Enhanced Inflammation and Nitrosative Stress in the Saliva and Plasma of Patients with Plaque Psoriasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Collection
2.2. Saliva Collection
2.3. Stomatological Examination
2.4. Biochemical Assays
2.5. Pro-Inflammatory Cytokines
2.6. Nitrosative Stress
2.7. Salivary Protein
2.8. Salivary Amylase
2.9. Statistical Analysis
3. Results
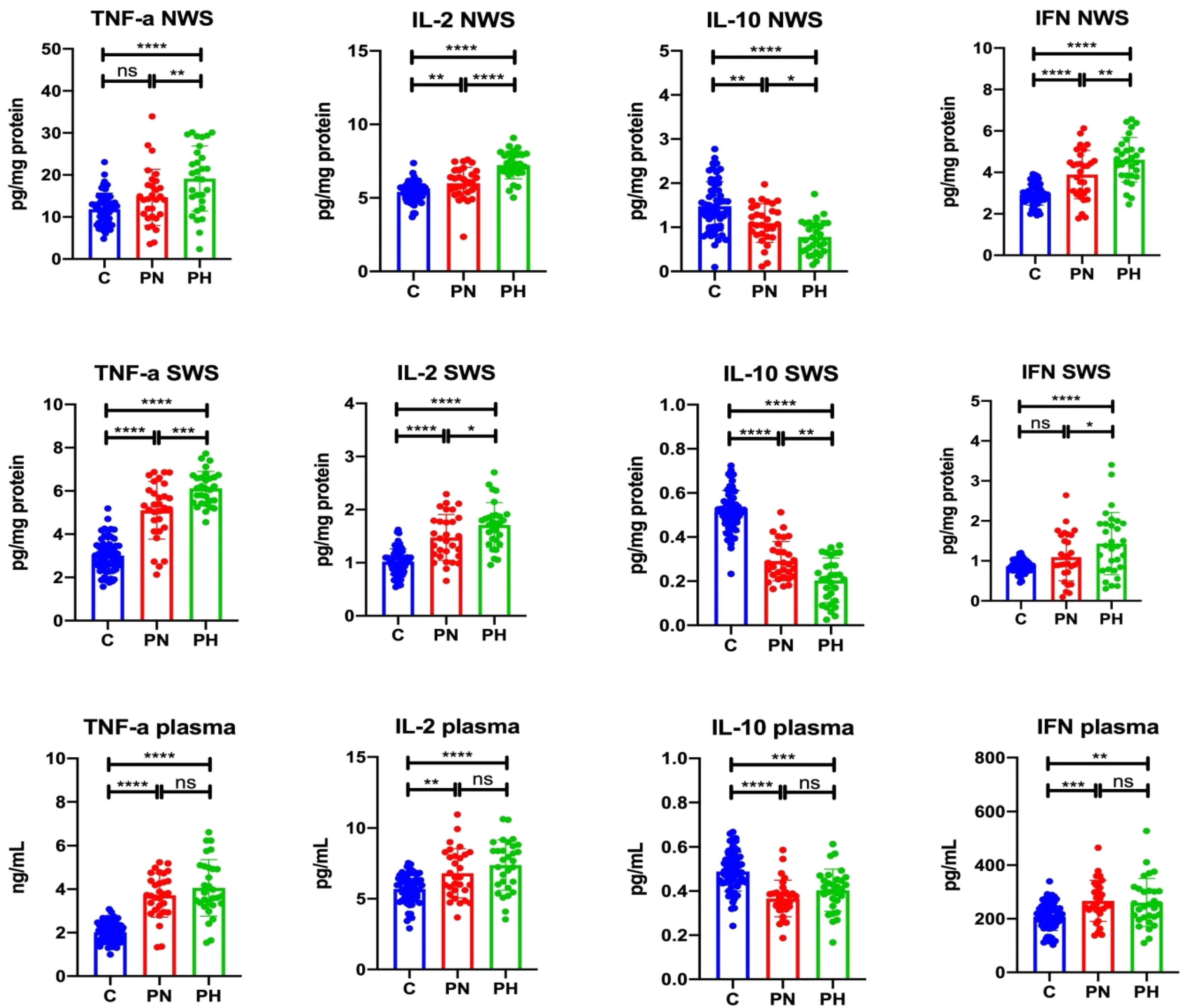
3.1. Inflammatory Cytokines
3.1.1. NWS
3.1.2. SWS
3.1.3. Plasma
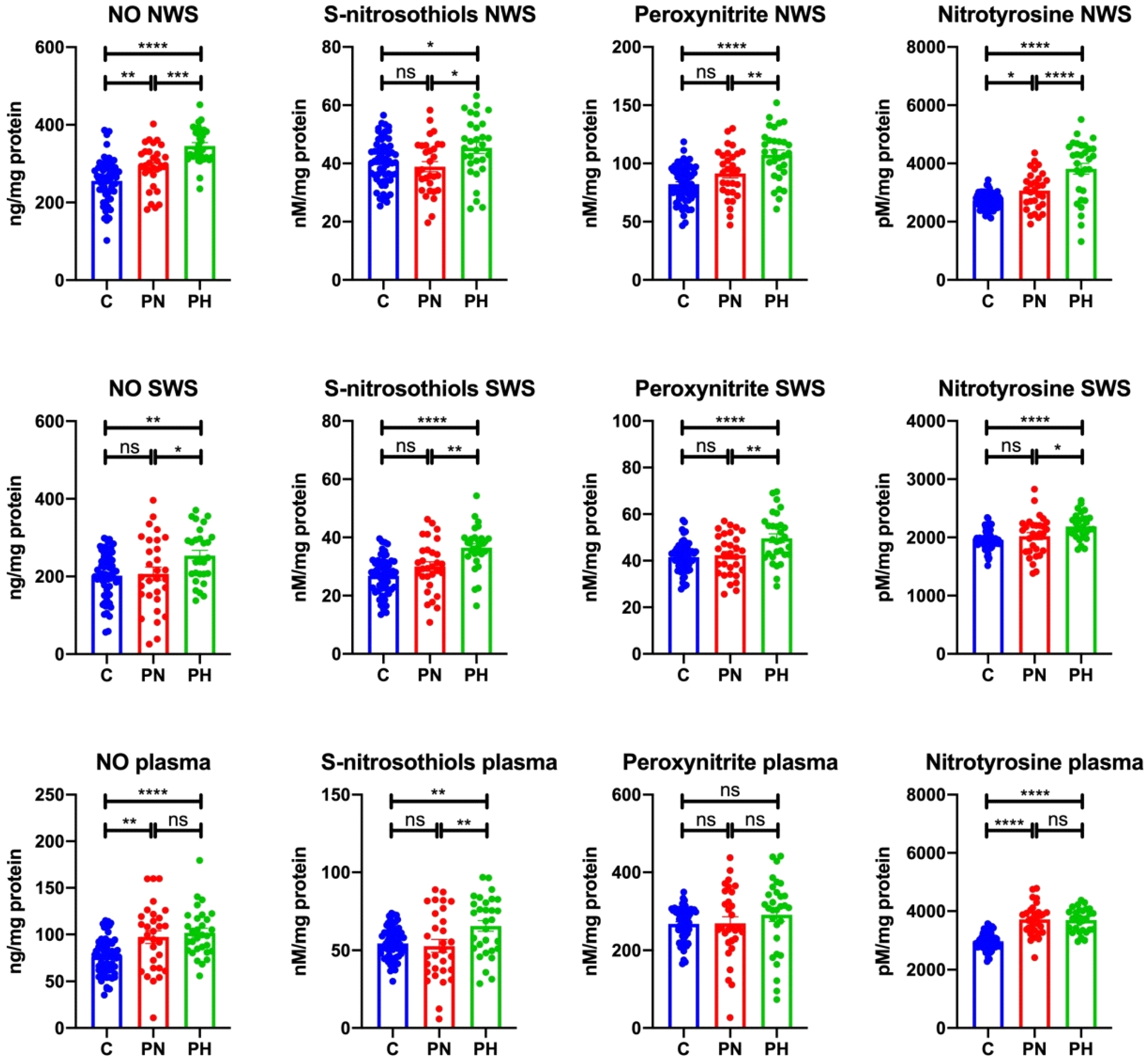
3.2. Nitrosative Stress
3.2.1. NWS
3.2.2. SWS
3.2.3. Plasma
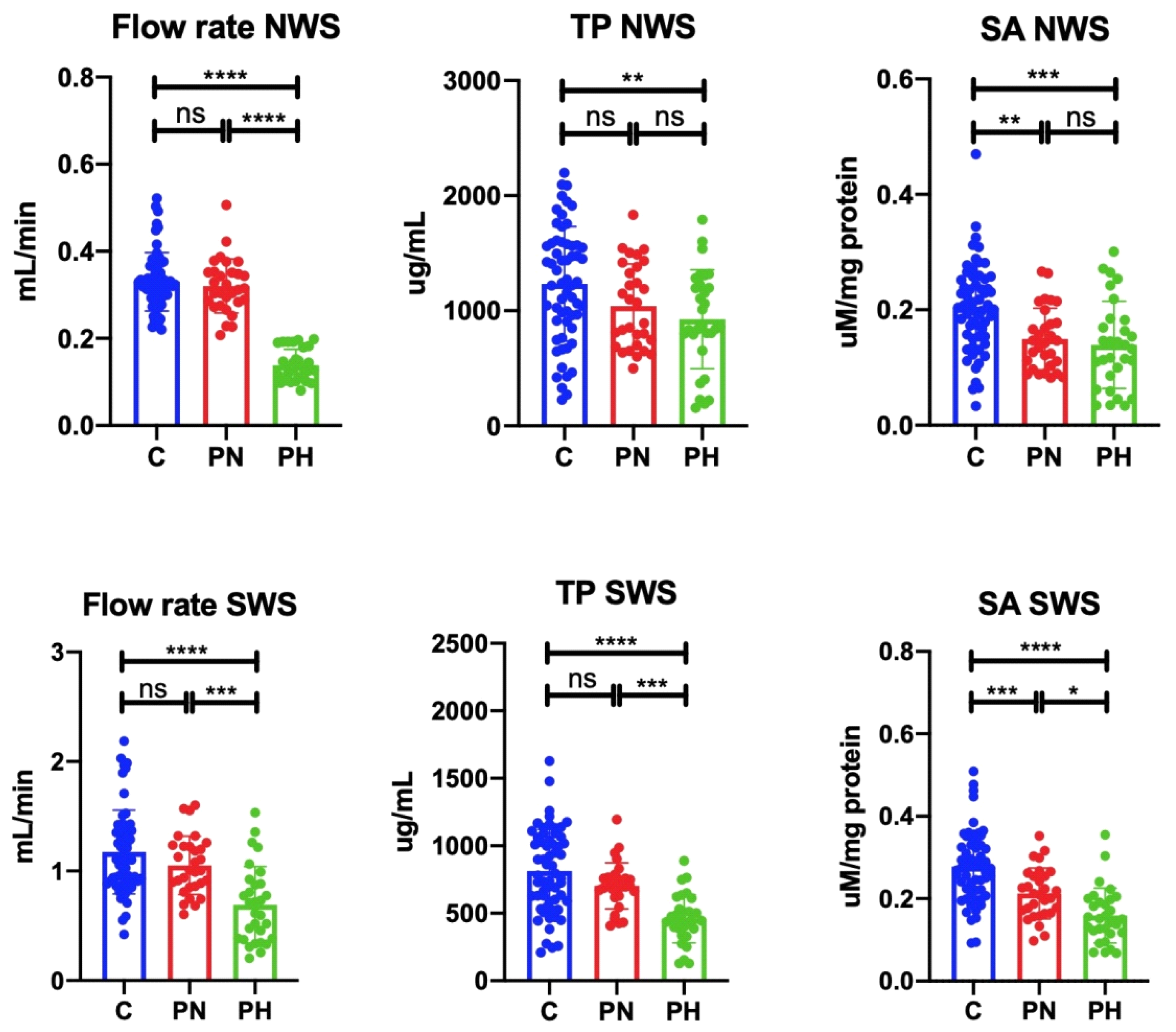
3.3. Salivary Gland Function
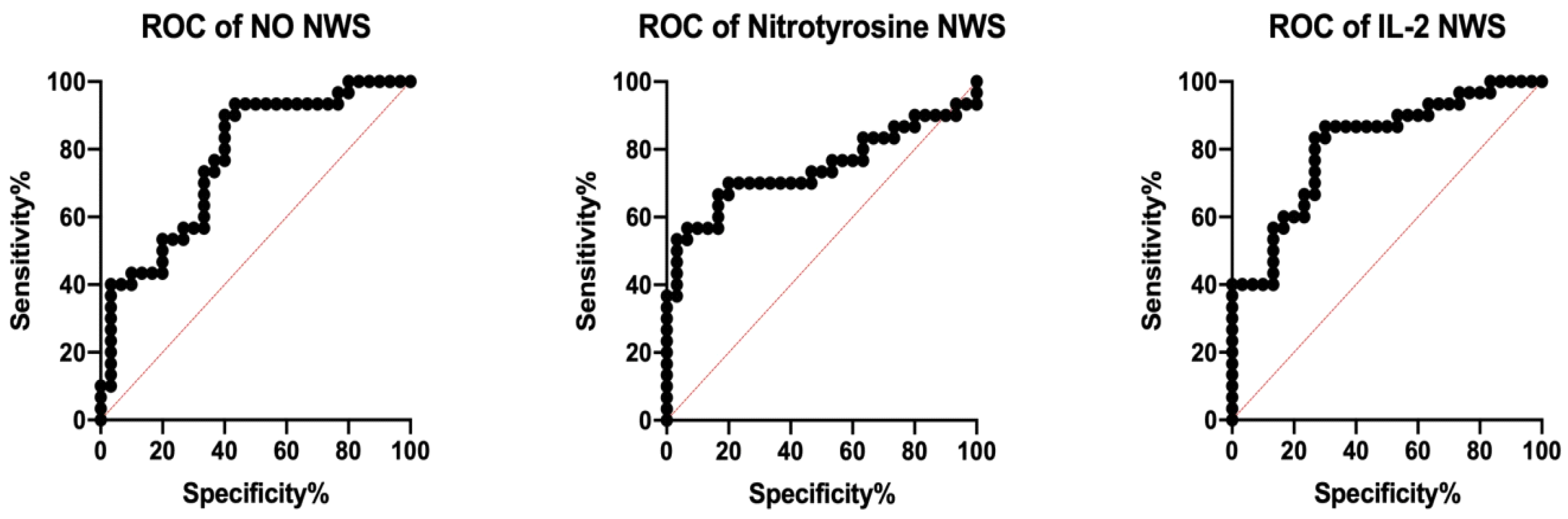
3.4. ROC Analysis
3.5. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Global Report on Psoriasis; WHO Library Cataloguing-in-Publication Data; World Health Organization: Geneva, Switzerland, 2016; pp. 10–11. [Google Scholar]
- Kupper, T.S. Immunologic Targets in Psoriasis. N. Engl. J. Med. 2003, 349, 1987–1990. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J. The immunologic and genetic basis of psoriasis. Arch. Dermatol. 1999, 135, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yang, Y.; Yan, X.; Zhang, K. Abnormalities in cytokine secretion from mesenchymal stem cells in psoriatic skin lesions. Eur. J. Dermatol. 2013, 23, 600–607. [Google Scholar] [CrossRef]
- Asha, K.; Singal, A.; Sharma, S.B.; Arora, V.K.; Aggarwal, A. Dyslipidaemia & oxidative stress in patients of psoriasis: Emerging cardiovascular risk factors. Indian J. Med. Res. 2017, 146, 708–713. [Google Scholar] [PubMed]
- Kadam, D.P.; Suryakar, A.N.; Ankush, R.D.; Kadam, C.Y.; Deshpande, K.H. Role of oxidative stress in various stages of psoriasis. Indian J. Clin. Biochem. 2010, 25, 388–392. [Google Scholar] [CrossRef]
- Houshang, N.; Reza, K.; Sadeghi, M.; Ali, E.; Mansour, R.; Vaisi-Raygani, A. Antioxidant status in patients with psoriasis. Cell Biochem. Funct. 2014, 32, 268–273. [Google Scholar] [CrossRef]
- Pujari, V.K.M. The Serum Levels of Malondialdehyde, Vitamin E and Erythrocyte Catalase Activity in Psoriasis Patients. J. Clin. Diagn. Res. 2014, 8, CC14–CC16. [Google Scholar] [CrossRef]
- Yildirim, M.; Inaloz, H.S.; Baysal, V.; Delibas, N. The role of oxidants and antioxidants in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 34–36. [Google Scholar] [CrossRef]
