Atrial Ectopy Increases Asynchronous Activation of the Endo- and Epicardium at the Right Atrium
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population
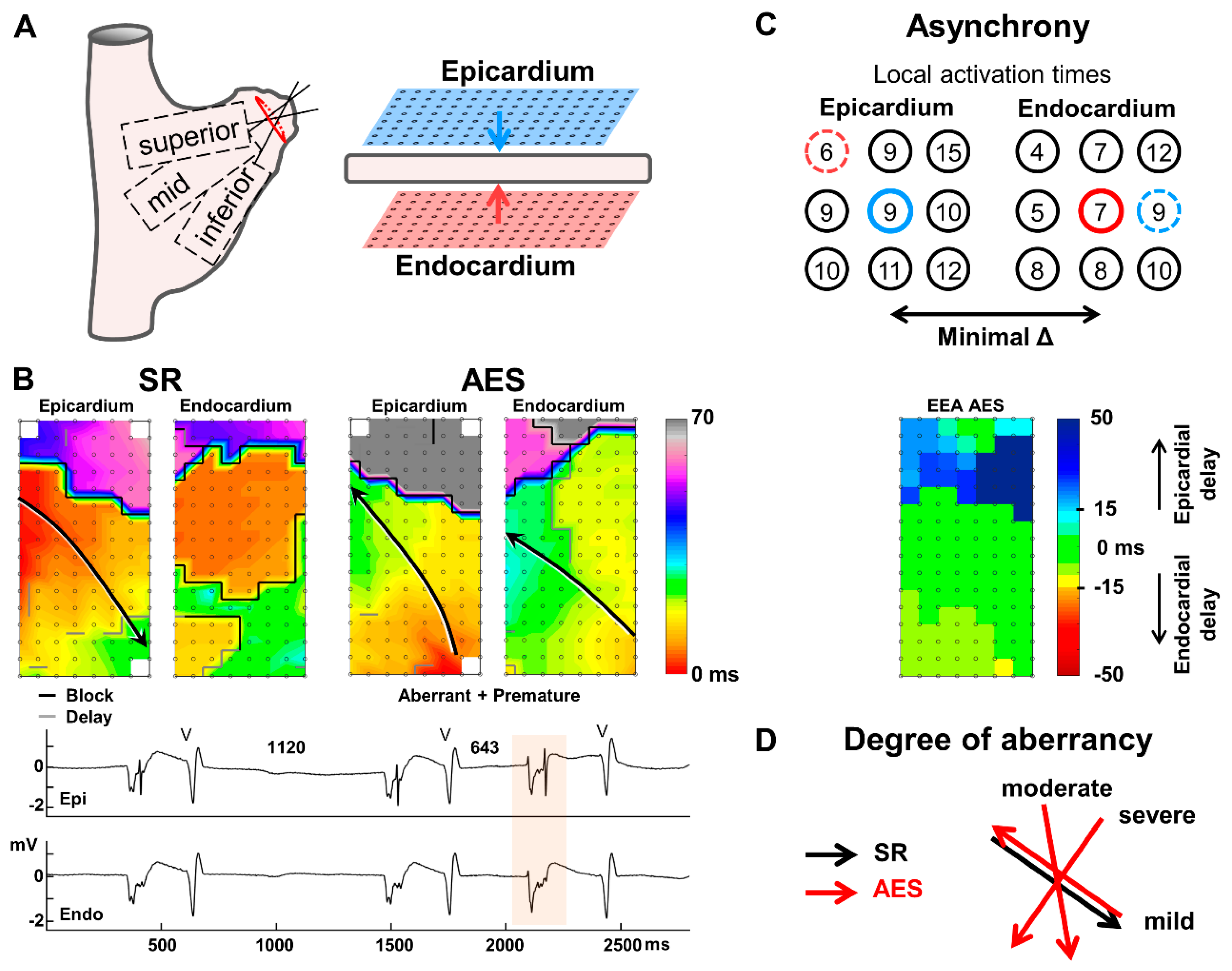
2.2. Endo-Epicardial Mapping Procedure
2.3. Data Analysis
