Urine Proteomic Study in OAB Patients—Preliminary Report
Abstract
:1. Introduction
2. Materials and Methods
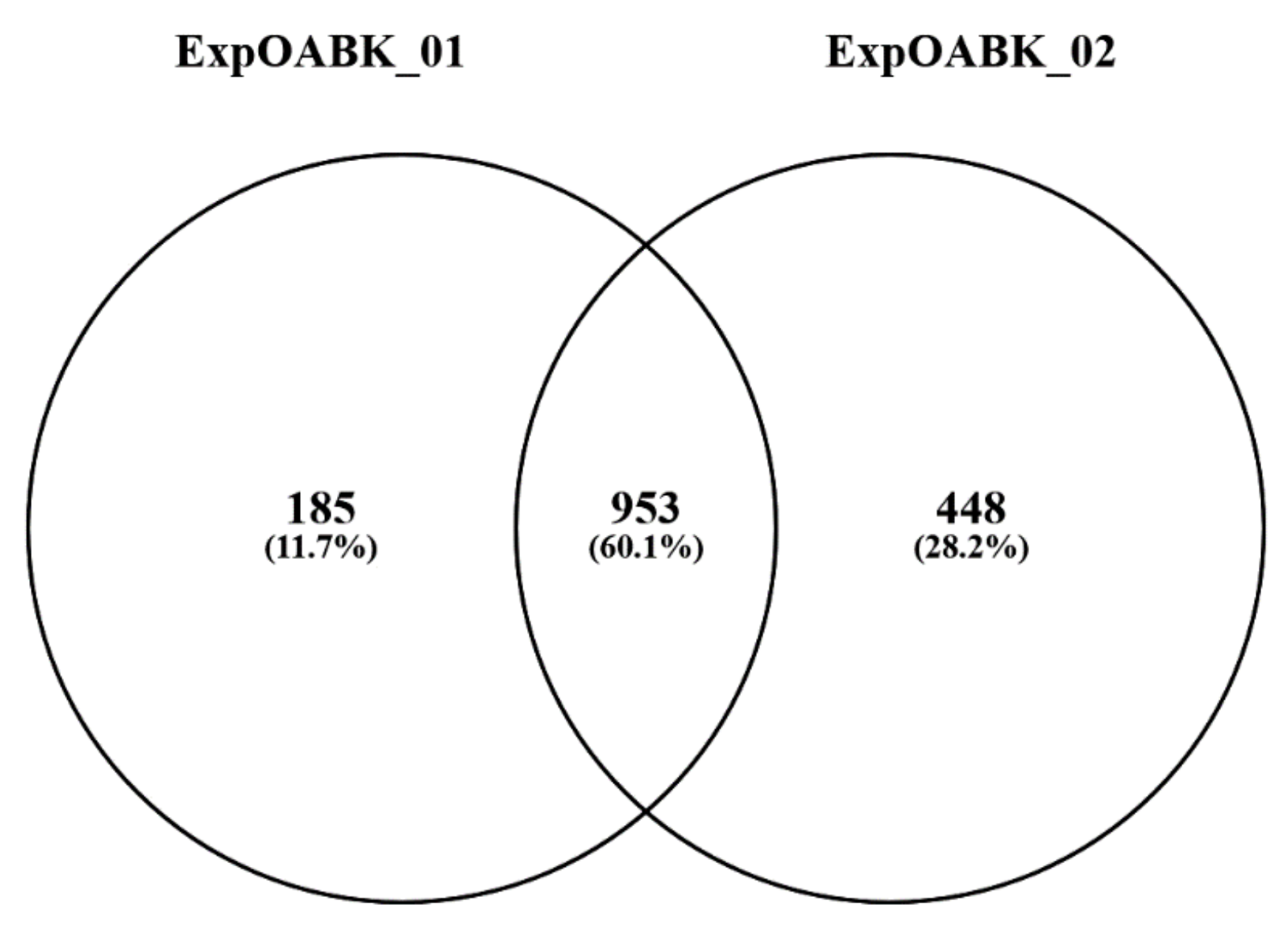
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol. Urodyn. 2010, 29, 4–20. [Google Scholar] [PubMed]
- Mannikarottu, A.S.; Disanto, M.E.; Zderic, S.A.; Wein, A.J.; Chacko, S. Altered expression of thin filament-associated proteins in hypertrophied urinary bladder smooth muscle. Neurourol. Urodyn. 2006, 25, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.T.; Yoshimura, N.; Tyagi, V.; Jacobs, B.; Leng, W.; Tyagi, P. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J. Urol. 2013, 31, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julian, B.A.; Wittke, S.; Haubitz, M.; Zürbig, P.; Schiffer, E.; McGuire, B.M.; Wyatt, R.J.; Novak, J. Urinary biomarkers of IgA nephropathy and other IgA-associated renal diseases. World J. Urol. 2007, 25, 467–476. [Google Scholar] [CrossRef]
- Urinology Think Tank Writing Group. Urine: Waste product or biologically active tissue? Neurourol. Urodyn. 2018, 37, 1162–1168. [Google Scholar] [CrossRef]
- Mossa, A.H.; Shamout, S.; Cammisotto, P.; Campeau, L. Urinary metabolomics predict the severity of overactive bladder syndrome in an aging female population. Int. Urogynecol. J. 2019. [Google Scholar] [CrossRef]
- Fry, C.H.; Vaha bi, B. The Role of the Mucosa in Normal and Abnormal Bladder Function. Basic Clin. Pharmacol. Toxicol. 2016, 119 (Suppl. S3), 57–62. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, J.D.; Koutros, S.; Colt, J.S.; Kogevinas, M.; Garcia-Closas, M.; Real, F.X.; Friesen, M.C.; Baris, D.; Stewart, P.; Schwenn, M.; et al. Modification of occupational exposures on bladder cancer risk by common genetic polymorphisms. J. Natl. Cancer Inst. 2015, 107, djv223. [Google Scholar] [CrossRef] [Green Version]
- Girard, B.M.; Malley, S.; May, V.; Vizzard, M.A. Effects of cyp-induced cystitis on growth factors and associated receptor expression in micturition pathways in mice with chronic overexpression of ngf in urothelium. J. Mol. Neurosci. 