- Barygina, V.V.; Becatti, M.; Soldi, G.; Prignano, F.; Lotti, T.; Nassi, P.; Wright, D.; Taddei, N.; Fiorillo, C. Altered redox status in the blood of psoriatic patients: Involvement of NADPH oxidase and role of anti-TNF-α therapy. Redox Rep. 2013, 18, 100–106. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Campanati, A.; Simonetti, O.; Liberati, G.; Offidani, A. Correlation between lipoprotein (a) and lipid peroxidation in psoriasis: Role of the enzyme paraoxonase-1. Br. J. Dermatol. 2012, 166, 204–207. [Google Scholar] [CrossRef]
- Drewa, G.; Krzyzynska-Malinowska, E.; Wozniak, A.; Protas-Drozd, F.; Mila-Kierzenkowska, C.; Rozwodowska, M.; Kowaliszyn, B.; Czajkowski, R. Activity of superoxide dismutase and catalase and the level of lipid peroxidation products reactive with TBA in patients with psoriasis. Med. Sci. Monit. 2002, 8, BR338–BR343. [Google Scholar] [PubMed]
- Lin, X.; Huang, T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free Radic. Res. 2016, 50, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, V.A.; Nebot, E.; Porres, J.M.; Sánchez, C.; Aranda, P.; García-del Moral, R.; Machado-Vílchez, M. High-protein diets and renal status in rats. Nutr. Hosp. 2013, 28, 232–237. [Google Scholar] [PubMed]
- Skutnik-Radziszewska, A.; Maciejczyk, M.; Fejfer, K.; Krahel, J.; Flisiak, I.; Kołodziej, U.; Zalewska, A. Salivary Antioxidants and Oxidative Stress in Psoriatic Patients: Can Salivary Total Oxidant Status and Oxidative Status Index Be a Plaque Psoriasis Biomarker? Oxidative Med. Cell. Longev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sondermann, W.; Djeudeu Deudjui, D.A.; Körber, A.; Slomiany, U.; Brinker, T.J.; Erbel, R.; Moebus, S. Psoriasis, cardiovascular risk factors and metabolic disorders: Sex-specific findings of a population-based study. J. Eur. Acad. Dermatol. Venereol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Raksasuk, S. Psoriasis and risk of incident chronic kidney disease and end-stage renal disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2018, 50, 1277–1283. [Google Scholar] [CrossRef]
- Zalewska, A.; Knaś, M.; Gińdzieńska-Sieśkiewicz, E.; Waszkiewicz, N.; Klimiuk, A.; Litwin, K.; Sierakowski, S.; Waszkiel, D. Salivary antioxidants in patients with systemic sclerosis. J. Oral Pathol. Med. 2014, 43, 61–68. [Google Scholar] [CrossRef]
