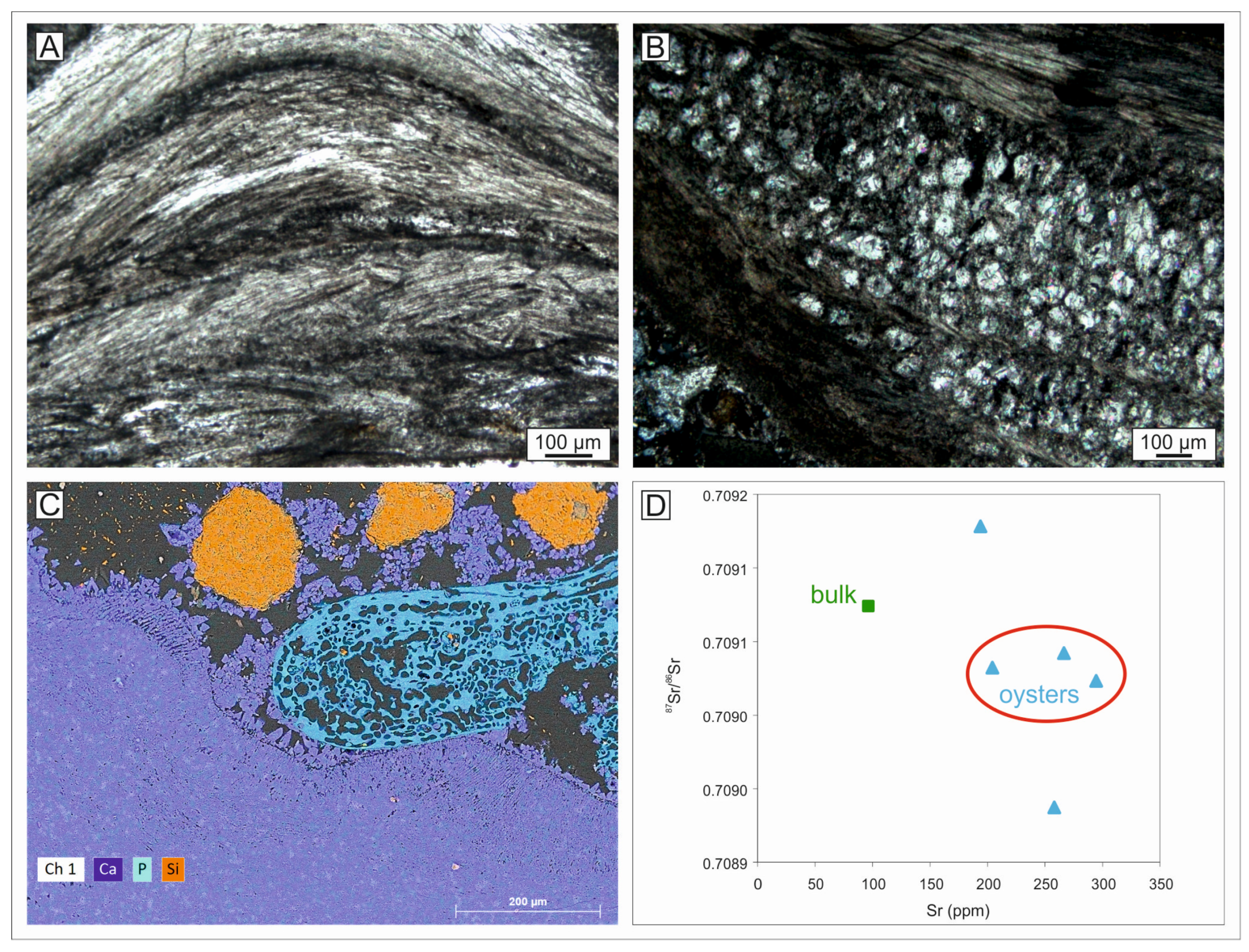
4. Results
4.1. Taxonomy and Ontogenetic Status
4.2. Articulation and Completeness
4.3. Disposition
4.4. Shark Tooth Marks and Associated Shark Teeth
4.5. Associated Macroinvertebrates and Macroinvertebrate Traces
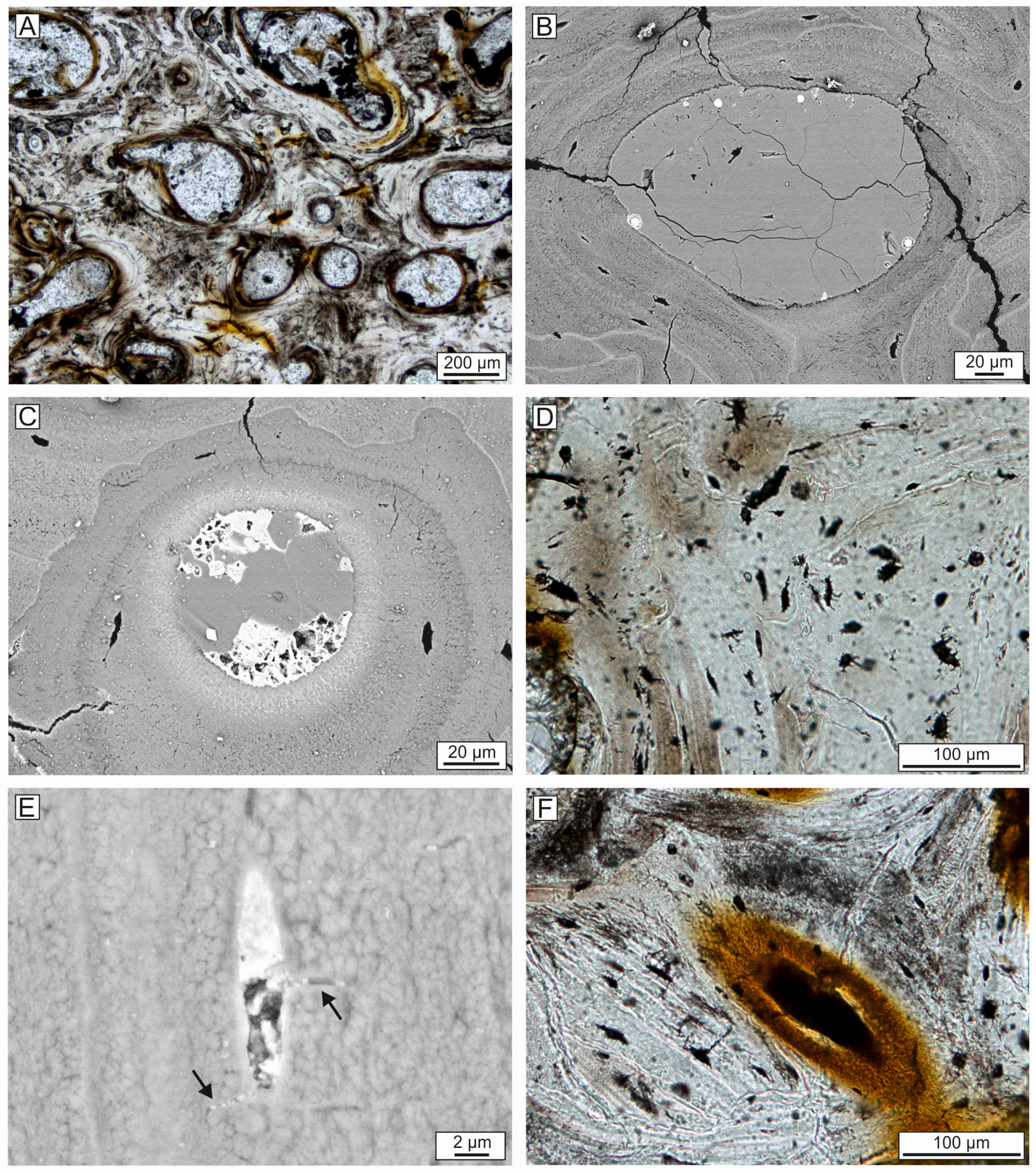
4.6. Bone Preservation and Mineralization
4.7. Composition of the Whale-Embedding Block
4.8. Strontium Isotope Stratigraphy
5. Taphonomic History
5.1. Transport and Deposition
5.2. Permanence on the Seafloor
5.3. Diagenesis and Mineralization
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, S.E.; Antar, M.S.M.; Zalmout, I.S.; Gingerich, P.D. Sequence stratigraphic control on preservation of late Eocene whales and other vertebrates at Wadi Al-Hitan, Egypt. Palaios 2009, 24, 290–302. [Google Scholar] [CrossRef]
- Pyenson, N.D. The high fidelity of the cetacean stranding record: Insights into measuring diversity by integrating taphonomy and macroecology. Proc. R. Soc. B Biol. Sci. 2011, 278, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.B. Geodiversity, palaeodiversity or biodiversity: Where is the place of palaeobiology and an understanding of taphonomy? Proc. Geol. Assoc. 2012, 123, 551–555. [Google Scholar] [CrossRef]
- Dominici, S.; Danise, S.; Cau, S.; Freschi, A. The awkward record of fossil whales. Earth-Sci. Rev. 2020, 205, 103057. [Google Scholar] [CrossRef]
- Bisconti, M.; Pellegrino, L.; Carnevale, G. The chronology of mysticete diversification (Mammalia, Cetacea, Mysticeti): Body size, morphological evolution and global change. Earth-Sci. Rev. 2023, 239, 104373. [Google Scholar] [CrossRef]
- Schäfer, W. Ecology and Palaeoecology of Marine Environments; Chicago University Press: Chicago, IL, USA, 1972. [Google Scholar]
- Reisdorf, A.G.; Bux, R.; Wyler, D.; Benecke, M.; Klug, C.; Maisch, M.W.; Fornaro, P.; Wetzel, A. Float, explode or sink: Postmortem fate of lung-breathing marine vertebrates. Palaeobiodiv. Palaeoenviron. 2012, 92, 67–81. [Google Scholar] [CrossRef]
- Moore, M.J.; Mitchell, G.H.; Rowles, T.K.; Early, G. Dead cetacean? Beach, bloat, float, sink. Front. Mar. Sci. 2020, 7, 333. [Google Scholar] [CrossRef]
- Gioncada, A.; Gariboldi, K.; Collareta, A.; Di Celma, C.; Bosio, G.; Malinverno, E.; Lambert, O.; Pike, J.; Urbina, M.; Bianucci, G. Looking for the key to preservation of fossil marine vertebrates in the Pisco Formation of Peru: New insights from a small dolphin skeleton. Andean Geol. 2018, 45, 379–398. [Google Scholar] [CrossRef]
- Bosio, G.; Gioncada, A.; Gariboldi, K.; Bonaccorsi, E.; Collareta, A.; Pasero, M.; Di Celma, C.; Malinverno, E.; Urbina, M.; Bianucci, G. Mineralogical and geochemical characterization of fossil bones from a Miocene marine Konservat-Lagerstätte. J. S. Am. Earth Sci. 2021, 105, 102924. [Google Scholar] [CrossRef]
