Effect of Simulated Tillage in Combination with Post-Shattering Temperature Conditions on Senna obtusifolia and Xanthium strumarium Seed Survival, Seedling Emergence and Seedbank Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Material and Experimental Set up
2.2. Monitoring Soil Seed Reserves
2.3. Germination Tests
2.4. Data Analysis
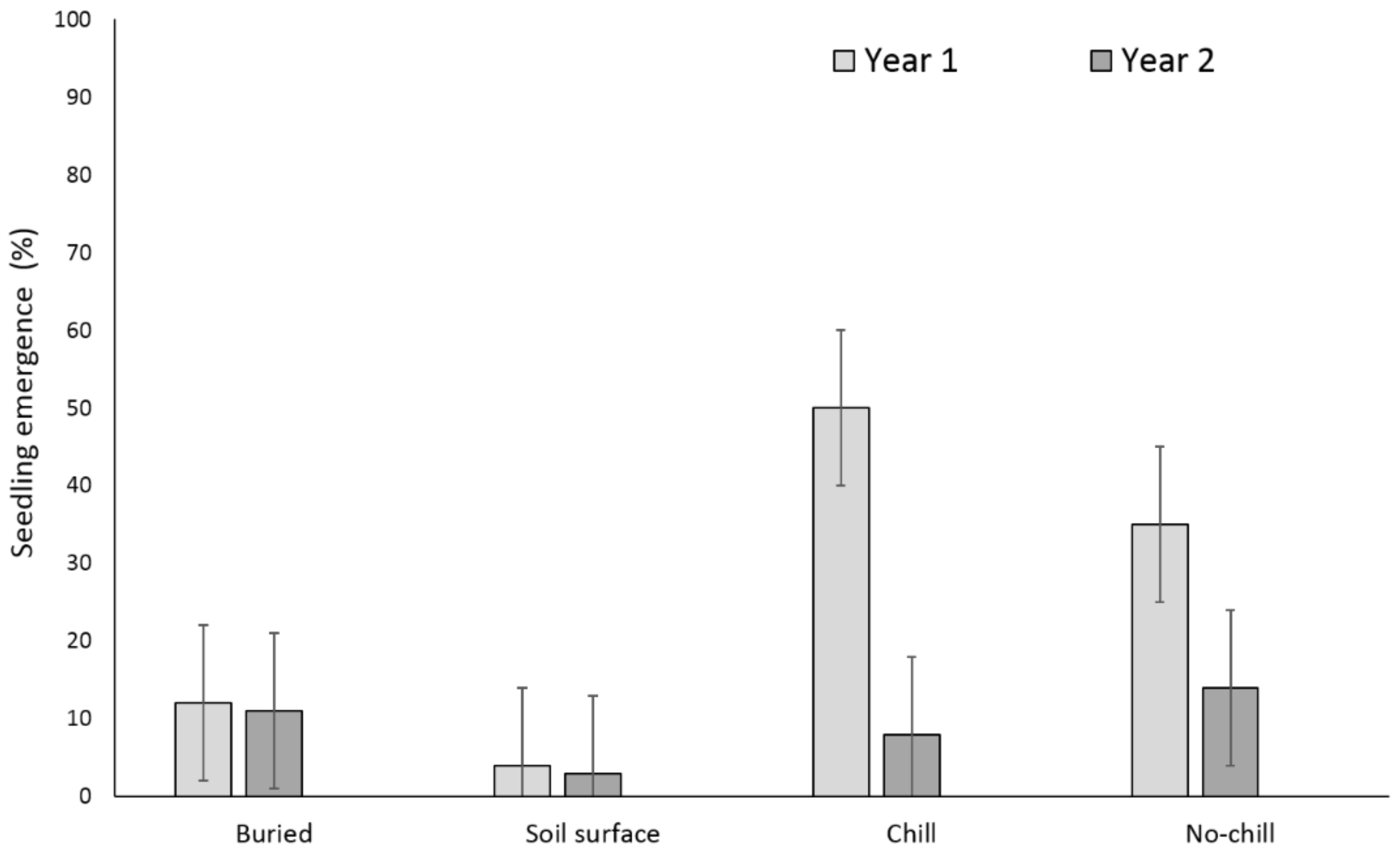
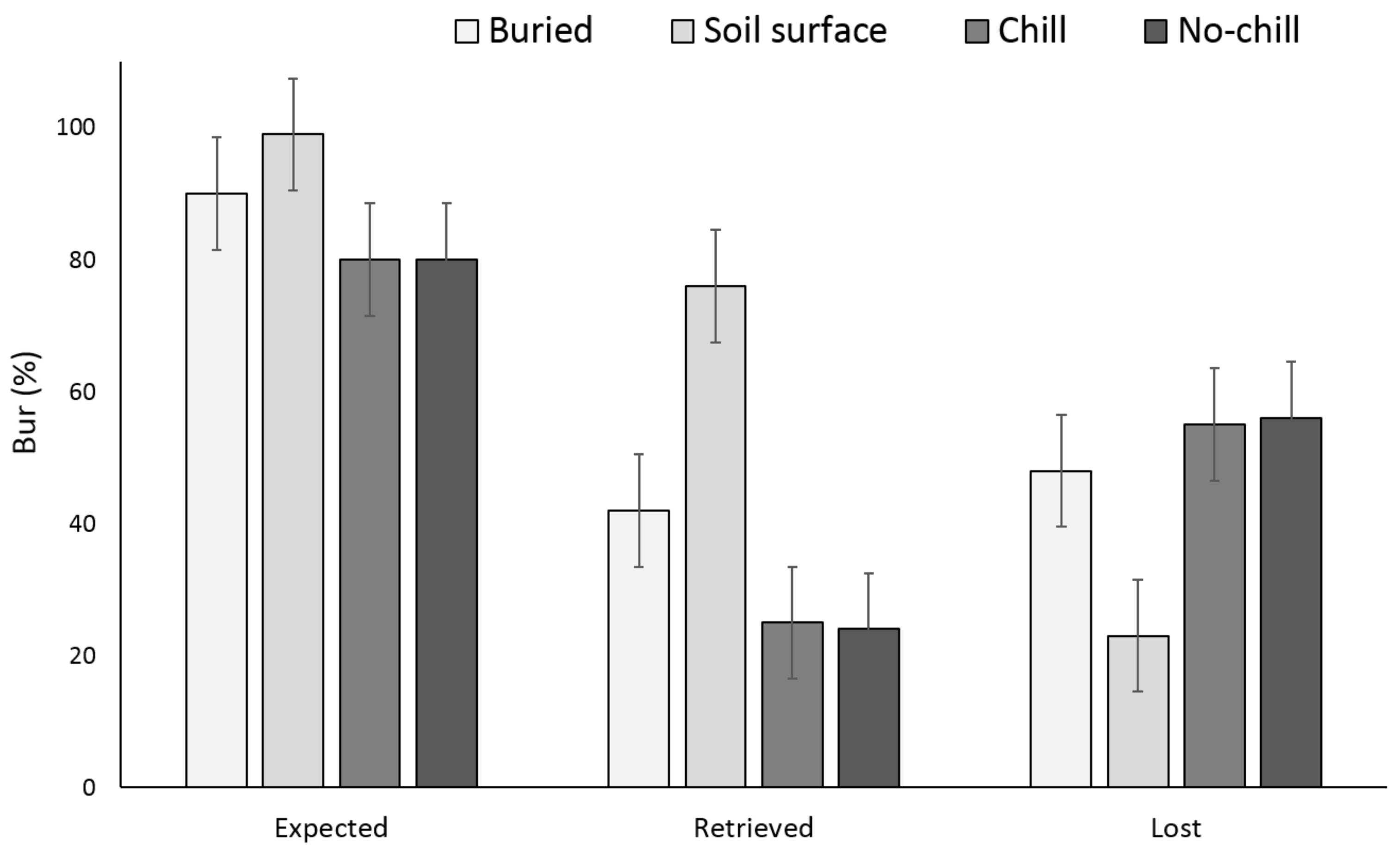
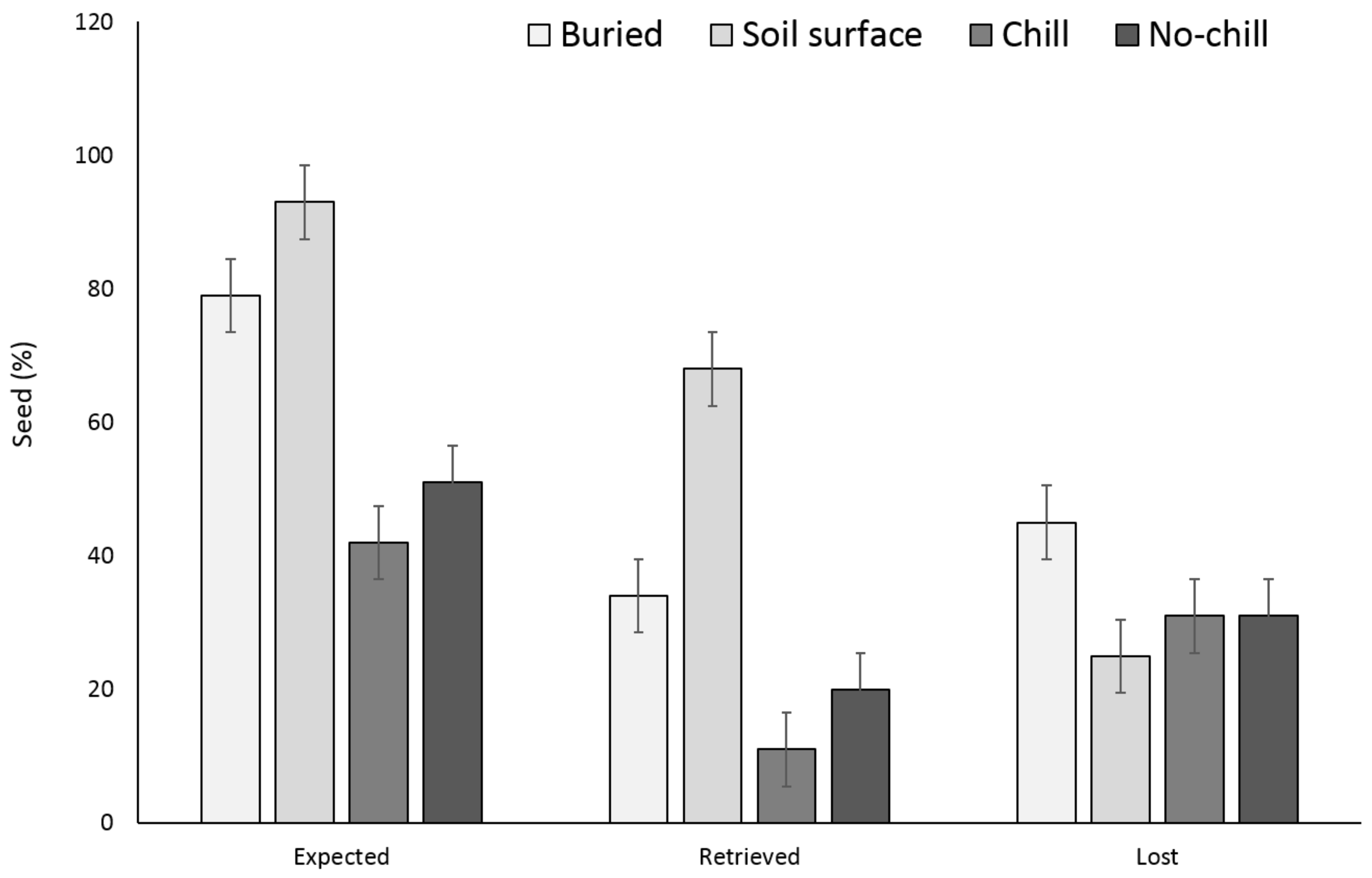
3. Results
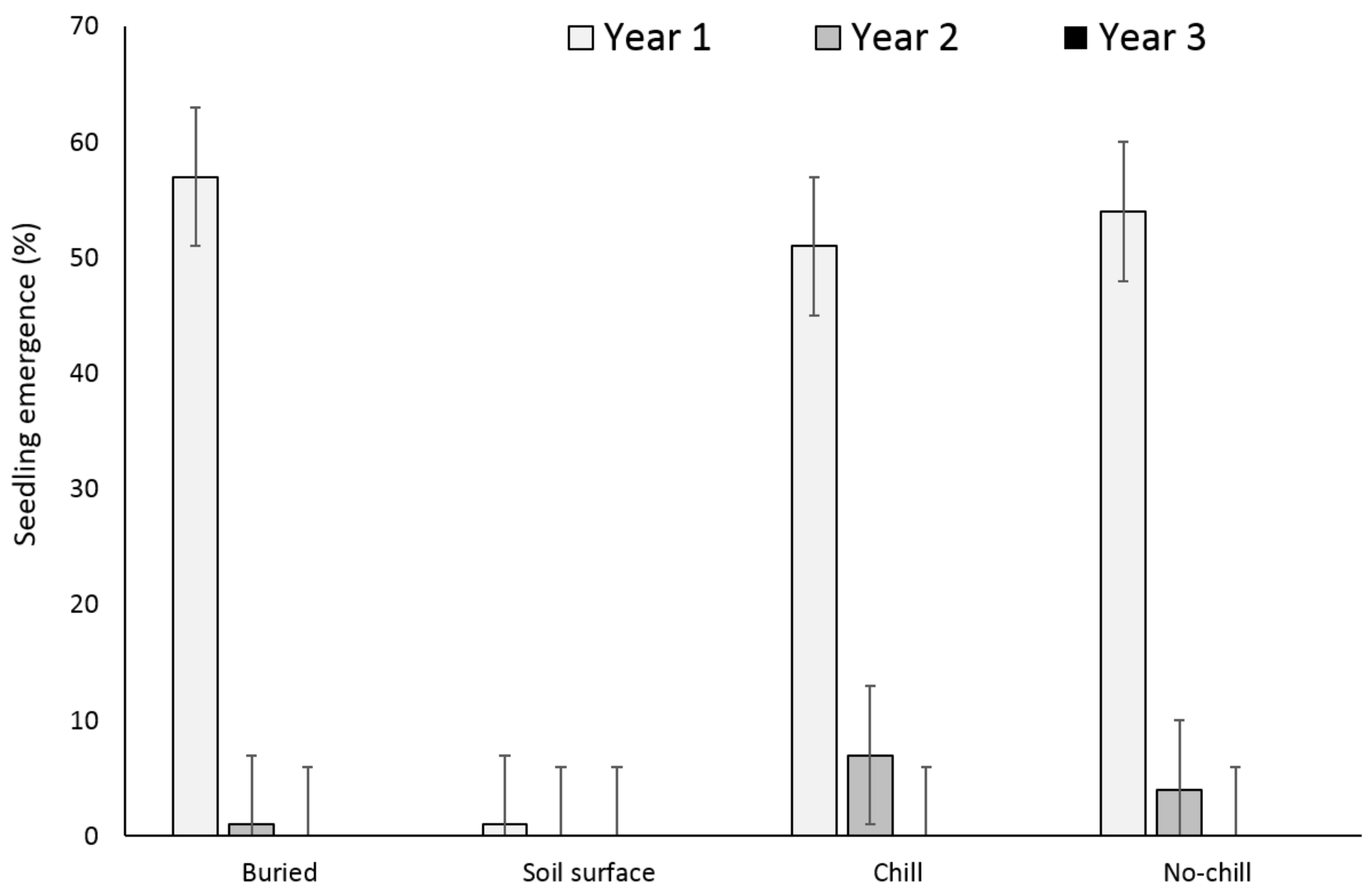
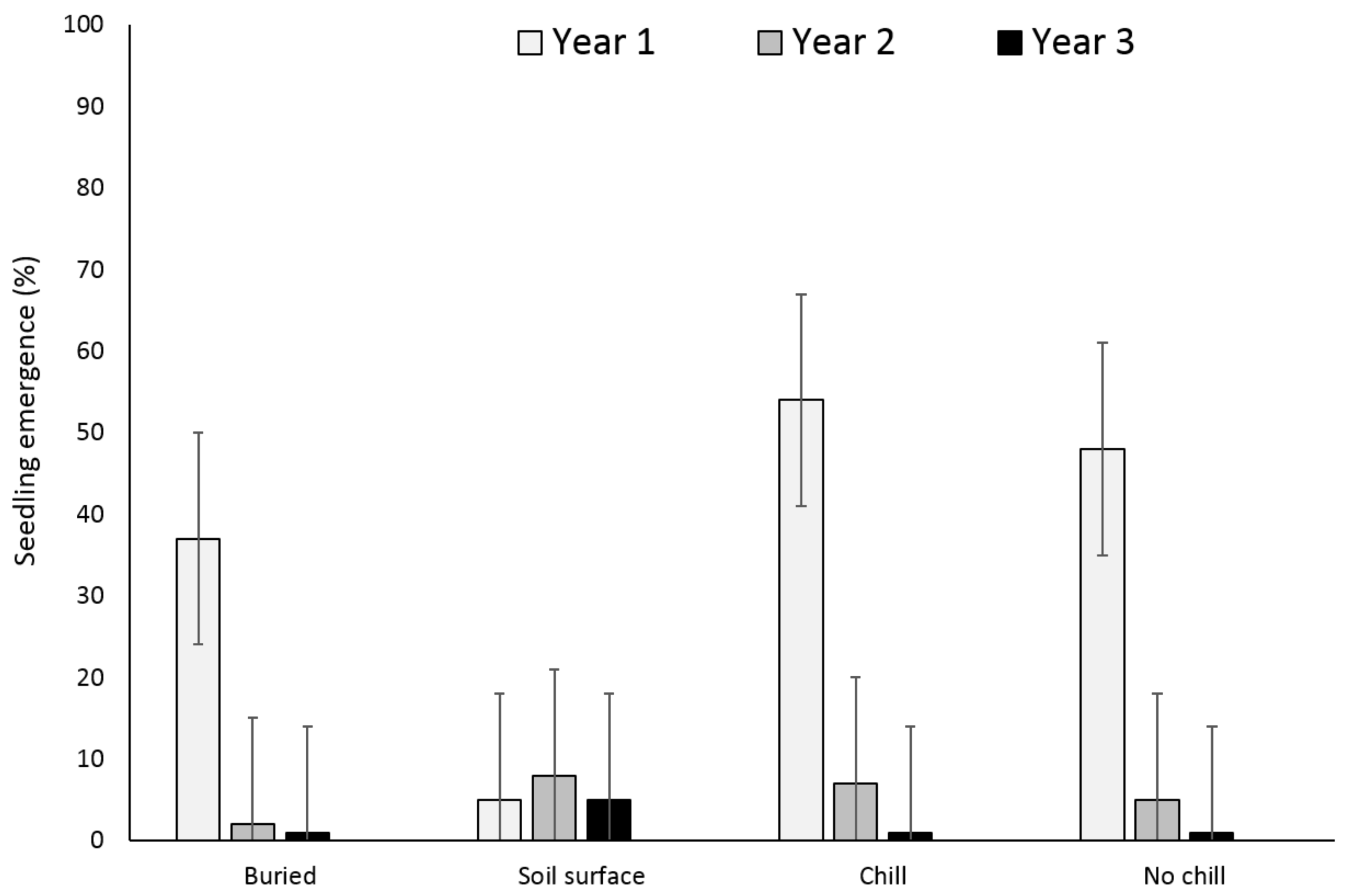
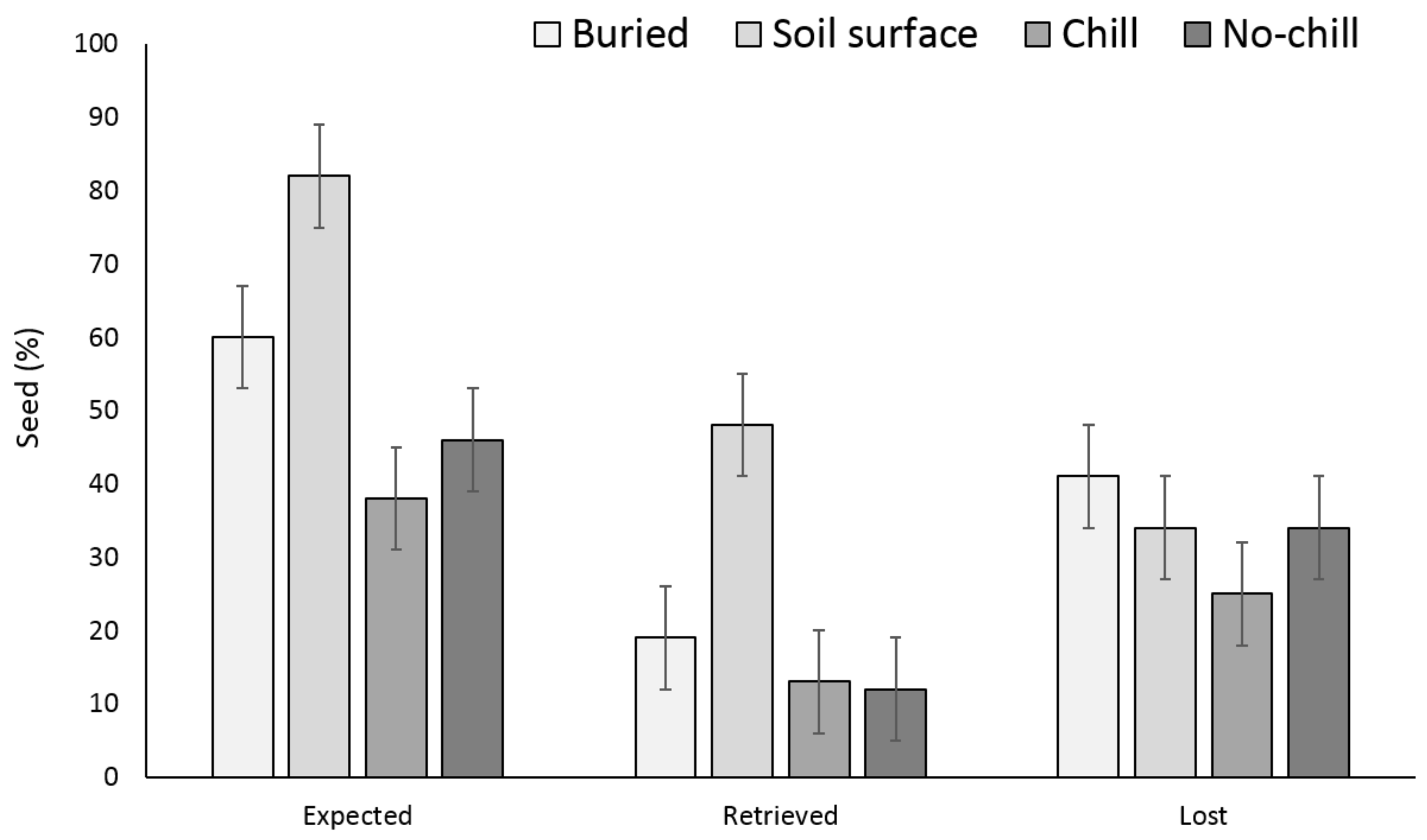
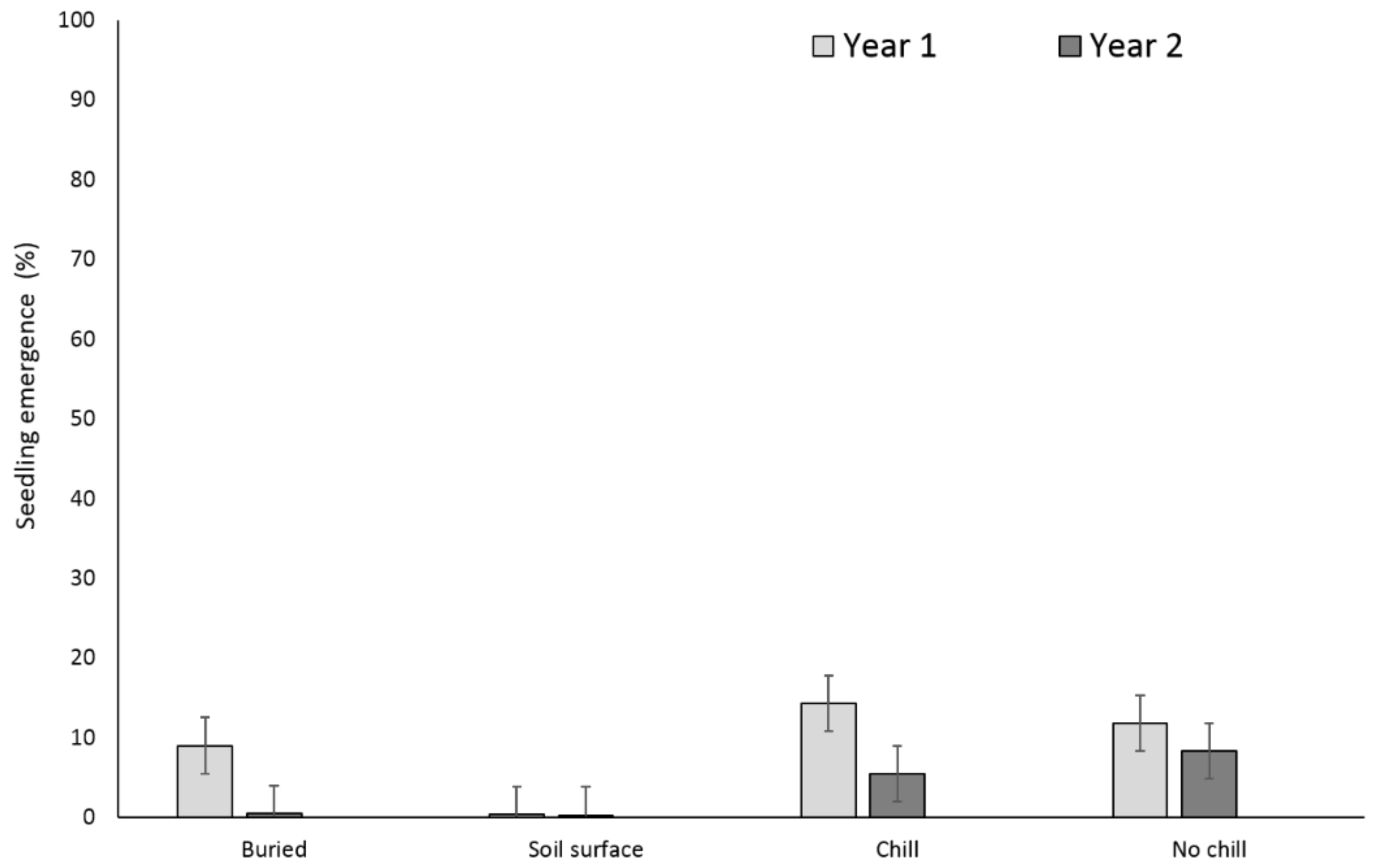
3.1. Single Maternal Seed Source
3.2. Multiple Maternal Seed Source
4. Discussion
5. Conclusions-Practical Applications