- Desoutter, A.; Soudain-Pineau, M.; Munsch, F.; Mauprivez, C.; Dufour, T.; Coeuriot, J.L. Xerostomia and medication: A cross-sectional study in long-term geriatric wards. J. Nutr. Health Aging 2012, 16, 575–579. [Google Scholar] [CrossRef]
- Zalewska, A.; Knaś, M.; Waszkiewicz, N.; Waszkiel, D.; Sierakowski, S.; Zwierz, K. Rheumatoid arthritis patients with xerostomia have reduced production of key salivary constituents. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 483–490. [Google Scholar] [CrossRef]
- Ganzetti, G.; Campanati, A.; Santarelli, A.; Pozzi, V.; Molinelli, E.; Minnetti, I.; Brisigotti, V.; Procaccini, M.; Emanuelli, M.; Offidani, A. Involvement of the oral cavity in psoriasis: Results of a clinical study. Br. J. Dermatol. 2015, 172, 282–285. [Google Scholar] [CrossRef]
- Stichtenoth, D.O.; Frölich, J.C. Nitric oxide and inflammatory joint diseases. Br. J. Rheumatol. 1998, 37, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Brune, B.; Lapetina, E.G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J. Biol. Chem. 1989, 264, 8455–8458. [Google Scholar] [PubMed]
- Modin, A.; Weitzberg, E.; Hökfelt, T.; Lundberg, J.M. Nitric oxide synthase in the pig autonomic nervous system in relation to the influence of NG-nitro-L-arginine on sympathetic and parasympathetic vascular control In Vivo. Neuroscience 1994, 62, 189–203. [Google Scholar] [CrossRef]
- Toda, N.; Ayajiki, K.; Okamura, T. Neurogenic and endothelial nitric oxide regulates blood circulation in lingual and other oral tissues. J. Cardiovasc. Pharmacol. 2012, 60, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Asa’ad, F.; Fiore, M.; Alfieri, A.; Pigatto, P.D.M.; Franchi, C.; Berti, E.; Maiorana, C.; Damiani, G. Saliva as a Future Field in Psoriasis Research. Biomed Res. Int. 2018. [Google Scholar] [CrossRef]
- Villanova, F.; Di Meglio, P.; Nestle, F.O. Biomarkers in psoriasis and psoriatic arthritis. Ann. Rheum. Dis. 2013, 72, 104–110. [Google Scholar] [CrossRef]
- Klimiuk, A.; Maciejczyk, M.; Choromańska, M.; Fejfer, K.; Waszkiewicz, N.; Zalewska, A. Salivary Redox Biomarkers in Different Stages of Dementia Severity. J. Clin. Med. 2019, 8, 840. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Proc. Ann. N. Y. Acad. Sci. 2007, 1098, 288–311. [Google Scholar] [CrossRef]
