Topical VEGF-C/D Inhibition Prevents Lymphatic Vessel Ingrowth into Cornea but Does Not Improve Corneal Graft Survival
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Anesthesia and Suture Model
2.2. Corneal Transplantations
2.3. Flow Cytometry
2.4. Immunohistochemistry of Neovascularization and Immune Cell Infiltrate
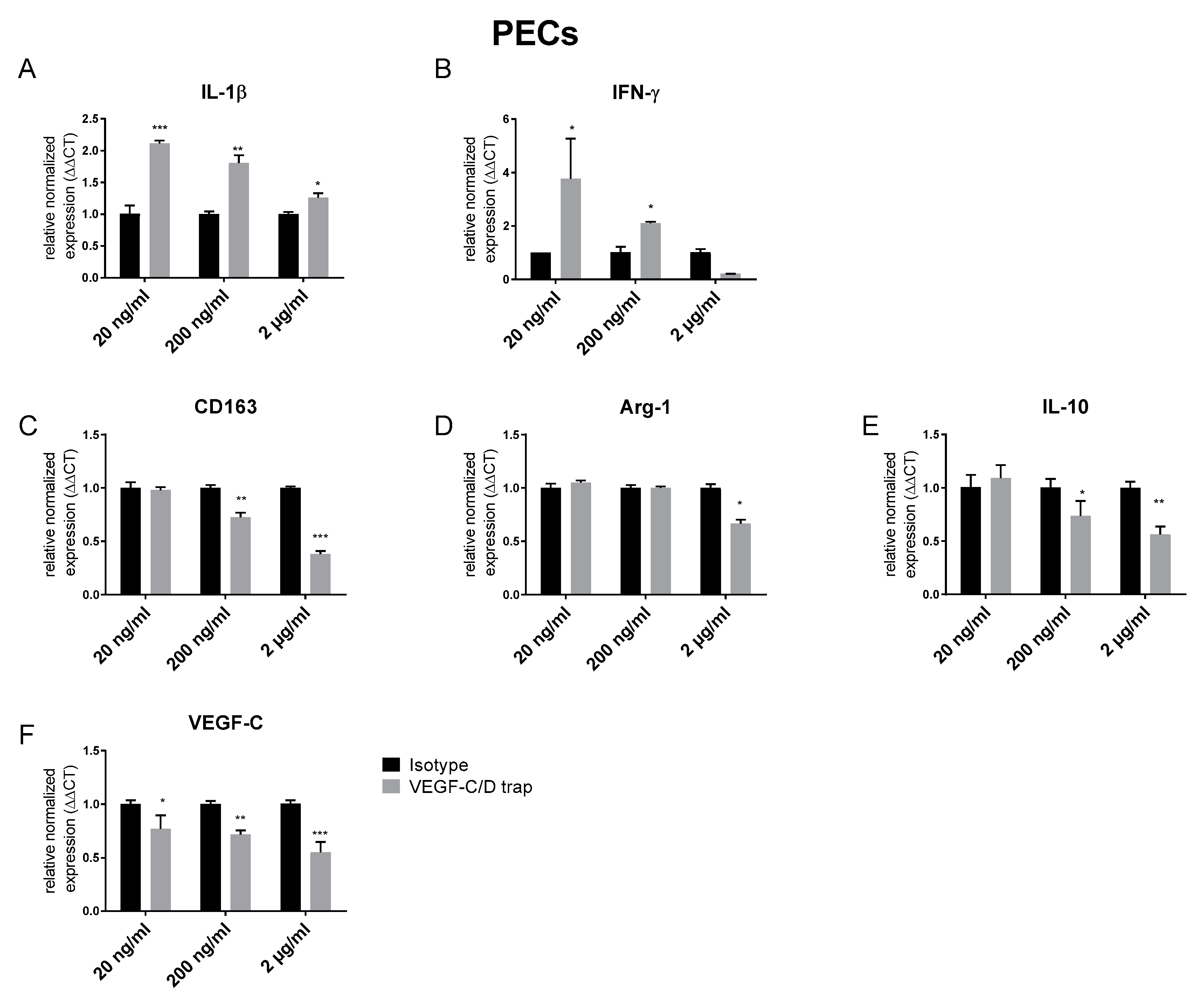
2.5. Purification and Analysis of Peritoneal Macrophages
2.6. RNA Isolation from Corneas or PECs and RT-PCR
2.7. Mixed Lymphocyte Reaction (MLR)
2.8. Statistical Analyses
3. Results
3.1. VEGF C/D Trap Specifically Inhibits Lymphatic Vessels while Affecting LYVE-1 Positive Macrophage Recruitment
3.2. Topical VEGF-C/D Trap Has No Beneficial Effect on Survival of Cornea Transplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bachmann, B.; Taylor, R.S.; Cursiefen, C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: An evidence-based meta-analysis. Ophthalmology 2010, 117, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C. Immune privilege and angiogenic privilege of the cornea. Chem. Immunol. Allergy 2007, 92, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. Corneal transplantation and immune privilege. Int. Rev. Immunol. 2013, 32, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, T.; Bock, F.; Yuen, D.; Hos, D.; Bachmann, B.O.; Zahn, G.; Wiegand, S.; Chen, L.; Cursiefen, C. Cutting edge: Lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J. Immunol. 2010, 184, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Kumar, A. Immunological aspects of corneal transplant. Immunol. Investig 2014, 43, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [Green Version]
- van Essen, T.H.; Roelen, D.L.; Williams, K.A.; Jager, M.J. Matching for Human Leukocyte Antigens (HLA) in corneal transplantation—To do or not to do. Prog. Retin. Eye Res. 2015, 46, 84–110. [Google Scholar] [CrossRef]
- Sonoda, Y.; Streilein, J.W. Orthotopic corneal transplantation in mice--evidence that the immunogenetic rules of rejection do not apply. Transplantation 1992, 54, 694–704. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, H.; Ling, S. Clinicopathological correlation analysis of (lymph) angiogenesis and corneal graft rejection. Mol. Vis. 2011, 17, 1694–1700. [Google Scholar]
- Inomata, T.; Mashaghi, A.; Di Zazzo, A.; Lee, S.M.; Chiang, H.; Dana, R. Kinetics of Angiogenic Responses in Corneal Transplantation. Cornea 2017, 36, 491–496. [Google Scholar] [CrossRef]
- Qazi, Y.; Hamrah, P. Corneal Allograft Rejection: Immunopathogenesis to Therapeutics. J. Clin. Cell Immunol. 2013, 2013. [Google Scholar] [CrossRef]