- Smith, C.R.; Glover, A.G.; Treude, T.; Higgs, N.D.; Amon, D.J. Whale-fall ecosystems: Recent insights into ecology, paleoecology, and evolution. Ann. Rev. Mar. Sc. 2015, 7, 571–596. [Google Scholar] [CrossRef]
- Allison, P.A.; Smith, C.R.; Kukert, H.; Deming, J.W.; Bennett, B.A. Deep-water taphonomy of vertebrate carcasses: A whale skeleton in the bathyal Santa Catalina Basin. Paleobiology 1991, 17, 78–89. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Spangler, E. Bacterial fossil record in whale-falls: Petrographic evidence of microbial sulfate reduction. Palaeogeogr. Palaeoclim. 2009, 274, 196–203. [Google Scholar] [CrossRef]
- Danise, S.; Cavalazzi, B.; Dominici, S.; Westall, F.; Monechi, S.; Guioli, S. Evidence of microbial activity from a shallow water whale fall (Voghera, northern Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 317, 13–26. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Perry, F.A.; Schmitt, J.G. Comparative taphonomy, taphofacies, and bonebeds of the Mio-Pliocene Purisima Formation, Central California: Strong physical control on marine vertebrate preservation in shallow marine settings. PLoS ONE 2014, 9, e91419. [Google Scholar] [CrossRef] [PubMed]
- Cuitiño, J.I.; Buono, M.R.; Viglino, M.; Farroni, N.D.; Bessone, S. Factors affecting the preservation and distribution of cetaceans in the lower Miocene Gaiman Formation of Patagonia, Argentina. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 526, 110–125. [Google Scholar] [CrossRef]
- Tucker, J.P.; Vercoe, B.; Santos, I.R.; Dujmovic, M.; Butcher, P.A. Whale carcass scavenging by sharks. Glob. Ecol. Conserv. 2019, 19, e00655. [Google Scholar] [CrossRef]
- Bosio, G.; Collareta, A.; Di Celma, C.; Lambert, O.; Marx, F.G.; de Muizon, C.; Gioncada, A.; Gariboldi, K.; Malinverno, E.; Varas-Malca, R.; et al. Taphonomy of marine vertebrates of the Pisco Formation (Miocene, Peru): Insights into the origin of an outstanding Fossil-Lagerstätte. PLoS ONE 2021, 16, e0254395. [Google Scholar] [CrossRef] [PubMed]
- Bianucci, G. A new record of baleen whale from the Pliocene of Tuscany (Italy). Atti. Soc. Tosc. Sci. Nat. Mem. Serie A 1995, 102, 101–104. [Google Scholar]
- Esperante, R.; Guinea, F.M.; Nick, K.E. Taphonomy of a Mysticeti whale in the Lower Pliocene Huelva Sands Formation (Southern Spain). Geol. Acta 2009, 7, 489–505. [Google Scholar]
- Zazzera, A.; Girone, A.; La Perna, R.; Marino, M.; Maiorano, P.; Sardella, R.; Montenegro, V.; Francescangeli, R.; Bianucci, G. Systematics, taphonomy and palaeobiogeography of a balaenopterid (Cetacea, Mysticeti) from the Early Pleistocene of southern Italy. Geobios 2022, 71, 51–65. [Google Scholar] [CrossRef]
- Bisconti, M.; Chicchi, S.; Monegatti, P.; Scacchetti, M.; Campanini, R.; Marsili, S.; Carnevale, G. Taphonomy, osteology and functional morphologyof a partially articulated skeleton of an archaic Pliocene right whale from Emilia Romagna (NW Italy). Boll. Soc. Paleontol. Ital. 2023, 62, 1–32. [Google Scholar]