- Choromańska, M.; Klimiuk, A.; Kostecka-Sochoń, P.; Wilczyńska, K.; Kwiatkowski, M.; Okuniewska, N.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M. Antioxidant defence, oxidative stress and oxidative damage in saliva, plasma and erythrocytes of dementia patients. Can salivary AGE be a marker of dementia? Int. J. Mol. Sci. 2017, 18, 2205. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Szulimowska, J.; Skutnik, A.; Taranta-Janusz, K.; Wasilewska, A.; Wiśniewska, N.; Zalewska, A. Salivary Biomarkers of Oxidative Stress in Children with Chronic Kidney Disease. J. Clin. Med. 2018, 7, 209. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Szulimowska, J.; Taranta-Janusz, K.; Werbel, K.; Wasilewska, A.; Zalewska, A. Salivary FRAP as a marker of chronic kidney disease progression in children. Antioxidants 2019, 8, 409. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Zalewska, A.; Ładny, J.R. Salivary Antioxidant Barrier, Redox Status, and Oxidative Damage to Proteins and Lipids in Healthy Children, Adults, and the Elderly. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Hefti, A.F. Periodontal probing. Crit. Rev. Oral Biol. Med. 1997, 8, 336–356. [Google Scholar] [CrossRef]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996, 268, 237–246. [Google Scholar]
- Wink, D.A.; Kim, S.; Coffin, D.; Cook, J.C.; Vodovotz, Y.; Chistodoulou, D.; Jourd’heuil, D.; Grisham, M.B. Detection of S-nitrosothiols by fluorometric and colorimetric methods. Methods Enzymol. 1999, 301, 201–211. [Google Scholar]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Sidun, J.; Świderska, M.; Zalewska, A. Free radical production, inflammation and apoptosis in patients treated with titanium mandibular fixations—An observational study. Front. Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Ischiropoulos, H.; Zhu, L.; van der Woerd, M.; Smith, C.; Chen, J.; Harrison, J.; Martin, J.C.; Tsai, M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch. Biochem. Biophys. 1992, 298, 438–445. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Kossakowska, A.; Szulimowska, J.; Klimiuk, A.; Knaś, M.; Car, H.; Niklińska, W.; Ładny, J.R.; Chabowski, A.; Zalewska, A. Lysosomal Exoglycosidase Profile and Secretory Function in the Salivary Glands of Rats with Streptozotocin-Induced Diabetes. J. Diabetes Res. 2017. [Google Scholar] [CrossRef]