2.4. Atrial Extrasystoles
2.5. Statistical Analysis
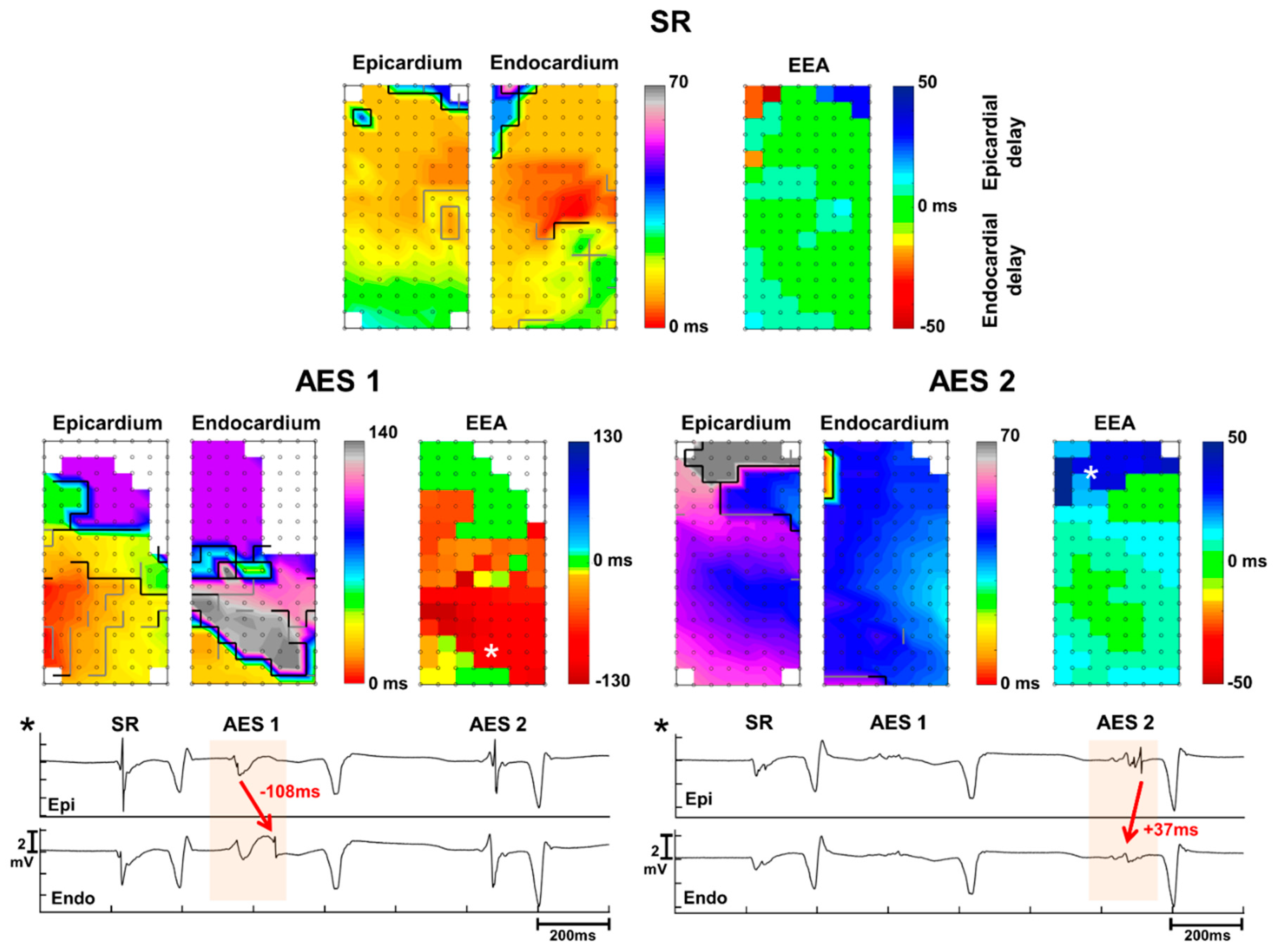
3. Results
3.1. Atrial Extrasystole Characteristics