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leon, R.G.; Ferrell, J.A.; Sellers, B.A. Seed production and control of sicklepod (Senna obtusifolia) and pitted morningglory (Ipomoea lacunosa) with 2,4-D, dicamba, and glyphosate combinations. Weed Technol. 2016, 30, 76–84. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Oliveira, M.J. Tillage and soybean canopy effects on common cocklebur (Xanthium strumarium) emergence. Weed Sci. 2007, 55, 474–480. [Google Scholar] [CrossRef]
- Anonymous. Sicklepods: Sicklepod (Senna obtusifolia), Foetid Senna (Senna tora) and Hairy Senna (Senna hirsuta). Restricted Invasive Plant. Department of Agriculture and Fisheries. Biosecurity Queensland. 2016. Available online: www.biosecurity.qld.gov.au (accessed on 27 December 2017).
- CABI. Invasive Species Compendium Online Data Sheet. Senna obtusifolia (sicklepod). CABI Publishing, 2011. Available online: www.cabi.org/isc (accessed on 27 December 2017).
- Buhler, D.D. Challenges and opportunities for integrated weed management. Weed Sci. 2002, 50, 273–280. [Google Scholar] [CrossRef]
- Cardina, J.; Webster, T.M.; Herms, C.P.; Regnier, E.E. Development of weed IPM: Levels of integration for weed management. In Expanding the Context of Weed Management; Buhler, D.D., Ed.; Haworth Press, Inc.: New York, NY, USA, 1999; pp. 239–267. [Google Scholar]
- Honda, Y. Ecological correlations between the persistence of the soil seed bank and several plant traits, including seed dormancy. Plant Ecol. 2008, 196, 301–309. [Google Scholar] [CrossRef]
- Ooi, M.K.J.; Auld, T.D.; Whelan, R.J. Distinguishing between persistence and dormancy in soil seed banks of three shrub species from fi re-prone southeastern Australia. J. Veg. Sci. 2007, 18, 405–412. [Google Scholar] [CrossRef]
- Egley, G.H.; Chandler, J.M. Germination and viability of weed seeds after 2.5 years in a 50-year buried seed study. Weed Sci. 1978, 26, 230–231. [Google Scholar]
