Normothermic Machine Perfusion (NMP) of the Liver as a Platform for Therapeutic Interventions during Ex-Vivo Liver Preservation: A Review
Abstract
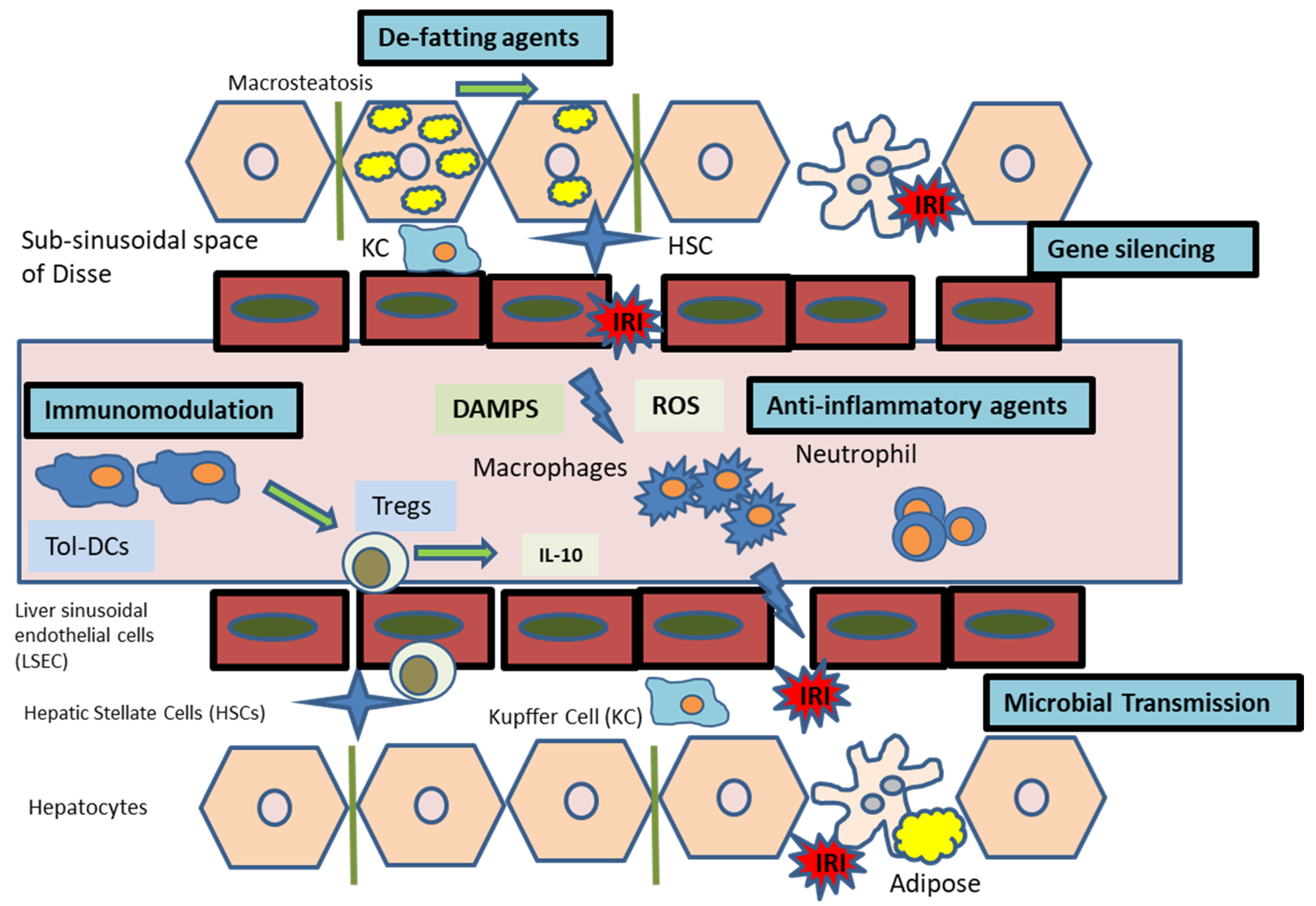
:1. Introduction
2. Normothermic Machine Perfusion of the Liver
3. Ischaemia Reperfusion Injury
Gene Silencing with RNAi
4. Immunomodulation
4.1. Cell Therapy during NMP
4.2. Extra-Cellular Vesicles (EVs) during NMP
4.3. Tolerance
5. Microcirculation and Endothelial Protection
6. Microbial Transmission
7. Hepatic Steatosis and Implications on Liver Transplantation
7.1. NMP, Hepatic Steatosis and Pre-Clinical Animal Studies
7.2. NMP, Hepatic Steatosis and Discarded Human Livers
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| °C | Degrees Celsius |
| % | Percent |
| 3R | Reduction, Refinement and Replacement |
| ARLD | Alcohol-related liver disease |
| ATP | Adenosine triphosphate |
| BMI | Body mass index |
| BQ-123 | D-tryptamine-D-aspartic acid-L-proline-D-valine-L-leucine |
| cAMP | Cyclic adenosine monophosphate |
| CD4 | Cluster of differentiation 4 |
| CD25 | Cluster of differentiation 25 |
| CD127 | Cluster of differentiation 127 |
| CIT | Cold ischaemia time |
| CO2 | Carbon dioxide |
| COPE | Consortium for Organ Preservation in Europe |
| Cy3 | Cyanine Cy3 |
| DAA | Direct-acting antivirals |
| DCD | Donors after circulatory death |
| DNA | Deoxyribonucleic acid |
| EAD | Early allograft dysfunction |
| ECD | Extended criteria donor |
| FOXP3 | Forkhead box P3 |
| GMP | Good manufacturing practice |
| GW7647 | 2-(4-(2-(1-Cyclohexanebutyl)-3-cyclohexylureido)ethyl)-phenyl-thio)-2-methyl-propionic acid |
| GW501516 | 2-[2-Methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl]methyl]thio]phenoxy]-acetic acid |
| h | Hour/hours |
| HBD | Heart-beating donor |
| HCV | Hepatitis C virus |
| HLSC | Human liver stem-like cells |
| HMP | Hypothermic machine perfusion |
| IFN-γ | Interferon gamma |
| IL-1 | Interleukin 1 |
| IL-6 | Interleukin 6 |
| IL-17 | Interleukin 17 |
| IRAK-4 | Interleukin-1 receptor-associated kinase 4 |
| IRI | Ischaemia reperfusion injury |
| kg/m2 | Kilogram per square meter |
| LD | Lipid droplet |
| LDLT | Living donor liver transplant |
| MaS | Macrovesicular steatosis |
| mRNA | Messenger RNA |
| miRNA | MicroRNA |
| MSC | Mesenchymal stem cells |
| MSC-EV | Extracellular vesicles of mesenchymal stem cells |
| NAFLD | Non-alcoholic fatty liver disease |
| NMP | Normothermic machine perfusion |
| O2 | Oxygen |
| PGE1 | Prostaglandin E1 |
| PNF | Primary non-function |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PPARδ | Peroxisome proliferator-activated receptor delta |
| PRS | Post reperfusion syndrome |
| RNA | Ribonucleic acid |
| RNAi | RNA interference |
| ROS | Reactive oxygen species |
| SCS | Static cold storage |
| SEC | Sinusoidal endothelial cell |
| shRNA | Short hairpin RNA |
| siRNA | Small interfering RNA |
| SNMP | Sub-normothermic machine perfusion |
| TG | Triacylglycerol |