- Jankowska, A.K.; Waszkiel, D.; Kobus, A.; Zwierz, K. Saliva as a main component of oral cavity ecosystem. Part II. Defense mechanisms. Wiad. Lek. 2007, 60, 253–257. [Google Scholar]
- Dilek, N.; Dilek, A.R.; Taskin, Y.; Erkinüresin, T.; Yalçin, Ö.; Saral, Y. Contribution of myeloperoxidase and inducible nitric oxide synthase to pathogenesis of psoriasis. Postepy Dermatol. Alergol. 2016, 33, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.I.; Kang, H.I.; Ando, D.; Abrams, J.; Pisa, E. Cytokine mRNA expression in salivary gland biopsies of Sjögren’s syndrome. J. Immunol. 1994, 152, 5532–5539. [Google Scholar] [PubMed]
- Looms, D.; Tritsaris, K.; Pedersen, A.M.; Nauntofte, B.; Dissing, S. Nitric oxide signalling in salivary glands. J. Oral Pathol. Med. 2002, 31, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Soinila, J.; Nuorva, K.; Soinila, S. Nitric oxide synthase in human salivary glands. Histochem. Cell Biol. 2006. [Google Scholar] [CrossRef]
- De La Cal, C.; Lomniczi, A.; Mohn, C.E.; De Laurentiis, A.; Casal, M.; Chiarenza, A.; Paz, D.; McCann, S.M.; Rettori, V.; Elverdín, J.C. Decrease in salivary secretion by radiation mediated by nitric oxide and prostaglandins. Neuroimmunomodulation 2006, 13, 19–27. [Google Scholar] [CrossRef]
- Slomiany, B.L.; Slomiany, A. Nitric oxide interferes with salivary mucin synthesis: Involvement of ERK and p38 mitogen-activated protein kinase. J. Physiol. Pharmacol. 2002, 53, 325–336. [Google Scholar]
- Zalewska, A.; Ziembicka, D.; Zendzian-Piotrowska, M.; MacIejczyk, M. The impact of high-fat diet on mitochondrial function, free radical production, and nitrosative stress in the salivary glands of wistar rats. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Azuma, M.; Aota, K.; Tamatani, T.; Motegi, K.; Yamashita, T.; Ashida, Y.; Hayashi, Y.; Sato, M. Suppression of tumor necrosis factor alpha-induced matrix metalloproteinase 9 production in human salivary gland acinar cells by cepharanthine occurs via down-regulation of nuclear factor kappaB: A possible therapeutic agent for preventing the destruction of the acinar structure in the salivary glands of Sjögren’s syndrome patients. Arthritis Rheum. 2002, 46, 1585–1594. [Google Scholar]
- Bozzato, A.; Burger, P.; Zenk, J.; Uter, W.; Iro, H. Salivary gland biometry in female patients with eating disorders. Eur. Arch. Oto Rhino Laryngol. 2008, 265, 1095–1102. [Google Scholar] [CrossRef]