- Bachmann, B.O.; Luetjen-Drecoll, E.; Bock, F.; Wiegand, S.J.; Hos, D.; Dana, R.; Kruse, F.E.; Cursiefen, C. Transient postoperative vascular endothelial growth factor (VEGF)-neutralisation improves graft survival in corneas with partly regressed inflammatory neovascularisation. Br. J. Ophthalmol. 2009, 93, 1075–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, F.; Maruyama, K.; Regenfuss, B.; Hos, D.; Steven, P.; Heindl, L.M.; Cursiefen, C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog. Retin. Eye Res. 2013, 34, 89–124. [Google Scholar] [CrossRef]
- Cursiefen, C.; Cao, J.; Chen, L.; Liu, Y.; Maruyama, K.; Jackson, D.; Kruse, F.E.; Wiegand, S.J.; Dana, M.R.; Streilein, J.W. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2666–2673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D′Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [Green Version]
- Dohlman, T.H.; Omoto, M.; Hua, J.; Stevenson, W.; Lee, S.M.; Chauhan, S.K.; Dana, R. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation 2015, 99, 678–686. [Google Scholar] [CrossRef]
- Hou, Y.; Le, V.N.H.; Clahsen, T.; Schneider, A.C.; Bock, F.; Cursiefen, C. Photodynamic Therapy Leads to Time-Dependent Regression of Pathologic Corneal (Lymph) Angiogenesis and Promotes High-Risk Corneal Allograft Survival. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5862–5869. [Google Scholar] [CrossRef] [Green Version]
- Le, V.N.H.; Schneider, A.C.; Scholz, R.; Bock, F.; Cursiefen, C. Fine Needle-Diathermy Regresses Pathological Corneal (Lymph)Angiogenesis and Promotes High-Risk Corneal Transplant Survival. Sci. Rep. 2018, 8, 5707. [Google Scholar] [CrossRef]
- Dastjerdi, M.H.; Al-Arfaj, K.M.; Nallasamy, N.; Hamrah, P.; Jurkunas, U.V.; Pineda, R., 2nd; Pavan-Langston, D.; Dana, R. Topical bevacizumab in the treatment of corneal neovascularization: Results of a prospective, open-label, noncomparative study. Arch. Ophthalmol. 2009, 127, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Usui, T.; Yamagami, S. Suppression of Allograft Rejection with Soluble VEGF Receptor 2 Chimeric Protein in a Mouse Model of Corneal Transplantation. Tohoku J. Exp. Med. 2016, 239, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Salabarria, A.C.; Braun, G.; Heykants, M.; Koch, M.; Reuten, R.; Mahabir, E.; Cursiefen, C.; Bock, F. Local VEGF-A blockade modulates the microenvironment of the corneal graft bed. Am. J. Transplant. 2019, 19, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, M.S.; Choi, M.Y.; Woo, S.Y.; Lee, H.K.; Lee, H.S.; Kim, K.J.; Jeoung, S.C.; Choi, J.S.; Joo, C.K.; Park, I.H. Femtosecond laser-assisted selective reduction of neovascularization in rat cornea. Lasers Med. Sci. 2014, 29, 1417–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D′Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005, 115, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.A.; Chan, S.W.S.; Zhou, X.; Glinka, Y.; Girard, E.; Yucel, Y.; Gupta, N. Lymphatic vessels identified in failed corneal transplants with neovascularisation. Br. J. Ophthalmol. 