| TNF-α | Tumour necrosis factor alpha |
| Treg | Regulatory T cell |
| UNHBD | Uncontrolled non-heart-beating donor |
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.P.; Fernandez, L.A.; Leverson, G.; Anderson, M.; Mezrich, J.; Sollinger, H.W.; D’Alessandro, A. Biliary complications after liver transplantation from donation after cardiac death donors: An analysis of risk factors and long-term outcomes from a single center. Ann. Surg. 2011, 253, 817–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayant, K.; Reccia, I.; Virdis, F.; Shapiro, A.M.J. Systematic Review and Meta-Analysis on the Impact of Thrombolytic Therapy in Liver Transplantation Following Donation after Circulatory Death. J Clin. Med. 2018, 7, 425. [Google Scholar] [CrossRef] [Green Version]
- Dar, W.A.; Sullivan, E.; Bynon, J.S.; Eltzschig, H.; Ju, C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019, 39, 788–801. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation-from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Wind, J.; Faut, M.; van Smaalen, T.C.; van Heurn, E.L.W. Variability in protocols on donation after circulatory death in Europe. Crit. Care 2013, 17, R217. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, C.J.; Charman, S.C.; Muiesan, P.; Powell, J.J.; Gimson, A.E.; van der Meulen, J.H.P. Outcomes of transplantation of livers from donation after circulatory death donors in the UK: A cohort study. BMJ Open 2013, 3, e003287. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, A.; Kalisvaart, M.; Scalera, I.; Laing, R.W.; Mergental, H.; Mirza, D.F.; Perera, T.; Isaac, J.; Dutkowski, P.; Muiesan, P. The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation. J. Hepatol. 2018, 68, 456–464. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Machuca, T.; Chen, M.; Singer, L.G.; Yasufuku, K.; de Perrot, M.; Pierre, A.; Waddel, T.K.; Keshavjee, S. Experience with the first 50 Ex Vivo lung perfusions in clinical transplantation. J. Thorac. Cardiovasc. Surg. 2012, 144, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Rojas, S.V.; Ius, F.; Schibilsky, D.; Kaufeld, T.; Sommer, W.; Benk, C.; Goecke, T.; Siemeni, T.; Poyanmehr, R.; Rümke, S.; et al. Cardiac Transplantation in Higher Risk Patients: Is Ex Vivo Heart Perfusion a Safe Preservation Technique? A Two Center Experience. J. Heart Lung Transplant. 2019, 38, S43. [Google Scholar] [CrossRef]
- Hosgood, S.A. Renal transplantation after Ex Vivo normothermic perfusion: The first clinical study. Am. J. Transplant. 2013, 13, 1246–1252. [Google Scholar] [CrossRef]
- Ravikumar, R.; Jassem, W.; Mergental, H.; Heaton, N.; Mirza, D.; Perera, M.T.P.R.; Quaglia, A.; Holroyd, D.; Vogel, T.; Coussios, C.C.; et al. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am. J. Transplant. 2016, 16, 1779–1787. [Google Scholar] [CrossRef]