2016, 59, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Wessel, D.; Fluegge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Bakun, M.; Niemczyk, M.; Domanski, D.; Jazwiec, R.; Perzanowska, A.; Niemczyk, S.; Kistowski, M.; Fabijanska, A.; Borowiec, A.; Paczek, L.; et al. Urine proteome of autosomal dominant polycystic kidney disease patients. Clin. Proteomics 2012, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Malinowska, A.; Kistowski, M.; Bakun, M.; Rubel, T.; Tkaczyk, M.; Mierzejewska, J.; Dadlez, M. Diffprot—software for non-parametric statistical analysis of differential proteomics data. J. Proteomics 2012, 75, 4062–4073. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, 442–450. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, K.D. Interstitial cells in the urinary bladder—Localization and function. Neurourol. Urodyn. 2010, 29, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Steers, W.D. Pathophysiology of overactive bladder and urge urinary incontinence. Rev. Urol. 2002, 4, 7–18. [Google Scholar]
- Mukerji, G.; Yiangou, Y.; Grogono, J.; Underwood, J.; Agarwal, S.K.; Khullar, V.; Anand, P. Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J. Urol. 2006, 176, 367–373. [Google Scholar] [CrossRef]
- De Groat, W.C. Highlights in basic autonomic neuroscience: Contribution of the urothelium to sensory mechanisms in the urinary bladder. Auton. Neurosci. 2013, 177, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Fry, C.; Hayashi, F.; Stolz, D.; Griffiths, D.; Kanai, A. Role of gap junctions in spontaneous activity of the rat bladder. Am. J. Physiol. Renal Physiol. 2007, 293, 1018–1025. [Google Scholar] [CrossRef] [Green Version]
- Biers, S.M.; Reynard, J.M.; Doore, T.; Brading, A.F. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006, 97, 612–616. [Google Scholar] [CrossRef]
- Kuo, H.C. Potential urine and serum biomarkers for patients with bladder pain syndrome/interstitial cystitis. Int. J. Urol. 2014, 21, 34–41. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Alvarez, S.; Andersen, L.; Nordling, J.; Horn, T.; Bouchelouche, P. Monocyte chemoattractant protein-1 production by human detrusor smooth muscle cells. J. Urol. 2004, 171, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Lintomen, L.; Franchi, G.; Nowill, A.; Condino-Neto, A.; de Nucci, G.; Zanesco, A.; Antunes, E. Human eosinophil adhesion and degranulation stimulated with eotaxin and RANTES in vitro: Lack of interaction with nitric oxide. BMC Pulm. Med. 2008, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Yamamoto, T.; Suzuki, Y.; Gotoh, M.; Egawa, S.; Yoshimura, N. Comparison of inflammatory urine markers in patients with interstitial cystitis and overactive bladder. Int. Urogynecol. J. 2018, 29, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Grundy, L.; Caldwell, A.; Brierley, S.M. Mechanisms underlying overactive bladder and interstitial cystitis/painful bladder syndrome. Front. Neurosci. 