- Heo, M.S.; Lee, S.C.; Lee, S.S.; Choi, H.M.; Choi, S.C.; Park, T.W. Quantitative analysis of normal major salivary glands using computed tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 240–244. [Google Scholar] [CrossRef]
- Hanemaaijer, R.; Visser, H.; Konttinen, Y.T.; Koolwijk, P.; Verheijen, J.H. A novel and simple immunocapture assay for determination of gelatinase-B (MMP-9) activities in biological fluids: Saliva from patients with Sjögren’s syndrome contain increased latent and active gelatinase-B levels. Matrix Biol. 1998, 17, 657–665. [Google Scholar] [CrossRef]
- Weiner, D.; Khankin, E.V.; Levy, Y.; Reznick, A.Z. Effects of cigarette smoke borne reactive nitrogen species on salivary alpha-amylase activity and protein modifications. J. Physiol. Pharmacol. 2009, 60, 127–132. [Google Scholar] [PubMed]
- Gryka, D.; Pilch, W.B.; Czerwińska-Ledwig, O.M.; Piotrowska, A.M.; Klocek, E.; Bukova, A. The influence of Finnish sauna treatments on the concentrations of nitric oxide, 3-nitrotyrosine and selected markers of oxidative status in training and non-training men. Int. J. Occup. Med. Environ. Health 2020. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Control n = 60 | PN n = 30 | PH n = 30 | |
|---|---|---|---|---|
| Sex | Male n (%) | 23 (38.33%) | 13 (43.33%) | 10 (33.33%) |
| Female n (%) | 37 (61.67%) | 17 (56.67%) | 20 (66.67%) | |
| Age (years) | 52 ± 5 | 49 ± 6 | 51 ± 3 | |
| Height (cm) | 172 ± 6 | 176 ± 3 | 169 ± 8 | |
| Weight (kg) Duration of psoriasis (years) | 75 ± 1 | 72 ± 3 | 74 ± 1 | |
| ND | 9.7 ± 3.4 | 18.52 ± 7.8 | ||
| PASI | ND | 10.39 ± 2.4 | 18.29 ± 4.6 | |
| Psoriasis in the family | <1 n (%) | ND | 18 (60%) | 6 (20%) |
| ≥1 n (%) | ND | 12 (40%) | 24 (80%) | |
| Blood Tests | ||||
| RBC (106/µL) | 4.54 ± 0.54 | 4.23 ± 0.65 | 4.89 ± 0.87 | |
| HCT (%) | 40.25 ± 6.5 | 42.45 ± 4.25 | 45.21 ± 0.89 | |
| PLT (103/µL) | 275 ± 56 | 245 ± 87 | 292 ± 23 | |
| WBC (103/µL) | 6.5 ± 2.2 | 7.05 ± 1.8 | 6.98 ± 1.86 | |
| CRP (mg/L) | 1.5 ± 0.5 | 8.56 ± 6.3 | 6.32 ± 7.56 | |
| Glc (mg/dL) | 69 ± 9.8 | 72 ± 9.8 | 75 ± 6.5 | |
| ALT (U/L) | 27.56 ± 12.3 | 24.36 ± 9.68 | 27.24 ± 6.35 | |
| AST (U/L) | 28.54 ± 12.25 | 30.23 ± 6.52 | 28.98 ± 8.64 | |
| Dental Characteristics | ||||
| DMFT | 20 ± 3 | 19 ± 6 | 21 ± 2 | |
| GI | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.2 | |