- Goodman, L.F.; Yasmine, E.S.; Maija, Y.; Benno, C.; Ozgediz, D.; Adoyi Ameh, E.; Bickler, S.; Poenaru, D.; Oldham, K.; Farmer, D. The Global Initiative for Children’s Surgery: Optimal Resources for Improving Care. Eur. J. Pediatr. Surg. 2018, 28, 58–59. [Google Scholar] [CrossRef]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; Garcia-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Ceresa, C.D.L.; Nasralla, D.; Watson, C.J.E.; Butler, A.J.; Coussios, C.C.; Crick, K.; Hodson, L.; Imber, C.; Jassem, W.; Knight, S.R.; et al. Transient Cold Storage Prior to Normothermic Liver Perfusion May Facilitate Adoption of a Novel Technology. Liver Transplant. 2019, 25, 1503–1513. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Jochmans, I. From “Gut Feeling” to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr. Transplant. Rep. 2018, 5, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Laing, R.W.; Mergental, H.; Yap, C.; Kirkham, A.; Whilku, M.; Darren, B.; Curbishley, S.; Boteon, Y.L.; Neil, D.A.; Hübscher, S.G.; et al. Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: Study protocol for an open-label, nonrandomised, prospective, singlearm trial. BMJ Open 2017, 7, e017733. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Becker, D.; Bautista Borrego, L.; Hefti, M.; Schuler, M.J.; Hagedorn, C.; Muller, X.; Mueller, M.; Onder, C.; Graf, R.; et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 2020, 38, 189–198. [Google Scholar] [CrossRef]
- Rusche, B. The 3Rs and animal welfare—Conflict or the way forward? Altex 2003, 20, 63–76. [Google Scholar]
- Vogel, T.; Brockmann, J.G.; Quaglia, A.; Morovat, A.; Jassem, W.; Heaton, N.D.; Coussios, C.C.; Friend, P.J. The 24-hour normothermic machine perfusion of discarded human liver grafts. Liver Transplant. 2017, 23, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, R.M.; Soeiro Teodoro, J.; Furtado, E.; Rolo, A.P.; Marques Palmeira, C.; Tralhão, J.G. Recent insights into mitochondrial targeting strategies in liver transplantation. Int. J. Med. Sci. 2018, 15, 248–256. [Google Scholar] [CrossRef] [Green Version]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Liu, Y.; Li, M.; Liu, Z.; Gong, J. IRAK-4-shRNA Prevents Ischemia/Reperfusion Injury Via Different Perfusion Periods Through the Portal Vein After Liver Transplantation in Rat. Transplant Proc. 2016, 48, 2803–2808. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.F.; Lu, M.Q.; Yang, Y.; Xu, C.; Li, H.; Wang, G.S.; Cai, C.J.; Chen, G.H. Alleviation of ischemia-reperfusion injury in rat liver transplantation by induction of small interference RNA targeting Fas. Langenbeck’s Arch. Surg. 2007, 392, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, M.F.; Brüggenwirth, I.M.A.; Gillooly, A.; Khvorova, A.; Kowalik, T.F.; Martins, P.N. Gene Silencing With siRNA (RNA Interference): A New Therapeutic Option During Ex Vivo Machine Liver Perfusion Preservation. Liver Transplant. 2019, 25, 140–151. [Google Scholar] [CrossRef]