- Forcella, F. Seedling emergence model for velvetleaf. Agron. J. 1993, 85, 929–933. [Google Scholar] [CrossRef]
- Vleeshouwers, L.M.; Kropff, M.J. Modelling field emergence patterns in arable weeds. New Phytol. 2000, 148, 445–457. [Google Scholar] [CrossRef]
- Dyer, W. Exploiting weed seed dormancy and germination requirements through agronomic practices. Weed Sci. 1995, 43, 498–503. [Google Scholar]
- Mulugeta, D.; Stoltenberg, D.E. Increased weed emergence and seedbank depletion by soil disturbance in a no-tillage system. Weed Sci. 1997, 45, 234–241. [Google Scholar]
- Webster, T.M.; Cardina, J.; Norquay, H.M. Tillage and seed depth effects on velvetleaf (Abutilon theophrasti) emergence. Weed Sci. 1998, 46, 76–82. [Google Scholar]
- Yenish, J.P.; Doll, J.D.; Buhler, D.D. Effects of tillage on vertical distribution and viability of weed seed in soil. Weed Sci. 1992, 40, 429–433. [Google Scholar]
- Bararpour, M.T.; Oliver, L.R. Effect of tillage and interference on common cocklebur (Xanthium strumarium) and sicklepod (Senna obtusifolia) population, seed production, and seedbank. Weed Sci. 1998, 46, 424–431. [Google Scholar]
- Norsworthy, J.K. Soybean canopy formation effects on pitted morningglory (Ipomoea lacunosa), common cocklebur (Xanthium strumarium), and sicklepod (Senna obtusifolia) emergence. Weed Sci. 2004, 52, 961–967. [Google Scholar] [CrossRef]
- Litch, M.A.; Al-Kaisi, M. Strip-tillage on seedbed soil temperature and other soil physical properties. Soil Tillage Res. 2005, 80, 233–249. [Google Scholar]
- Bararpour, M.T. Effects of Tillage and Interference on Sicklepod (Senna obtusifolia L.) and Common Cocklebur (Xanthium strumarium