| PPD | 1.5 ± 0.5 | 1.0 ± 0.5 | 1.0 ± 0.5 | |
| Dental implants | 0 | 0 | 0 | |
| NWS | SWS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | AUC | 95% Confidence Interval | p-Value | Cut-Off | Sensitivity (%) | Specificity (%) | AUC | 95% Confidence Interval | p-Value | Cut-Off | Sensitivity (%) | Specificity (%) | AUC |
| Cytokines | |||||||||||||
| TNF-α (pg/mg protein) | 0.68 | 0.5417–0.8183 | 0.0166 | >15.83 | 63.33 | 63.33 | 0.7311 | 0.6025–0.8597 | 0.0021 | >5.717 | 66.67 | 66.67 | 0.5611 |
| IL-2 (pg/mg protein) | 0.8111 | 0.7024–0.9198 | <0.0001 | >6.789 | 73.33 | 73.33 | 0.6478 | 0.5071–0.7884 | 0.0493 | >1.595 | 63.33 | 63.33 | 0.6122 |
| IL-10 (pg/mg protein) | 0.7089 | 0.5760–0.8418 | 0.0054 | <0.9704 | 63.33 | 63.33 | 0.7267 | 0.5997–0.8536 | 0.0026 | <0.2410 | 66.67 | 66.67 | 0.6556 |
| INF-γ (pg/mg protein) | 0.6622 | 0.5246–0.7999 | 0.0309 | >4.358 | 60.00 | 60.00 | 0.6278 | 0.4848–0.7708 | 0.0891 | >1.142 | 60.00 | 60.00 | 0.5378 |
| Nitrosative Stress | |||||||||||||
| NO (ng/mg protein) | 0.77 | 0.6507–0.8893 | 0.0003 | >318.90 | 66.67 | 66.67 | 0.6464 | 0.5443–0.7885 | 0.0556 | >224.00 | 60.71 | 60.00 | 0.5244 |
| S–nitrosothiols (nM/mg protein) | 0.6844 | 0.5479–0.8209 | 0.0141 | >42.73 | 60.00 | 60.00 | 0.7211 | 0.5857–0.8565 | 0.0033 | >34.60 | 70.00 | 70.00 | 0.6644 |
| Peroxynitrite (nM/mg protein) | 0.7056 | 0.5744–0.8367 | 0.0062 | >101.50 | 63.33 | 63.33 | 0.69 | 0.5569–0.8231 | 0.0115 | >45.31 | 60.00 | 60.00 | 0.5744 |
| Nitrotyrosine (pM/mg protein) | 0.7444 | 0.6120–0.8769 | 0.0011 | >3398.00 | 70.00 | 70.00 | 0.6611 | 0.5223–0.8000 | 0.0321 | >2139.00 | 60.00 | 60.00 | 0.5011 |
| Plasma | ||||||
|---|---|---|---|---|---|---|
| Parameter | AUC | 95% Confidence Interval | p-Value | Cut-Off | Sensitivity (%) | Specificity (%) |
| Cytokines | ||||||
| TNF-α (pg/mL) | 0.5611 | 0.4135–0.7087 | 0.4161 | >3.748 | 50.00 | 50.00 |
| IL-2 (pg/mL) | 0.6122 | 0.4685–0.7559 | 0.1354 | >7.201 | 60.00 | 60.00 |
| IL-10 (pg/mL) | 0.6556 | 0.5127–0.7984 | 0.0385 | >0.3863 | 63.33 | 63.33 |
| INF-γ (pg/mL) | 0.5378 | 0.3897–0.6858 | 0.6152 | <251.20 | 50.00 | 50.00 |
| Nitrosative Stress | ||||||
| NO (ng/mg protein) | 0.5244 | 0.3743–0.6746 | 0.745 | >99.49 | 46.67 | 46.67 |
| S–nitrosothiols (nM/mg protein) | 0.6644 | 0.5261–0.8028 | 0.0287 | >57.03 | 60.00 | 60.00 |