- Moore, C.; Thijssen, M.; Wang, X.; Mandrekar, P.; Xiaofei, E.; Abdi, R.; Porte, R.; Bozorgzadeh, A.; Leuvenink, H.; Kowalik, T.; et al. Gene Silencing with p53 si-RNA Downregulates Inflammatory Markers in the Liver: Potential Utilization during Normothermic Machine Preservation. Am. J. Transplant. 2017, 18, 503. [Google Scholar]
- Buchwald, J.E.; Xu, J.; Bozorgzadeh, A.; Martins, P.N. Therapeutics administered during Ex Vivo liver machine perfusion: An overview. World J. Transplant. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Gillooly, A.R.; Perry, J.; Martins, P.N. First Report of siRNA Uptake (for RNA Interference) during Ex Vivo Hypothermic and Normothermic Liver Machine Perfusion. Transplantation 2019, 103, e56–e57. [Google Scholar] [CrossRef]
- Beal, E.W.; Kim, J.L.; Reader, B.F.; Akateh, C.; Maynard, K.; Washburn, W.K.; Zweier, J.L.; Whitson, B.A.; Black, S.M. [D-Ala 2, D-Leu 5] Enkephalin Improves Liver Preservation During Normothermic Ex Vivo Perfusion. J. Surg. Res. 2019, 241, 323–335. [Google Scholar] [CrossRef]
- Vogel, T.; Brockmann, J.G.; Pigott, D.; Neil, D.A.H.; Muthusamy, A.S.R.; Coussios, C.C.; et al. Successful transplantation of porcine liver grafts following 48-hour normothermic preservation. PLoS ONE 2017, 12, e0188494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bral, M.; Dajani, K.; Leon Izquierdo, D.; Bigam, D.; Kneteman, N.; Ceresa, C.D.L.; Friend, P.J.; Shapiro, A.M.J. A Back-to-Base Experience of Human Normothermic Ex Situ Liver Perfusion: Does the Chill Kill? Liver Transplant. 2019, 25, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Jassem, W.; Xystrakis, E.; Ghnewa, Y.G.; Yuksel, M.; Pop, O.; Martinez-Llordella, M.; Jabri, Y.; Huang, X.; Lozano, J.J.; Quaglia, A.; et al. Normothermic machine perfusion (NMP) inhibits proinflammatory responses in the liver and promotes regeneration. Hepatology 2018, 70, 682–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safinia, N.; Grageda, N.; Scottà, C.; Thirkell, S.; Fry, L.J.; Vaikunthanathan, T.; Lechler, R.I.; Lombardi, G. Cell Therapy in Organ Transplantation: Our Experience on the Clinical Translation of Regulatory T Cells. Front. Immunol. 2018, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Beriou, G.; Moreau, A.; Cuturi, M.C. Tolerogenic dendritic cells: Applications for solid organ transplantation. Curr. Opin. Organ. Transplant. 2012, 17, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Detry, O.; Vandermeulen, M.; Delbouille, M.H.; Somja, J.; Bletard, N.; Briquet, A.; et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. J. Hepatol. 2017, 67, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Todo, S.; Yamashita, K.; Goto, R.; Zaitsu, M.; Nagatsu, A.; Oura, T.; Watanabe, M.; Ayoura, T.; Suzuki, T.; Shimamura, T.; et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 2016, 64, 632–643. [Google Scholar] [CrossRef] [Green Version]
- Global Observatory on Donation and Transplantation (GODT). 2017 Global Report. Available online: http://www.transplant-observatory.org/download/2017-activity-data-report/ (accessed on 25 February 2020).