- Esperante, R.; Brand, L.R.; Chadwick, A.; Poma, O. Taphonomy of fossil whales in the diatomaceous sediments of the Miocene/Pliocene Pisco Formation, Peru. In Current Topics on Taphonomy and Fossilization; De Renzi, M., Pardo Alonso, M.V., Belinchón, M., Peñalver, E., Montoya, P., Márquez-Aliaga, A., Eds.; Ayuntamiento de Valencia: Valencia, Spain, 2002; pp. 337–343. [Google Scholar]
- Danise, S.; Dominici, S. A record of fossil shallow-water whale falls from Italy. Lethaia 2014, 47, 229–243. [Google Scholar] [CrossRef]
- Esperante, R.; Brand, L.R.; Chadwick, A.V.; Poma, O. Taphonomy and paleoenvironmental conditions of deposition of fossil whales in the diatomaceous sediments of the Miocene/Pliocene Pisco Formation, southern Peru—A new fossil-lagerstätte. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 417, 337–370. [Google Scholar] [CrossRef]
- Bianucci, G.; Collareta, A.; Bosio, G.; Landini, W.; Gariboldi, K.; Gioncada, A.; Lambert, O.; Malinverno, E.; de Muizon, C.; Varas-Malca, R.; et al. Taphonomy and palaeoecology of the lower Miocene marine vertebrate assemblage of Ullujaya (Chilcatay Formation, East Pisco Basin, Southern Peru). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 511, 256–279. [Google Scholar] [CrossRef]
- Bisconti, M.; Damarco, P.; Santagati, P.; Pavia, M.; Carnevale, G. Taphonomic patterns in the fossil record of baleen whales from the Pliocene of Piedmont, north-west Italy (Mammalia, Cetacea, Mysticeti). Boll. Soc. Paleontol. Ital. 2021, 60, 183–211. [Google Scholar]
- Farroni, N.D.; Cuitiño, J.I.; Pérez, D.E.; Buono, M.R. Sedimentological and stratigraphically controlled preservation styles and distribution of fossil cetaceans from a new Early Miocene fossiliferous locality, Patagonia, Argentina. Hist. Biol. 2023, 1–15. [Google Scholar] [CrossRef]
- Pilleri, G. Contributions to the Paleontology of Some Tethyan Cetacea and Sirenia: Record of Eurhinodelphis sp. (Cetacea: Rhabdosteidae) from the Lower Miocene of Catalonia, Spain; Brain Anatomy Institute: Bern, Switzerland, 1988. [Google Scholar]
- Pilleri, G.; Biosca, L.; Via, L. The Tertiary Sirenia of Catalonia; Brain Anatomy Institute: Bern, Switzerland, 1989. [Google Scholar]
- Mendiola, C. Hallazgo de Carcharodon carcharias (Linnaeus 1758) en el Pliocceno superior de Conil de la Frontera (Cádiz, Spain). Rev. Soc. Paleontol. D’Elx 2001, 7, 1–9. [Google Scholar]
- Sendra, J.; Bajo, I. Scaldicetus degiorgii Varola, Landini & Pilleri,1988 (Mammalia, Cetacea, Scaldicetidae) del Mioceno (Tortoniense) de la Cuenca del Guadalquivir. In XXIX Jornadas de Paleontología. Libro de Resúmenes; Álvarez-Vázquez, C., Ed.; Córdoba, Spain, 2013; Available online: https://www.researchgate.net/publication/257237909_Scaldicetus_degiorgii_Varola_Landini_y_Pilleri_1988_Mammalia_Cetacea_Scaldicetidae_del_Mioceno_Tortoniense_de_la_Cuenca_del_Guadalquivir (accessed on 17 December 2023).