2018, 12, 931. [Google Scholar] [CrossRef] [Green Version]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Lin, S.X.; Amin, S.; Overbergh, L.; Maggiolino, G.; Chan, L.S. VCAM-1 blockade delays disease onset, reduces disease severity and inflammatory cells in an atopic dermatitis model. Immunol. Cell Biol. 2010, 88, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Chin, J.E.; Hatfield, C.A.; Winterrowd, G.E.; Brashler, J.R.; Vonderfecht, S.L.; Fidler, S.F.; Griffin, R.L.; Kolbasa, K.P.; Krzesicki, R.F.; Sly, L.M.; et al. Airway recruitment of leukocytes in mice is dependent on alpha4-integrins and vascular cell adhesion molecule-1. Am. J. Physiol. 1997, 272, 219–229. [Google Scholar] [CrossRef]
- Hakugawa, J.; Bae, S.J.; Tanaka, Y.; Katayama, I. The inhibitory effect of anti-adhesion molecule antibodies on eosinophil infiltration in cutaneous late phase response in Balb/c mice sensitized with ovalbumin (OVA). J. Dermatol. 1997, 24, 73–79. [Google Scholar] [CrossRef]
| Parameter | OAB Group (n = 8) | Control Group (n = 8) | p Value |
|---|---|---|---|
| Age (years) | 57.4 | 62.0 | 0.08 |
| BMI (kg/m2) | 24.1 | 25.2 | 0.69 |
| Parity (n) | 1.9 | 1.5 | 0.44 |
| Experiment 1 (ExpOABK_01) | ||||||
| No. | Protein | q Value | Ratio | Fold Change | Peptides | Description |
| 1 | P25311 | 0.0002 | 1.46 | 1.46 | 40 | Zinc-alpha-2-glycoprotein |
| 2 | P12830 | 0.0002 | 1.45 | 1.45 | 28 | Cadherin-1 |
| 3 | P06396 | 0.0002 | 1.37 | 1.37 | 30 | Gelsolin |
| 4 | P02760 | 0.0009 | 1.15 | 1.15 | 50 | Protein AMBP |
| 5 | P41222 | 0.00391 | 1.21 | 1.21 | 53 | Prostaglandin-H2 D-isomerase |
| 6 | P08246 | 0.00397 | 0.43 | 2.35 | 4 | Neutrophil elastase |
| 7 | Q9Y279 | 0.00465 | 2.17 | 2.17 | 4 | V-set and immunoglobulin domain-containing protein 4 |
| 8 | P19320 | 0.00723 | 1.36 | 1.36 | 10 | Vascular cell adhesion molecule 1 |
| 9 | P0DOX8 | 0.06346 | 1.1 | 1.1 | 32 | Immunoglobulin lambda-1 light chain |
| 10 | Q5T013 | 0.02613 | 1.25 | 1.25 | 18 | Putative hydroxypyruvate isomerase |
| 11 | P01619 | 0.02754 | 1.19 | 1.19 | 9 | Immunoglobulin kappa variable 3–20 |
| Experiment 2 (ExpOABK_02) | ||||||