- Girdlestone, J. Mesenchymal stromal cells with enhanced therapeutic properties. Immunotherapy 2016, 8, 1405–1416. [Google Scholar] [CrossRef] [Green Version]
- Obermajer, N.; Popp, F.C.; Johnson, C.L.; Benseler, V.; Dahlke, M.H. Rationale and prospects of mesenchymal stem cell therapy for liver transplantation. Curr. Opin. Organ. Transplant. 2014, 19, 60–64. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug. Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Stone, M.L.; Zhao, Y.; Smith, J.R.; Weiss, M.L.; Kron, I.L.; Laubach, V.E.; Sharma, A.K. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir. Res. 2017, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Lonati, C.; Bassani, G.A.; Brambilla, D.; Leonardi, P.; Carlin, A.; Maggioni, M.; Zanella, A.; Dondossola, D.; Fonsato, V.; Grange, C.; et al. Mesenchymal stem cell–derived extracellular vesicles improve the molecular phenotype of isolated rat lungs during ischemia/reperfusion injury. J. Heart Lung Transplant. 2019, 38, 1306–1316. [Google Scholar] [CrossRef]
- Varchetta, S.; Oliviero, B.; Francesca Donato, M.; Agnelli, F.; Rigamonti, C.; Paudice, E.; Arosio, E.; Berra, M.; Rossi, G.; Tinelli, C.; et al. Prospective study of natural killer cell phenotype in recurrent hepatitis C virus infection following liver transplantation. J. Hepatol. 2009, 50, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Dondossola, D.; Santini, A.; Lonati, C.; Zanella, A.; Merighi, R.; Vivona, L.; Battistin, M.; Galli, A.; Biancolilli, O.; Maggioni, M.; et al. Human Red Blood Cells as Oxygen Carriers to Improve Ex-Situ Liver Perfusion in a Rat Model. J. Clin. Med. 2019, 8, 1918. [Google Scholar] [CrossRef] [Green Version]
- Chin, L.Y.; Carroll, C.; Raigani, S.; Detelich, D.M.; Tessier, S.N.; Wojtkiewicz, G.R.; Schmidt, S.P.; Weissleder, R.; Yeh, H.; Uygun, K.; et al. Ex Vivo perfusion-based engraftment of genetically engineered cell sensors into transplantable organs. PLoS ONE 2019, 14, e0225222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, S.P.; Bhattacharjya, S.; Maniakin, N.; Greenwood, J.; Guerreiro, D.; Hughes, D.; Imber, C.J.; Piggot, D.W.; Susan, F.; Richard, T.; et al. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation 2004, 77, 1328–1332. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Berendsen, T.; Kim, K.; Soto-Gutiérrez, A.; Bertheium, F.; Yarmush, M.L.; Hertl, M. Excorporeal normothermic machine perfusion resuscitates pig DCD livers with extended warm ischemia. J. Surg. Res. 2012, 173, e83–e88. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, A.; De Rougemont, O.; Graf, R.; Clavien, P.A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef]
- Goldaracena, N.; Echeverri, J.; Spetzler, V.N.; Kaths, J.M.; Barbas, A.S.; Louis, K.S.; Adeyi, O.A.; Grant, D.R.; Selzner, N.; Selzner, R. Anti-inflammatory signaling during Ex Vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transplant. 2016, 22, 1573–1583. [Google Scholar] [CrossRef]