- Reolid, M.; Molina, J.M. Registro de Carcharocles megalodon en el sector oriental de la Cuenca del Guadalquivir (Mioceno superior, Sur de España). Estud. Geol. 2015, 71, e032. [Google Scholar] [CrossRef]
- Reolid, M.; García-García, F.; Reolid, J.; De Castro, A.; Bueno, J.F.; Martín-Suárez, E. Palaeoenvironmental interpretation of a sand-dominated coastal system of the Upper Miocene of eastern Guadalquivir Basin (South Spain): Fossil assemblages, ichnology and taphonomy. J. Iber. Geol. 2016, 42, 275–290. [Google Scholar] [CrossRef]
- Sendra, J. Los yacimientos de mamíferos marinos del Neógeno del Surde la provincia de Alicante. In Resúmenes XIII Jornadas de Paleontología; Grandal D’Anglade, A., Gutiérrez-Marco, J.C., Santos Fidalgo, L., Eds.; Sociedad Española de Paleontología: La Coruña, Spain, 1997; pp. 237–240. [Google Scholar]
- Mercadal, B.; Pilleri, G.; Casinos, A. A tooth of Scaldicetus grandis (Du Bus, 1872) (Physeteridae) from Aire Island (Menorca, Spain). Investig. Cetacea 1985, 17, 31–33. [Google Scholar]
- Pilleri, G. Miocene cetacean remains from Mediterranean Spain. Treb. Del Mus. Geol. Barc. 1990, 1, 43–76. [Google Scholar]
- Miján, I. Hallazgos de restos fósiles de Hyperoodon sp. (Cetacea, Ziphiidae) en las costas gallegas (NO España). Rev. Biol. Mar. Oceanogr. 2007, 42, 253–260. [Google Scholar] [CrossRef]
- Bianucci, G.; Miján, I.; Lambert, O.; Post, K.; Mateus, O. Bizarre fossil beaked whales (Odontoceti, Ziphiidae) fished from the Atlantic Ocean floor off the Iberian Peninsula. Geodiversitas 2013, 35, 105–153. [Google Scholar] [CrossRef]
- Bianucci, G.; Llàcer, S.; Cardona, J.Q.; Collareta, A.; Florit, A.R. A new beaked whale record from the upper Miocene of Menorca, Balearic Islands, based on CT-scan analysis of limestone slabs. Acta Palaeontol. Pol. 2019, 64, 291–302. [Google Scholar] [CrossRef]
- Mas, G.; Escalante, F.; Quintana, J. Primera cita de un Delphinidae en el Neógeno de las Islas Baleares. Batalleria 2013, 18, 45–51. [Google Scholar]
- Toscano, A.; Abad, M.; Ruiz, F.; Muñiz, F.; Álvarez, G.; García, E.X.M.; Caro, J.A. Nuevos restos de Scaldicetus (Cetacea, Odontoceti, Physeteridae) del Mioceno superior, sector occidental de la Cuenca del Guadalquivir (sur de España). Rev. Mex. Cienc. Geol. 2013, 30, 436–445. [Google Scholar]
- Miján, I.; Louwye, S.; Lambert, O. A new Beneziphius beaked whale from the ocean floor off Galicia, Spain and biostratigraphic reassessment of the type species. Acta Palaeont. Pol. 2017, 62, 211–220. [Google Scholar] [CrossRef]
- Sendra, S.J.; Hodgson, D. Astadelphis gastaldii (Brandt, 1874)-Mammalia, Cetacea, Delphinidae-en el Plioceno Espanol. Libro Resúmenes XIV J. Paleontol. 1998, 14, 169–172. [Google Scholar]
- López Amador, J.J.; Ruiz Gil, J.A. Patrimonio natural de la Bahía de Cádiz: El yacimiento terciario de El Manatial. Rev. Atlánt.-Mediterr. Prehist. Arqueol. Soc. 2016, 18, 163–169. [Google Scholar] [CrossRef]
- Sendra, J.; Reolid, M.; Reolid, J. Palaeoenvironmental interpretation of the Pliocene fan-delta system of the Vera Basin (SE Spain): Fossil assemblages, ichnology and taphonomy. Palaeoworld 2020, 29, 769–788. [Google Scholar] [CrossRef]
- Belaústegui, Z.; de Gibert, J.M.; Domènech, R.; Muñiz, F.; Martinell, J. Tafonomía y contexto paleoambiental de los restos de un cetáceo del Mioceno medio de Tarragona (NE España). Geobios 2011, 44, 19–31. [Google Scholar] [CrossRef]
- Belaústegui, Z.; de Gibert, J.M.; Domènech, R.; Muñiz, F.; Martinell, J. Clavate borings in a Miocene cetacean skeleton from Tarragona (NE Spain) and the fossil record of marine bone bioerosion. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 323, 68–74. [Google Scholar] [CrossRef]
- Sendra, J.; Bajo Campos, I. Evidencias tafonómicas de carroñeo o predación sobre cetáceos: El neurocráneo del Tortoniense de Burguillos (Sevilla). In Proceedings of the XXIX Jornadas de Paleontología de la Sociedad Española de Paleontología, Córdoba, Spain, 2–5 October 2013. [Google Scholar]
- Sendra, J.; Bajo, I.; Cardenas, J. Un ejemplar de Misticeto (Mammalia: Cetacea) del Plioceno inferior de Alcalá de Guadaira (Sevilla). J. Paleontol. 1996, 12, 116–117. [Google Scholar]
- Sendra, J.; Stokes, M.; Oltra, V. Excavación de un misticeto (Mammalia, Cetacea) en el Plioceno del sector norte de la Cuenca Vera (Almería). In Proceedings of the XIV Jornadas de Paleontología, Sociedad Española de Paleontología, Tenerife, Spain, 7–11 October 1998. [Google Scholar]
- Sendra, J.; Muñiz, F.; Mayoral, E. Primeros datos sobre misticetos (Mammalia, Cetacea, Balaenopteridae) en el Plioceno Inferior de la Cuencadel Guadalquivir (Lepe, Huelva). Temas Geológico-Min. 1999, 26, 356–361. [Google Scholar]
- Sendra, J.; Muñiz, F.; Mayoral, E.; De Renzi, M. Nuevos datos sobre Misticetos (Mammalia, Cetacea) en el Plioceno Inferior de La Cuenca del Guadalquivir (Lepe, Huelva, España). In Proceedings of the Libro de Resúmenes del I Congreso Ibérico de Paleontología y XVI Jornadas de La Sociedad Española de Paleontología, Spain, 12–14 October 2000. [Google Scholar]