| No. | Protein | q Value | Ratio | Fold Change | Peptides | Description |
| 1 | P98160 | 0.00018 | 0.76 | 1.32 | 60 | Basement membrane-specific heparan sulfate proteoglycan core protein |
| 2 | Q9HCU0 | 0.00018 | 0.49 | 2.05 | 5 | Endosialin |
| 3 | P02787 | 0.00018 | 1.4 | 1.4 | 47 | Serotransferrin |
| 4 | P00738 | 0.00018 | 2.07 | 2.07 | 13 | Haptoglobin |
| 5 | P20742 | 0.00029 | 1.36 | 1.36 | 43 | Pregnancy zone protein |
| 6 | P0DOX7 | 0.00172 | 1.23 | 1.23 | 34 | Immunoglobulin kappa light chain |
| 7 | P06870 | 0.00652 | 0.53 | 1.87 | 7 | Kallikrein-1 |
| 8 | P05155 | 0.00704 | 0.75 | 1.34 | 38 | Plasma protease C1 inhibitor |
| 9 | P04083 | 0.00735 | 1.53 | 1.53 | 15 | Annexin A1 |
| 10 | O95460 | 0.01472 | 0.47 | 2.11 | 3 | Matrilin-4 |
| 11 | P02750 | 0.01886 | 1.37 | 1.37 | 25 | Leucine-rich alpha-2-glycoprotein |
| 12 | P36957 | 0.01934 | 0.34 | 2.97 | 2 | Dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex. mitochondrial |
| 13 | P13647 | 0.01991 | 0.56 | 1.78 | 7 | Keratin. type II cytoskeletal 5 |
| 14 | P0DOX5 | 0.01992 | 1.33 | 1.33 | 11 | Immunoglobulin gamma-1 heavy chain |
| 15 | P19320 | 0.02104 | 1.24 | 1.24 | 12 | Vascular cell adhesion molecule 1 |
| 16 | P01861 | 0.02134 | 1.47 | 1.47 | 9 | Immunoglobulin heavy constant gamma 4 |
| 17 | Q99715 | 0.03727 | 0.8 | 1.26 | 27 | Collagen alpha-1(XII) chain |
| 18 | P22105 | 0.03768 | 0.78 | 1.28 | 38 | Tenascin-X |
| Sample | Total Queries | Queries | Peptides | Proteins |
|---|---|---|---|---|
| ExpOABK_01 | 1,388,534 | 19,605 | 5529 | 1138 |
| ExpOABK_02 | 1,434,226 | 22,496 | 7003 | 1401 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Futyma, K.; Nowakowski, Ł.; Ziętek-Strobl, A.; Kamińska, A.; Taoussi, N.; Rechberger, T. Urine Proteomic Study in OAB Patients—Preliminary Report. J. Clin. Med. 2020, 9, 1389. https://doi.org/10.3390/jcm9051389
Futyma K, Nowakowski Ł, Ziętek-Strobl A, Kamińska A, Taoussi N, Rechberger T. Urine Proteomic Study in OAB Patients—Preliminary Report. Journal of Clinical Medicine. 2020; 9(5):1389. https://doi.org/10.3390/jcm9051389
Chicago/Turabian StyleFutyma, Konrad, Łukasz Nowakowski, Alicja Ziętek-Strobl, Aleksandra Kamińska, Nadia Taoussi, and Tomasz Rechberger. 2020. "Urine Proteomic Study in OAB Patients—Preliminary Report" Journal of Clinical Medicine 9, no. 5: 1389. https://doi.org/10.3390/jcm9051389