- Hara, Y.; Akamatsu, Y.; Maida, K.; Kashiwadate, T.; Kobayashi, Y.; Ohuchi, N.; et al. A new liver graft preparation method for uncontrolled non-heart-beating donors, combining short oxygenated warm perfusion and prostaglandin E1. J. Surg. Res. 2013, 184, 1134–1142. [Google Scholar] [CrossRef]
- Lin, M.; Mah, A.; Wright, A.J. Infectious complications of liver transplantation. AME Med. J. 2018, 3, 5. [Google Scholar] [CrossRef]
- Phillips, B.L.; Chandak, P.; Uwechue, R.; Van Nispen Tot Pannerden, C.; Hemsley, C.; Callaghan, C.J. Microbial contamination during kidney Ex Vivo normothermic perfusion. Transplantation 2018, 102, E186–E188. [Google Scholar] [CrossRef] [PubMed]
- Spearman, C.W.; Dusheiko, G.M.; Hellard, M.; Sonderup, M. Hepatitis C. Lancet 2019, 394, 1451–1466. [Google Scholar] [CrossRef]
- Herzer, K.; Gerken, G. Hepatitis C virus reinfection after liver transplantation. New chances and new challenges in the era of direct-acting antiviral agents. World J. Hepatol. 2015, 7, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Goldaracena, N.; Spetzler, V.N.; Echeverri, J.; Kaths, J.M.; Cherepanov, V.; Persson, R.; Hodges, M.R.; Janssen, H.L.A.; Selzner, N.; Grant, D.R.; et al. Inducing Hepatitis C Virus Resistance After Pig Liver Transplantation—A Proof of Concept of Liver Graft Modification Using Warm Ex Vivo Perfusion. Am. J. Transplant. 2017, 17, 970–978. [Google Scholar] [CrossRef]
- Durand, F.; Renz, J.; Alkofer, B.; Burra, P.; Clavien, P.; Porte, R.; Freeman, R.B.; Belghiti, J. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transplant. 2008, 14, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Parekh, S.; Anania, F. Abnormal Lipid and Glucose Metabolism in Obesity: Implications for Nonalcoholic Fatty Liver Disease. Gastroenterology 2007, 132, 2191–2207. [Google Scholar] [CrossRef]
- Chu, M.; Dare, A.; Phillips, A.; Bartlett, A. Donor Hepatic Steatosis and Outcome After Liver Transplantation: A Systematic Review. J. Gastrointest. Surg. 2015, 19, 1713–1724. [Google Scholar] [CrossRef]
- Spitzer, A.L.; Lao, O.B.; Dick, A.A.S.; Bakthavatsalam, R.; Halldorson, J.B.; Yeh, M.M.; Upton, M.P.; Reyes, J.D.; Perkins, J.D. The biopsied donor liver: Incorporating macrosteatosis into high-risk donor assessment. Liver Transplant. 2010, 16, 874–884. [Google Scholar] [CrossRef]
- Nativ, N.I.; Maguire, T.J.; Yarmush, G.; Brasaemle, D.L.; Henry, S.D.; Guarrera, J.; Berthiaume, F.; Yarmush, M.L. Liver Defatting: An Alternative Approach to Enable Steatotic Liver Transplantation. Am. J. Transplant. 2012, 12, 3176–3183. [Google Scholar] [CrossRef] [Green Version]
- NHS Blood and Transfusion (NHSBT). Annual Report on Liver Transplantation: Report for 2014/2015/2016. Available online: http://www.odt.nhs.uk/pdf/organ_specific_report_liver_2014.pdf (accessed on 22 November 2019).
- Dyson, J.; Anstee, Q.; McPherson, S. Non-alcoholic fatty liver disease: A practical approach to diagnosis and staging. Front. Gastroenterol. 2013, 5, 211–218. [Google Scholar] [CrossRef] [PubMed]
- NHS Digital. Statistics on Obesity, Physical Activity and Diet: England 2015. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/statistics-on-obesity-physical-activity-and-diet-england-2015 (accessed on 4 November 2019).