- Muñiz, F.; De Gibert, J.M.; Esperante, R. First trace-fossil evidence of bone-eating worms in whale carcasses. Palaios 2010, 25, 269–273. [Google Scholar] [CrossRef]
- Mayoral, E.; Muñiz, F.; Sendra, J.; Bajo, I.; Cárdenas, J. Prospección paleontológica superficial en las márgenes del Río Guadaira, en el término municipal de Alcalá de Guadaira (Sevilla). Anu. Arqueol. Andal. 2001, 97, 1651–1659. [Google Scholar]
- Mayoral, E.; Muñiz, F.; Sendra, J. Sondeo estratigráfico de urgencia enel Cabezo del Tío Parra, Lepe (Huelva). Anu. Arqueol. Andal. 2001, 97, 370–374. [Google Scholar]
- Berástegui, X.; Banks, C.J.; Puig, C.; Taberner, C.; Waltham, D.; Fernández, M. Lateral diapiric emplacement of Triassic evaporites at the southern margin of the Guadalquivir Basin, Spain. Geol. Soc. Spec. Publ. 1998, 134, 49–68. [Google Scholar] [CrossRef]
- Garcia-Castellanos, D.; Fernandez, M.; Torné, M. Modeling the evolution of the Guadalquivir foreland basin (Southern Spain). Tectonics 2002, 21, 1–17. [Google Scholar] [CrossRef]
- Braga, J.C.; Martín, J.M.; Riding, R.; Aguirre, J.; Sánchez-Almazo, I.M.; Dinarès-Turell, J. Testing models for the Messinian salinity crisis: The Messinian record in Almería, SE Spain. Sediment. Geol. 2006, 188, 131–154. [Google Scholar] [CrossRef]
- Martín, J.M.; Braga, J.C.; Aguirre, J.; Puga-Bernabéu, Á. History and evolution of the North-Betic Strait (Prebetic Zone, Betic Cordillera): A narrow, early Tortonian, tidal-dominated, Atlantic–Mediterranean marine passage. Sediment. Geol. 2009, 216, 80–90. [Google Scholar] [CrossRef]
- Krijgsman, W.; Capella, W.; Simon, D.; Hilgen, F.J.; Kouwenhoven, T.J.; Meijer, P.T.; Sierro, F.J.; Tulbure, M.A.; van den Berg, B.C.J.; van der Schee, M.; et al. The Gibraltar corridor: Watergate of the Messinian salinity crisis. Mar. Geol. 2018, 403, 238–246. [Google Scholar] [CrossRef]
- Reolid, J.; Aguirre, J.; Pérez-Asensio, J.N.; Puga-Bernabéu, Á.; Braga, J.C.; Martín, J.M. Mixed carbonate-siliciclastic contourite drift deposits associated with the entrance of an Atlantic-Mediterranean corridor (Late Miocene, Southwest Spain). Sediment. Geol. 2022, 439, 106233. [Google Scholar] [CrossRef]
- Rouchy, J.M.; Caruso, A. The Messinian salinity crisis in the Mediterranean basin: A reassessment of the data and an integrated scenario. Sediment. Geol. 2006, 188, 35–67. [Google Scholar] [CrossRef]
- Viguier, C. Le Néogéne de l’Andalousie Nord-Occidental (Espagne). Histoire Géologique du bas Guadalquivir. Ph.D. Thesis, Thése Bordeaux, Burdeos, France, 1974; p. 449. [Google Scholar]
- Aguirre, J. Implicaciones paleoambientales y paleogeográficas de dos discontinuidades estratigráficas en los depósitos pliocénicos de Cádiz (SW de España). Rev. Soc. Geol. España 1995, 8, 153–166. [Google Scholar]
- Droser, M.L.; Bottjer, D.J. Ichnofabric of sandstones deposited in high-energy nearshore environments: Measurement and utilization. Palaios 1989, 4, 598–604. [Google Scholar] [CrossRef]
- Aguirre, J.; De Gibert, J.M.; Puga-Bernabéu, A. Proximal–distal ichnofabric changes in a siliciclastic shelf, Early Pliocene, Guadalquivir Basin, southwest Spain. Palaeogeogr. Palaeocl. 2010, 291, 328–337. [Google Scholar] [CrossRef]
- Aguirre Rodriguez, J. Estratigrafía del Plioceno de la costa de Cádiz entre Chiclana y Conil. Geogaceta 1991, 9, 84–87. [Google Scholar]
- Gutiérrez-Mas, J.M.; Mas, R. Record of very high energy events in Plio-Pleistocene marine deposits of the Gulf of Cadiz (SW Spain): Facies and processes. Facies 2013, 59, 679–701. [Google Scholar] [CrossRef]
- Yoshida, H.; Shirakihara, M.; Takemura, A.; Shirakihara, K. Development, sexual dimorphism, and individual variation in the skeleton of the finless porpoise, Neophocaena phocaenoides, in the coastal waters of western Kyushu, Japan. Mar. Mamm. Sci. 1994, 10, 266–282. [Google Scholar] [CrossRef]
- Tsai, C.H. A Miocene breeding ground of an extinct baleen whale (Cetacea: Mysticeti). PeerJ 2017, 5, e3711. [Google Scholar] [CrossRef]
- Baccelle, L.; Bosellini, A. Diagrammi per la Stima Visiva Della Composizione Percentuale Nelle Rocce Sedimentary; Nuova Serie, Sezione IX, Scienze Geologiche e Paleontologiche; Annali Università di Ferrara: Ferrara, Italy, 1965; pp. 59–89. [Google Scholar]
- Flügel, E. Microfacies of Carbonate Rocks: Analysis Interpretation and Application; Springer: New York, NY, USA, 2010; p. 984. [Google Scholar]
- Bosio, G.; Malinverno, E.; Collareta, A.; Di Celma, C.; Gioncada, A.; Parente, M.; Berra, F.; Marx, F.G.; Vertino, A.; Urbina, M.; et al. Strontium isotope stratigraphy and the thermophilic fossil fauna from the middle Miocene of the East Pisco Basin (Peru). J. S. Am. Earth Sci. 2020, 97, 102399. [Google Scholar] [CrossRef]
- McArthur, J.M.; Howarth, R.J.; Shields, G.A.; Zhou, Y. Strontium isotope stratigraphy. In Geologic Time Scale 2020; Gradstein, F.M., Ogg, J.G., Schmitz, M.D., Ogg, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 211–238. [Google Scholar]