L.) Growth Population, Seed Production, and Seedbank Potential. Ph.D. Dissertation, University of Arkansas, Fayetteville, AR, USA, 1995. [Google Scholar]
- Cavers, P.B. Seed demography. Can. J. Bot. 1983, 61, 3578–3590. [Google Scholar] [CrossRef]
- Davis, A.S.; Anderson, K.I.; Hallett, S.G.; Renner, K.A. Weed seed mortality in soils with contrasting agricultural management histories. Weed Sci. 2006, 54, 291–297. [Google Scholar] [CrossRef]
- Bararpour, M.T.; Oliver, L.R. Survival mechanisms of sicklepod (Senna obtusifolia L.) seed. In Proceedings of the Southern Weed Science Society, Houston, TX, USA, 20–22 January 1997; p. 151. [Google Scholar]
- Korres, N.E.; Norsworthy, J.K.; Young, B.G.; Reynolds, D.B.; Johnson, W.G.; Conley, S.P.; Smeda, R.J.; Mueller, T.C.; Bagavathiannan, M.V. Seedbank persistence of Palmer amaranth and waterhemp in the mid-south United States. In Proceedings of the Weed Science Society of America, Annual Meeting, Arlington, VA, USA, 29 January–1 February 2008. [Google Scholar]
- Sosnoskie, L.M.; Herms, C.P.; Cardina, J. Weed seedbank community composition in a 35-yr-old tillage and rotation experiment. Weed Sci. 2006, 54, 263–273. [Google Scholar] [CrossRef]

| Scarification * | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| % germination | |||
| Buried | 78 | 93 | 100 |
| Soil surface | 30 | 61 | 100 |
| Chill | 72 | 91 | 100 |
| No-chill | 76 | 82 | 100 |
| LSD | 20 | NS | NS |
| Scarification * | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| % germination | |||
| Buried | 75 | 100 | 100 |
| Soil surface | 60 | 91 | 100 |
| Chill | 56 | 91 | 100 |
| No-chill | 43 | 88 | 100 |
| LSD | 19 | NS | NS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bararpour, T.; Korres, N.E. Effect of Simulated Tillage in Combination with Post-Shattering Temperature Conditions on Senna obtusifolia and Xanthium strumarium Seed Survival, Seedling Emergence and Seedbank Potential. Agriculture 2018, 8, 61. https://doi.org/10.3390/agriculture8040061
Bararpour T, Korres NE. Effect of Simulated Tillage in Combination with Post-Shattering Temperature Conditions on Senna obtusifolia and Xanthium strumarium Seed Survival, Seedling Emergence and Seedbank Potential. Agriculture. 2018; 8(4):61. https://doi.org/10.3390/agriculture8040061
Chicago/Turabian StyleBararpour, Taghi, and Nicholas E. Korres. 2018. "Effect of Simulated Tillage in Combination with Post-Shattering Temperature Conditions on Senna obtusifolia and Xanthium strumarium Seed Survival, Seedling Emergence and Seedbank Potential" Agriculture 8, no. 4: 61. https://doi.org/10.3390/agriculture8040061