- Brockmann, J.; Reddy, S.; Coussios, C.; Pigott, D.; Guirriero, D.; Hughes, D.; Morovat, A.; Roy, D.; Winter, L.; Friend, P.J. Normothermic Perfusion. Ann. Surg. 2009, 250, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; et al. Observations on the Ex Situ perfusion of livers for transplantation. Am. J. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, R.W.; Zilvetti, M.; Roy, D.; Hughes, D.; Morovat, A.; Coussios, C.C.; Friend, P.J. Hepatic Steatosis and Normothermic Perfusion—Preliminary Experiments in a Porcine Model. Transplantation 2011, 92, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, D.; Xu, H.; Tanimura, Y.; Zuo, R.; Berthiaume, F.; Avila, M.; Yarmush, R.; Yarmush, M.L. Metabolic preconditioning of donor organs: Defatting fatty livers by normothermic perfusion Ex Vivo. Metab. Eng. 2009, 11, 274–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boteon, Y.L.; Attard, J.; Boteon, A.P.C.S.; Wallace, L.; Reynolds, G.; Hubscher, S.; Mirza, D.F.; Mergenthal, H.; Bhogal, R.H.; Afford, S.C. Manipulation of Lipid Metabolism During Normothermic Machine Perfusion: Effect of Defatting Therapies on Donor Liver Functional Recovery. Liver Transplant. 2019, 25, 1007–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banan, B.; Watson, R.; Xu, M.; Lin, Y.; Chapman, W. Development of a normothermic extracorporeal liver perfusion system toward improving viability and function of human extended criteria donor livers. Liver Transplant. 2016, 22, 979–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceresa, C.; Nasralla, D.; Neil, D.; Mergental, H.; Jassem, W.; Butler, A.; Imber, C.; Barrett, A.; Clark, A.; Coussios, C.; et al. A Histological and Biochemical Assessment of Steatotic Livers Undergoing Normothermic Machine Perfusion. In Proceedings of the 18th Congress of the European Society for Organ Transplantation, Barcelona, Spain, 24–27 September 2017; European Society for Organ Transplantation: Padova, Italy, 2017. [Google Scholar]
- Raigani, S.; Carrol, C.; Cronin, S.; Pendexter, C.; Rosales, I.; Yarmush, M.; Uygun, K.Y.H. Defatting Steatotic Rat Livers during Ex Situ Normothermic Perfusion Improves Lactate Clearance and Bile Quality—ATC Abstracts. In Proceedings of the 2019 American Transplant Congress, Boston, MA, USA, 1–5 June 2019; American Society of Transplantation: Mt. Laurel, NJ, USA, 2019; pp. 14–17. [Google Scholar]
- Liu, Q.; Nassar, A.; Buccini, L.; Iuppa, G.; Soliman, B.; Pezzati, D.; Hassan, A.; Blum, M.; Baldwin, W.; Bennett, A.; et al. Lipid metabolism and functional assessment of discarded human livers with steatosis undergoing 24 hours of normothermic machine perfusion. Liver Transplant. 2018, 24, 233–245. [Google Scholar] [CrossRef]
- Laing, R.W.; Boteon, Y.L.; Kirkham, A.; Perera, T.; Attard, J.; Barton, D.; Curbishley, S.; Neil, D.A.; Hubscher, S.G.; Musien, P.; et al. Transplantation of Discarded Livers Following Viability Testing With Normothermic Machine Perfusion: The VITTAL (VIability Testing and Transplantation of mArginal Livers) Trial Outcomes. Transplantation 2019, 103, 3–4. [Google Scholar]
- Raigani, S.; Markmann, J.F.; Yeh, H. Rehabilitation of Discarded Steatotic Livers Using Ex Situ Normothermic Machine Perfusion: A Future Source of Livers for Transplantation. Liver Transplant. 2019, 25, 991–992. [Google Scholar] [CrossRef]
- Boteon, Y.; Wallace, L.; Boteon, A.; Mirza, D.; Mergental, H.; Bhogal, R.; Afford, S. An effective protocol for pharmacological defatting of primary human hepatocytes which is non-toxic to cholangiocytes or intrahepatic endothelial cells. PLoS ONE 2018, 13, e0201419. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Goodwin, B.; Jones, S.; Wisely, G.; Serabjit-Singh, C.; Willson, T.; Collins, J.L.; Kliewer, S.A., St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 7500–7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, C.; Rodriguez, O.; Martin, P.; Albanese, C.; Li, X.; Kopelovich, L.; Glazer, R.I. Induction of Metastatic Gastric Cancer by Peroxisome Proliferator-Activated Receptorδ Activation. PPAR Res. 2010, 2010, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Study | Model | N | Preservation Details/Groups | Length of NMP | Therapeutic Agent(s) | Outcome |
|---|---|---|---|---|---|---|
| Gillooly et al. 2019 [29] | Rat | N/A | HMP ± siRNA, NMP ± SiRNA, | 4 h | Fas-short interfering RNA (siRNA) | Diffuse uptake of siRNA in both NMP and HMP groups, with increased uptake in the latter. |