2019, 103, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, L.M.; Hicklin, D.J. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Schlereth, S.L.; Bock, F.; Heindl, L.M.; Cursiefen, C. Antilymphangiogenic therapy to promote transplant survival and to reduce cancer metastasis: What can we learn from the eye? Semin. Cell Dev. Biol. 2015, 38, 117–130. [Google Scholar] [CrossRef]
- Goyal, S.; Chauhan, S.K.; Dana, R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch. Ophthalmol. 2012, 130, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Yamada, J.; Streilein, J.W. Fate of orthotopic corneal allografts in C57BL/6 mice. Transpl. Immunol. 1998, 6, 161–168. [Google Scholar] [CrossRef]
- Hos, D.; Regenfuss, B.; Bock, F.; Onderka, J.; Cursiefen, C. Blockade of insulin receptor substrate-1 inhibits corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5778–5785. [Google Scholar] [CrossRef] [Green Version]
- Hos, D.; Saban, D.R.; Bock, F.; Regenfuss, B.; Onderka, J.; Masli, S.; Cursiefen, C. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch. Ophthalmol. 2011, 129, 445–452. [Google Scholar] [CrossRef]
- Makinen, T.; Jussila, L.; Veikkola, T.; Karpanen, T.; Kettunen, M.I.; Pulkkanen, K.J.; Kauppinen, R.; Jackson, D.G.; Kubo, H.; Nishikawa, S.; et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 2001, 7, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Reuten, R.; Patel, T.R.; McDougall, M.; Rama, N.; Nikodemus, D.; Gibert, B.; Delcros, J.G.; Prein, C.; Meier, M.; Metzger, S.; et al. Structural decoding of netrin-4 reveals a regulatory function towards mature basement membranes. Nat. Commun. 2016, 7, 13515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inomata, T.; Mashaghi, A.; Di Zazzo, A.; Dana, R. Ocular surgical models for immune and angiogenic responses. J. Biol. Methods 2015, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hos, D.; Bucher, F.; Regenfuss, B.; Dreisow, M.L.; Bock, F.; Heindl, L.M.; Eming, S.A.; Cursiefen, C. IL-10 Indirectly Regulates Corneal Lymphangiogenesis and Resolution of Inflammation via Macrophages. Am. J. Pathol. 2016, 186, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, M.; Alard, P.; Streilein, J.W. TGF-beta promotes immune deviation by altering accessory signals of antigen-presenting cells. J. Immunol. 1998, 160, 1589–1597. [Google Scholar]
- Emami-Naeini, P.; Dohlman, T.H.; Omoto, M.; Hattori, T.; Chen, Y.; Lee, H.S.; Chauhan, S.K.; Dana, R. Soluble vascular endothelial growth factor receptor-3 suppresses allosensitization and promotes corneal allograft survival. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1755–1762. [Google Scholar] [CrossRef] [Green Version]