- Frijia, G.; Parente, M.; Di Lucia, M.; Mutti, M. Carbon and strontium isotope stratigraphy of the Upper Cretaceous (Cenomanian-Campanian) shallow-water carbonates of southern Italy: Chronostratigraphic calibration of larger foraminifera biostratigraphy. Cretac. Res. 2015, 53, 110–139. [Google Scholar] [CrossRef]
- Bosio, G.; Bianucci, G.; Collareta, A.; Landini, W.; Urbina, M.; Di Celma, C. Ultrastructure, composition, and 87Sr/86Sr dating of shark teeth from lower Miocene sediments of southwestern Peru. J. S. Am. Earth Sci. 2022, 118, 103909. [Google Scholar] [CrossRef]
- Lowe, D.G. Object recognition from local scale-invariant features. In Proceedings of the Seventh IEEE International Conference on Computer Vision, Kerkyra, Greece, 20–27 September 1999; Volume 2, pp. 1150–1157. [Google Scholar]
- Carabassa, V.; Montero, P.; Crespo, M.; Padró, J.C.; Pons, X.; Balagué, J.; Brotons, L.; Alcañiz, J.M. Unmanned aerial system protocol for quarry restoration and mineral extraction monitoring. J. Environ. Manag. 2020, 270, 110717. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, J.P.; Fiorillo, A.R.; Hasiotis, S.; Gingras, M.K. Tooth marks, gnaw marks, claw-marks, bite marks, scratch marks, etc.: Terminology in ichnology. Ichnos 2022, 29, 93–101. [Google Scholar] [CrossRef]
- Cigala Fulgosi, F. Predation (or possible scavenging) by a great white shark on an extinct species of bottlenosed dolphin in the Italian Pliocene. Tert. Res. 1990, 12, 17–36. [Google Scholar]
- Bianucci, G.; Sorce, B.; Storai, T.; Landini, W. Killing in the Pliocene: Shark attack on a dolphin from Italy. Palaeontology 2010, 53, 457–470. [Google Scholar] [CrossRef]
- Collareta, A.; Lambert, O.; Landini, W.; Di Celma, C.; Malinverno, E.; Varas-Malca, R.; Urbina, M.; Bianucci, G. Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine mammal remains from the late Miocene of Peru. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 469, 84–91. [Google Scholar] [CrossRef]
- Kiel, S.; Goedert, J.L.; Kahl, W.A.; Rouse, G.W. Fossil traces of the bone-eating worm Osedax in early Oligocene whale bones. Proc. Natl. Acad. Sci. USA 2010, 107, 8656–8659. [Google Scholar] [CrossRef]
- Danise, S.; Higgs, N.D. Bone-eating Osedax worms lived on Mesozoic marine reptile deadfalls. Biol. Lett. 2015, 11, 20150072. [Google Scholar] [CrossRef] [PubMed]
- Higgs, N.D.; Little, C.T.; Glover, A.G.; Dahlgren, T.G.; Smith, C.R.; Dominici, S. Evidence of Osedax worm borings in Pliocene (∼3 Ma) whale bone from the Mediterranean. Hist. Biol. 2012, 24, 269–277. [Google Scholar]
- Higgs, N.D.; Pokines, J.T. Marine environmental alterations to bone. In Manual of Forensic Taphonomy; Pokines, J.T., Symes, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 143–179. [Google Scholar]
- Boessenecker, R.W.; Fordyce, R.E. Trace fossil evidence of predation upon bone-eating worms on a baleen whale skeleton from the Oligocene of New Zealand. Lethaia 2015, 48, 326–331. [Google Scholar] [CrossRef]
- Bloebaum, R.D.; Skedros, J.G.; Vajda, E.G.; Bachus, K.N.; Constantz, B.R. Determining mineral content variations in bone using backscattered electron imaging. Bone 1997, 20, 485–490. [Google Scholar] [CrossRef]
- Pfretzschner, H.U. Pyrite in fossil bone. N. Jb. Geol. Paläont. Abh. 2001, 220, 1–23. [Google Scholar] [CrossRef]
- Pfretzschner, H.U. Iron oxides in fossil bone. N. Jb. Geol. Paläont. Abh. 2001, 220, 417–429. [Google Scholar] [CrossRef]
- Bodzioch, A. Idealized model of mineral infillings in bones of fossil freshwater animals, on the example of Late Triassic metoposaurs from Krasiejów (Poland). Austin J. Earth Sci. 2015, 2, 1008. [Google Scholar]
- Pfretzschner, H.U. Microcracks and fossilization of Haversian bone. N. Jb. Geol. Paläont. Abh. 2000, 216, 413–432. [Google Scholar] [CrossRef]
- Gariboldi, K.; Gioncada, A.; Bosio, G.; Malinverno, E.; Di Celma, C.; Tinelli, C.; Cantalamessa, G.; Landini, W.; Urbina, M.; Bianucci, G. The dolomite nodules enclosing fossil marine vertebrates in the East Pisco Basin, Peru: Field and petrographic insights into the Lagerstätte formation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 438, 81–95. [Google Scholar] [CrossRef]
- Jans, M.M.E. Microbial bioerosion of bone—A review. In Current Developments in Bioerosion; Wisshak, M., Tapanila, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 397–413. [Google Scholar]
- Cox, L.R.; Newell, N.D.; Boyd, D.W.; Branson, C.C.; Casey, R.; Chavan, A.; Coogan, A.H.; Dechaseaux, C.; Fleming, C.A.; Haas, F.; et al. Mollusca (Bivalvia). In Treatise of Invertebrate Paleontology; Moore, R.C., Ed.; The University of Kansas Printing Service: Lawrence, KS, USA; Meriden, CT, USA; New York, NY, USA, 1971; pp. N1–N1224. [Google Scholar]
- Van der Zwaan, G.J.; Jorissen, F.J.; De Stigter, H.C. The depth dependency of planktonic/benthic foraminiferal ratios: Constraints and applications. Mar. Geol. 1990, 95, 1–16. [Google Scholar] [CrossRef]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006; p. 318. [Google Scholar]
- Bromley, R.G.; Frey, R.W. Redescription of the trace fossil Gyrolithes and taxonomic evaluation of Thalassinoides, Ophiomorpha and Spongeliomorpha. Bull. Geol. Soc. Den. 1974, 23, 311–335. [Google Scholar]