| Beal et al. 2019 [30] | Rat | 6 per group | NMP, NMP + Enkephalin | 4 h | Enkephalin | Reduced hepatocyte oxidative stress and mitochondrial dysfunction via opioid receptor signalling. |
| Moore et al. 2017 [27] | Rat | N/A | NMP | 6 h | p53 siRNA-cy3 | Positive fluorescence for cy3 detected in NMP livers. |
| Study | Model | N | Perfusion Details/Groups | Length of NMP | Therapeutic Agent(s) | Outcome |
|---|---|---|---|---|---|---|
| Hara et al. 2013 [51] | Rat (Reperfusion) | 5 per group | HBD (SCS), UNHBD + NMP, UNHBD + NMP +PGE1 | 30 min | Prostaglandin E1 (PGE1) | Improved mitochondrial function and reduced inflammatory cytokines in NMP + PGE1 group |
| Goldaracena et al. 2016 [50] | Porcine (Transplant) | 5 per group | NMP, SNMP + anti-inflammatory agents, HBD (SCS) | 4 h | Anti-inflammatory additives: BQ123, prostaglandin E1, Acetylcystine, prostacycline, gas composition 95% O2 and 5% CO2 | Significantly lower markers of hepatocellular damage in NMP groups, Improved endothelial (microcirulatory) function |
| Key Benefits: |
|---|
| (i) Allows recovery from acute injury (hypoxia) sustained prior to or during retrieval [65]; |
| (ii) Permits objective assessment of organ function prior to transplantation: a number of studies have shown that this enables identification of organs in the ‘high-risk’ category that can safely be transplanted [14,17,66]; |
| (iii) Enables extended preservation times (up to 24 h) [14] |
| (iv) Provides the opportunity for therapeutic intervention to a functioning organ before it is transplanted. |
| Defatting Agent | Function |
|---|---|
| PPARδ ligand GW501516 | Increase fatty acid β-oxidation |
| Peroxisome proliferator-activated receptor (PPAR) α ligand GW7647 | Increase mitochondrial fatty acid oxidation |
| Cyclic adenosine monophosphate (cAMP) activator forskolin | A glucagon mimetic cAMP activator, increases lipolysis and fatty acid oxidation |
| Pregnane X receptor ligand hypericin | Increase β-oxidation (very long chain fatty acids) |
| Visfatin | An insulin-memetic adipokine, role not fully understood |
| Scorparone | An androstane receptor ligand, upregulates PPAR |
| Ref. | Defatting Interventions | Model | Total Ex-Situ Perfusion Time (h) | Percentage (%) Reduction in Macrosteatosis (MaS) | Main Outcomes |
|---|---|---|---|---|---|
| Jamieson et al. 2011 [67] | NMP alone | Porcine | 48 | 13 | Reduction in hepatic triglyceride content of 31% and markers of hepatocyte injury comparable to lean counterparts |
| Nagarth et al. 2009 [68] | GW501516, GW7647, forskolin, hypericin, visfatin and scorparone | Zucker rats | 3 | 50 | Reduction in hepatic triglyceride content of 65% Increased hepatic lipid metabolism |
| Raigani et al. 2019 [72] | GW501516, GW7647, forskolin, hypericin, visfatin, scorparone and L-carnitine | Zucker rats | 6 | 33 | Hepatic triglyceride content not reported Increased perfusate ketone content, bile bicarbonate content and lactate clearance |
| Boteon et al. 2019 [69] | GW501516, GW7647, forskolin, hypericin, visfatin, scorparone and L-carnitine | Discarded human livers | 6 12 | 40 50 | Reduction in hepatic triglyceride level of 38% at 6 h and 30% at 12 h Increased hepatic lipid metabolism, improved metabolic liver function, reduced vascular resistance and reduced markers of hepatocyte injury Reduced immune cell activation and release of inflammatory cytokines |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dengu, F.; Abbas, S.H.; Ebeling, G.; Nasralla, D. Normothermic Machine Perfusion (NMP) of the Liver as a Platform for Therapeutic Interventions during Ex-Vivo Liver Preservation: A Review. J. Clin. Med. 2020, 9, 1046. https://doi.org/10.3390/jcm9041046
Dengu F, Abbas SH, Ebeling G, Nasralla D. Normothermic Machine Perfusion (NMP) of the Liver as a Platform for Therapeutic Interventions during Ex-Vivo Liver Preservation: A Review. Journal of Clinical Medicine. 2020; 9(4):1046. https://doi.org/10.3390/jcm9041046
Chicago/Turabian StyleDengu, Fungai, Syed Hussain Abbas, Georg Ebeling, and David Nasralla. 2020. "Normothermic Machine Perfusion (NMP) of the Liver as a Platform for Therapeutic Interventions during Ex-Vivo Liver Preservation: A Review" Journal of Clinical Medicine 9, no. 4: 1046. https://doi.org/10.3390/jcm9041046