- Touyz, R.M.; Herrmann, J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis. Oncol. 2018, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Feizi, S.; Azari, A.A.; Safapour, S. Therapeutic approaches for corneal neovascularization. Eye Vis. 2017, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Chung, E.S.; Chauhan, S.K.; Jin, Y.; Nakao, S.; Hafezi-Moghadam, A.; van Rooijen, N.; Zhang, Q.; Chen, L.; Dana, R. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am. J. Pathol. 2009, 175, 1984–1992. [Google Scholar] [CrossRef] [Green Version]
- Kiesewetter, A.; Cursiefen, C.; Eming, S.A.; Hos, D. Phase-specific functions of macrophages determine injury-mediated corneal hem- and lymphangiogenesis. Sci. Rep. 2019, 9, 308. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Dietrich, T.; Bachmann, B.; Pytowski, B.; Cursiefen, C. Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 115–119. [Google Scholar] [CrossRef] [PubMed]




| mRNA | Sequence | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| HPRT | F: 5′-TTGGATACAGGCCAGACTTTGTTG-3′ | 163 | 60-63 |
| R: 5′-GATTCAACTTGCGCTCATCTTAGGC-3′ | |||
| TNF-a | F: 5′- AGGACTCAAATGGGCTTTCC-3′ | 63 | 63 |
| R: 5′-CAGAGGCAACCTGACCACTC-3′ | |||
| IL-1ß | F: 5′-GTCCTGTGTAATGAAAGACGGC-3′ | 176 | 62.4 |
| R: 5′-CTGCTTGTGAGGTGCTGATGTA-3′ | |||
| IL-10 | F: 5′-CAGTACAGCCGGGAAGACAATA-3′ | 151 | 63 |
| R: 5′-GCATTAAGGAGTCGGTTAGCAG-3′ | |||
| IFN-g | F: 5′-GCTTTGCAGCTCTTCCTCAT-3′ | 61 | |
| R: 5′-GTCACCATCCTTTTGCCAGT-3′ | |||
| VEGF-C | F: 5′-AGAACGTGTCCAAGAAATCAGC-3′ | 219 | 60 |
| R: 5′-ATGTGGCCTTTTCCAATACG-3′ | |||
| Arg-1 | F: 5′-GCAGAGGTCCAGAAGAATGG-3′ | 126 | 60 |
| R: 5′-GTGAGCATCCACCCAAATG-3′ | |||
| CD163 | F: 5′-GGCACTCTTGGTTTGTGGAG-3′ | 153 | 60 |
| R: 5′-GCCTTTGAATCCATCTCTTGG-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salabarria, A.-C.; Koch, M.; Schönberg, A.; Zinser, E.; Hos, D.; Hamdorf, M.; Imhof, T.; Braun, G.; Cursiefen, C.; Bock, F. Topical VEGF-C/D Inhibition Prevents Lymphatic Vessel Ingrowth into Cornea but Does Not Improve Corneal Graft Survival. J. Clin. Med. 2020, 9, 1270. https://doi.org/10.3390/jcm9051270
Salabarria A-C, Koch M, Schönberg A, Zinser E, Hos D, Hamdorf M, Imhof T, Braun G, Cursiefen C, Bock F. Topical VEGF-C/D Inhibition Prevents Lymphatic Vessel Ingrowth into Cornea but Does Not Improve Corneal Graft Survival. Journal of Clinical Medicine. 2020; 9(5):1270. https://doi.org/10.3390/jcm9051270
Chicago/Turabian StyleSalabarria, Ann-Charlott, Manuel Koch, Alfrun Schönberg, Elisabeth Zinser, Deniz Hos, Matthias Hamdorf, Thomas Imhof, Gabriele Braun, Claus Cursiefen, and Felix Bock. 2020. "Topical VEGF-C/D Inhibition Prevents Lymphatic Vessel Ingrowth into Cornea but Does Not Improve Corneal Graft Survival" Journal of Clinical Medicine 9, no. 5: 1270. https://doi.org/10.3390/jcm9051270