- Coleman, N.; Poore, G.C.B. The distribution of Callianassa species (Crustacea, Decapoda) in Western Port, Victoria. Proc. R. Soc. Vic. 1980, 91, 73–78. [Google Scholar]
- Coletti, G.; Bosio, G.; Collareta, A.; Bialik, O.M.; Regattieri, E.; Cornacchia, I.; Buckeridge, J. Barnacle-rich facies as a tool for palaeoenvironmental reconstructions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 634, 111914. [Google Scholar] [CrossRef]
- MacEachern, J.A.; Raychaudhuri, I.; Pemberton, S.G. Stratigraphic applications of the Glossifungites ichnofacies: Delineating discontinuities in the rock record. In Applications of Ichnology to Petroleum Exploration; Pemberton, S.G., Ed.; Core Workshop Notes; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1992; Volume 17, pp. 169–198. [Google Scholar]
- Abdel-Fattah, Z.A.; Gingras, M.K.; Caldwell, M.W.; Pemberton, S.G.; MacEachern, J.A. The Glossifungites ichnofacies and sequence stratigraphic analysis: A case study from middle to upper Eocene successions in Fayum, Egypt. Ichnos 2016, 23, 157–179. [Google Scholar] [CrossRef]
- Coletti, G.; Bosio, G.; Collareta, A.; Buckeridge, J.; Consani, S.; El Kateb, A. Palaeoenvironmental analysis of the Miocene barnacle facies: Case studies from Europe and South America. Geol. Carpathica 2018, 69, 573–592. [Google Scholar] [CrossRef]
- Coletti, G.; Bosio, G.; Collareta, A. Lower Pliocene barnacle facies of western Liguria (NW Italy): A peek into a warm past and a glimpse of our incoming future. Riv. Ital. Paleontol. Stratigr. 2021, 127, 103–131. [Google Scholar]
- Westphal, H.; Halfar, J.; Freiwald, A. Heterozoan carbonates in subtropical to tropical settings in the present and past. Int. J. Earth Sci. 2010, 99, 153–169. [Google Scholar]
- Bialik, O.M.; Coletti, G.; Mariani, L.; Commissario, L.; Desbiolles, F.; Meroni, A.N. Availability and type of energy regulate the global distribution of neritic carbonates. Sci. Rep. 2023, 13, 19687. [Google Scholar] [CrossRef]
- Martín, J.M.; Braga, J.C.; Aguirre, J.; Betzler, C. Contrasting models of temperate carbonate sedimentation in a small Mediterranean embayment: The Pliocene Carboneras Basin, SE Spain. J. Geol. Soc. 2004, 161, 387–399. [Google Scholar] [CrossRef]
- Braga, J.C.; Martín, J.M.; Betzler, C.; Aguirre, J. Models of temperate carbonate deposition in Neogene basins in SE Spain: A synthesis. Geol. Soc. Lond. Spec. Publ. 2006, 255, 121–135. [Google Scholar] [CrossRef]
- Aguirre, J.; Martín, J.M.; Braga, J.C.; Betzler, C.; Berning, B.; Buckeridge, J.S. Densely packed concentrations of sessile barnacles (Cirripedia: Sessilia) from the Early Pliocene of SE Spain. Facies 2008, 54, 193–206. [Google Scholar] [CrossRef]
- Rico-García, A.; Aguirre, J.; González-Delgado, J.Á. Taphonomy and taphofacies models of the Pliocene deposits of Vejer de la Frontera (Cádiz, SW Spain). Geobios 2008, 41, 543–558. [Google Scholar] [CrossRef]
- Curtis, T.H.; Kelly, J.T.; Menard, K.L.; Laroche, R.K.; Jones, R.E.; Klimley, A.P. Observations on the behavior of white sharks scavenging from a whale carcass at Point Reyes, California. Calif Fish Game 2006, 92, 113–124. [Google Scholar]
- Fallows, C.; Gallagher, A.J.; Hammerschlag, N. White sharks (Carcharodon carcharias) scavenging on whales and its potential role in further shaping the ecology of an apex predator. PLoS ONE 2013, 8, e60797. [Google Scholar] [CrossRef] [PubMed]
- Lea, J.S.E.; Daly, R.; Leon, C.; Daly, C.A.K.; Clarke, C.R. Life after death: Behaviour of multiple shark species scavenging a whale carcass. Mar. Freshw. Res. 2018, 70, 302–306. [Google Scholar] [CrossRef]
- Goffredi, S.K.; Orphan, V.J.; Rouse, G.W.; Jahnke, L.; Embaye, T.; Turk, K.; Lee, R.; Vrijenhoek, R.C. Evolutionary innovation: A bone-eating marine symbiosis. Environ. Microbiol. 2005, 7, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Vietti, L.A.; Bailey, J.V.; Fox, D.L.; Rogers, R.R. Rapid formation of framboidal sulfides on bone surfaces from a simulated marine carcass fall. Palaios 2015, 30, 327–334. [Google Scholar] [CrossRef]
- Crisp, D.J.; Bourget, E. Growth in barnacles. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 1985; Volume 22, pp. 199–244. [Google Scholar]
- Nishizaki, M.T.; Carrington, E. The effect of water temperature and velocity on barnacle growth: Quantifying the impact of multiple environmental stressors. J. Therm. Biol. 2015, 54, 37–46. [Google Scholar] [CrossRef]
- Lundsten, L.; Schlining, K.L.; Frasier, K.; Johnson, S.B.; Kuhnz, L.A.; Harvey, J.B.; Clague, G.; Vrijenhoek, R.C. Time-series analysis of six whale-fall communities in Monterey Canyon, California, USA. Deep Sea Res. Part I 2010, 57, 1573–1584. [Google Scholar] [CrossRef]
- Allison, P.A. The role of anoxia in the decay and mineralization of proteinaceous macro-fossils. Paleobiology 1988, 14, 139–154. [Google Scholar] [CrossRef]
- Berna, F.; Matthews, A.; Weiner, S. Solubilities of bone mineral from archaeological sites: The recrystallization window. J. Archaeol. Sci. 2004, 31, 867–882. [Google Scholar] [CrossRef]