| Peroxynitrite (nM/mg protein) | 0.5744 | 0.4261–0.7228 | 0.3219 | >302.90 | 60.00 | 60.00 |
| Nitrotyrosine (pM/mg protein) | 0.5011 | 0.3528–0.6494 | 0.9882 | <3747.00 | 50.00 | 50.00 |
| Pair of Variables | Group | r | p |
|---|---|---|---|
| NO NWS and NWS flow rate | PH | −0.68 | 0.001 |
| Peroxynitrite SWS and total protein SWS | PH | −0.56 | 0.0015 |
| TNF-α NWS and NWS flow rate | PH | −0.60 | 0.004 |
| IL-2 SWS and SWS flow rate | PH | −0.54 | 0.002 |
| Peroxynitrite NWS and amylase NWS | PN | −0.58 | 0.0008 |
| Peroxynitrite SWS and amylase SWS | PN | −0.68 | <0.0001 |
| TNF-α NWS and NO NWS | PH | 0.60 | 0.004 |
| IL-2 NWS and NO NWS | PN | 0.64 | 0.002 |
| TNF-α NWS and PASI | PH | 0.59 | 0.0006 |
| IL-2 NWS and PASI | PH | 0.63 | 0.0029 |
| Nitrotyrosine NWS and disease duration | PN | 0.53 | 0.003 |
| Nitrotyrosine SWS and disease duration | PN | 0.58 | 0.001 |
| Nitrotyrosine NWS and disease duration | PH | 0.61 | 0.004 |
| Nitrotyrosine SWS and disease duration | PH | 0.60 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skutnik-Radziszewska, A.; Maciejczyk, M.; Flisiak, I.; Krahel, J.; Kołodziej, U.; Kotowska-Rodziewicz, A.; Klimiuk, A.; Zalewska, A. Enhanced Inflammation and Nitrosative Stress in the Saliva and Plasma of Patients with Plaque Psoriasis. J. Clin. Med. 2020, 9, 745. https://doi.org/10.3390/jcm9030745
Skutnik-Radziszewska A, Maciejczyk M, Flisiak I, Krahel J, Kołodziej U, Kotowska-Rodziewicz A, Klimiuk A, Zalewska A. Enhanced Inflammation and Nitrosative Stress in the Saliva and Plasma of Patients with Plaque Psoriasis. Journal of Clinical Medicine. 2020; 9(3):745. https://doi.org/10.3390/jcm9030745
Chicago/Turabian StyleSkutnik-Radziszewska, Anna, Mateusz Maciejczyk, Iwona Flisiak, Julita Krahel, Urszula Kołodziej, Anna Kotowska-Rodziewicz, Anna Klimiuk, and Anna Zalewska. 2020. "Enhanced Inflammation and Nitrosative Stress in the Saliva and Plasma of Patients with Plaque Psoriasis" Journal of Clinical Medicine 9, no. 3: 745. https://doi.org/10.3390/jcm9030745
APA StyleSkutnik-Radziszewska, A., Maciejczyk, M., Flisiak, I., Krahel, J., Kołodziej, U., Kotowska-Rodziewicz, A., Klimiuk, A., & Zalewska, A. (2020). Enhanced Inflammation and Nitrosative Stress in the Saliva and Plasma of Patients with Plaque Psoriasis. Journal of Clinical Medicine, 9(3), 745. https://doi.org/10.3390/jcm9030745