- Creveling, J.R.; Johnston, D.T.; Poulton, S.W.; Kotrc, B.; März, C.; Schrag, D.P.; Knoll, A.H. Phosphorus sources for phosphatic Cambrian carbonates. GSA Bull. 2014, 126, 145–163. [Google Scholar] [CrossRef]







| Formation/Period | Locality (Province) | Suborder, Family | Material | References |
|---|---|---|---|---|
| Tortonian | Burguillos (Seville) | Mysticeti | Neurocranium | Sendra and Bajo 2013a [49] |
| Transitional Unit Messinian | Alcalá de Guadaíra (Seville) | Mysticeti | Cranium, vertebrae, ribs, scapulae, tympanic bullae, jaws | Sendra et al. 1996 [50] |
| Lower Pliocene | Lepe (Huelva) | Mysticeti (Cethoteridae) | Fragments of neurocranium, jaws, tympanic bulla | Sendra et al. 1999, 2000 [52,53] |
| Lower Pliocene Huelva Sands | Bonares (Huelva) | Mysticeti | Fragments of neurocranium, jaws, vertebrae | Esperante et al. 2009 [20] |
| Sample | Type | P2O5 | CaO | MgO | FeO | Na2O | SiO2 | Al2O3 | SO3 | Cl | Sum | Mineral |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA-OS1-1 | cement | - | 68.17 | 0.53 | - | - | - | - | - | - | 68.70 | calcite |
| CA-OS1-2 | bone | 24.82 | 42.84 | - | - | 0.99 | - | - | - | 0.62 | 69.27 | apatite |
| CA-OS1-3 | bone | 23.84 | 45.13 | - | - | 1.26 | - | - | - | 0.79 | 71.03 | apatite |
| CA-OS1-4 | bone | 25.45 | 47.49 | - | - | 1.23 | - | - | - | - | 74.16 | apatite |
| CA-OS1-5 | cement | - | 70.05 | 1.01 | - | - | - | - | - | - | 71.06 | calcite |
| CA-OS1-6 | framboid | - | 1.33 | - | 70.24 | - | 4.35 | 0.98 | - | - | 76.90 | Fe oxides |
| CA-OS1-7 | bright bone | 19.05 | 39.96 | - | 18.66 | - | - | - | - | - | 77.68 | apatite |
| CA-OS1-8 | bone | 21.25 | 46.81 | 0.73 | - | 0.90 | - | - | - | 0.59 | 70.28 | apatite |
| CA-OS2-1 | bone | 25.79 | 44.07 | 0.76 | - | 1.31 | - | - | 1.51 | 0.21 | 73.66 | apatite |
| CA-OS2-2 | bone | 23.15 | 48.71 | 0.71 | 1.94 | 1.07 | - | - | 0.46 | - | 76.04 | apatite |
| CA-OS2-3 | cement | - | 48.66 | 0.58 | 3.05 | - | 11.09 | 4.53 | - | - | 68.73 | calcite |
| CA-OS2-4 | bone | 25.68 | 52.19 | - | - | 1.10 | - | - | 0.85 | - | 79.82 | apatite |
| CA-OS2-5 | microboring infill | 21.67 | 50.47 | - | 2.85 | 0.94 | - | - | - | - | 75.94 | apatite |
| CA-OS2-6 | microboring infill | 14.9 | 40.72 | - | 6.31 | - | - | - | - | - | 61.94 | apatite |
| CA-OS2-7 | microboring infill | 24.44 | 38.82 | - | 5.40 | - | 0.46 | 0.73 | 0.56 | 0.86 | 73.44 | apatite |
| CA-OS2-8 | microboring infill | 1.24 | 2.07 | 0.91 | 59.22 | - | 2.24 | 1.49 | - | 1.32 | 68.48 | Fe oxides |
| CA-OS2-9 | microboring infill | 21.66 | 38.31 | 0.68 | 3.54 | 1.09 | - | - | 0.89 | 0.88 | 67.06 | apatite |
| CA-OS2-10 | microboring infill | 14.83 | 40.83 | 0.73 | 6.85 | 1.07 | - | 0.58 | 0.55 | 0.67 | 66.12 | apatite |
| CA-OS2-11 | cement | - | 76.33 | - | - | - | - | - | - | - | 76.33 | calcite |
| CA-OS2-12 | bone | 22.60 | 51.68 | 0.78 | - | 2.67 | - | - | - | - | 77.72 | apatite |
| CA-OS2-13 | bone | 27.68 | 48.73 | - | - | 0.91 | - | - | 0.69 | 0.22 | 78.23 | apatite |
| CA-OS2-14 | bright bone | 17.78 | 53.54 | 0.56 | 8.06 | 0.76 | - | - | 0.53 | - | 81.21 | apatite |
| CA-OS2-15 | framboid | - | 1.59 | - | 65.96 | - | 4.86 | 1.64 | - | - | 75.70 | Fe oxides |
| CA-OS2-16 | cement | - | 74.38 | - | - | - | - | - | - | - | 74.38 | calcite |
| CA-OS3-1 | cement | - | 75.38 | - | - | - | - | - | - | - | 75.38 | calcite |
| CA-OS3-2 | cement | - | 73.02 | - | - | - | - | - | - | - | 73.02 | calcite |
| Sample | Measured 87Sr/86Sr | Corrected 87Sr/86Sr | ±2σmean | Max Age (Ma) | Preferred Age (Ma) | Min Age (Ma) |
|---|---|---|---|---|---|---|
| CA-OST1 | 0.708931 | 0.708937 | 0.000005 | 8.26 | 7.62 | 7.19 |
| CA-OST2 | 0.709026 | 0.709032 | 0.000004 | 5.38 | 5.14 | 4.86 |
| CA-OST3 | 0.709122 | 0.709128 | 0.000006 | 1.29 | 1.16 | 1.03 |
| CA-OST4 | 0.709017 | 0.709023 | 0.000005 | 5.61 | 5.40 | 5.13 |
| CA-OST5 | 0.709036 | 0.709042 | 0.000005 | 5.11 | 4.79 | 4.01 |
| CA-BULK6 | 0.709068 | 0.709074 | 0.000005 | 2.68 | 2.37 | 2.10 |
| Mean values of the horizon | - | 0.709033 | 0.000013 | 5.59 | 5.11 | 4.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosio, G.; Bajo-Campos, I.; Collareta, A.; Ros-Montoya, S.; de la Torre, D.; Coletti, G.; Bianucci, G. Taphonomy of a Mysticete Whale from the Lower Pliocene of the Coast of Cádiz (Spain). J. Mar. Sci. Eng. 2024, 12, 17. https://doi.org/10.3390/jmse12010017
Bosio G, Bajo-Campos I, Collareta A, Ros-Montoya S, de la Torre D, Coletti G, Bianucci G. Taphonomy of a Mysticete Whale from the Lower Pliocene of the Coast of Cádiz (Spain). Journal of Marine Science and Engineering. 2024; 12(1):17. https://doi.org/10.3390/jmse12010017
Chicago/Turabian StyleBosio, Giulia, Ildefonso Bajo-Campos, Alberto Collareta, Sergio Ros-Montoya, Daniel de la Torre, Giovanni Coletti, and Giovanni Bianucci. 2024. "Taphonomy of a Mysticete Whale from the Lower Pliocene of the Coast of Cádiz (Spain)" Journal of Marine Science and Engineering 12, no. 1: 17. https://doi.org/10.3390/jmse12010017