3.2. Conduction Delay and Block
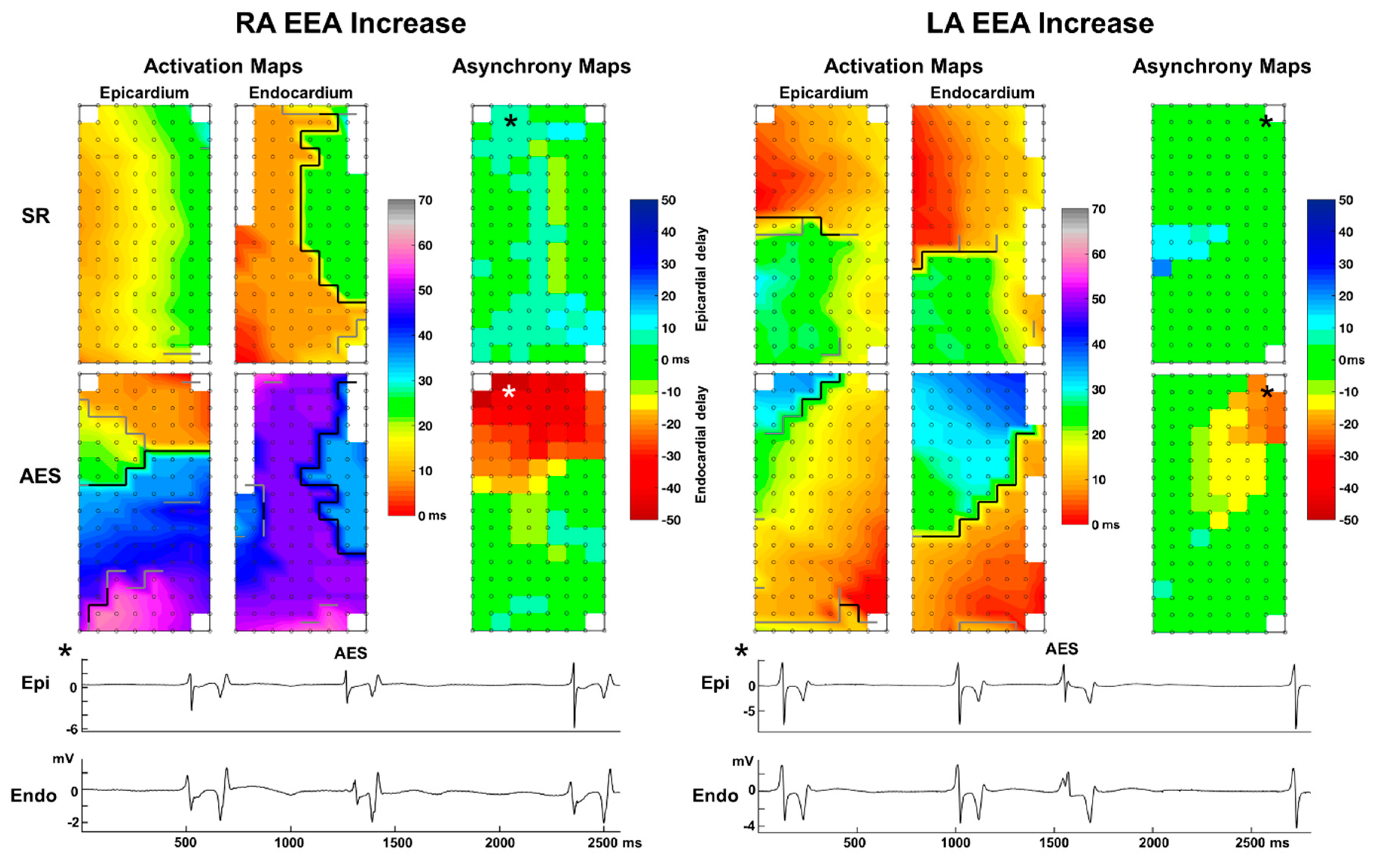
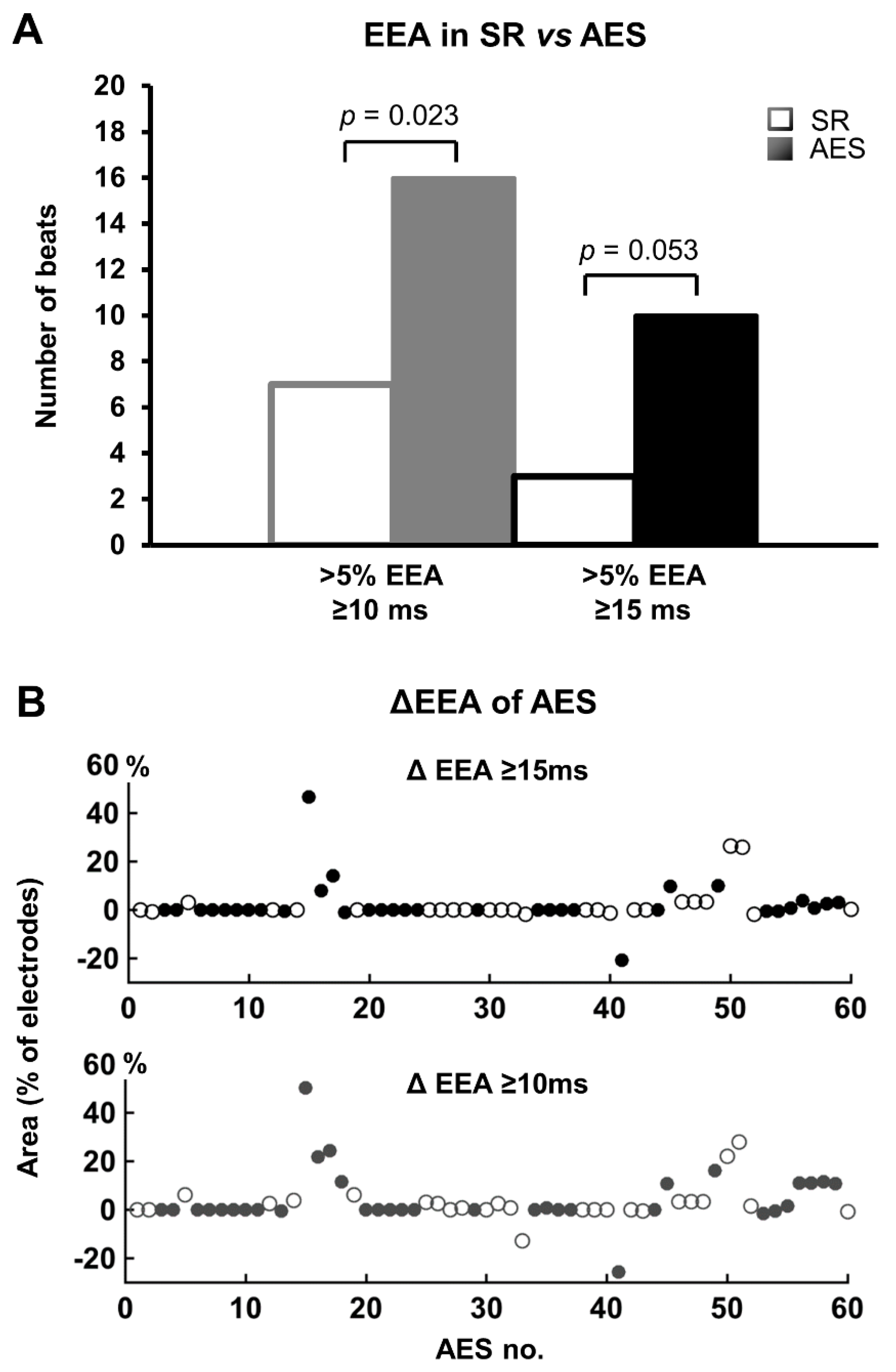
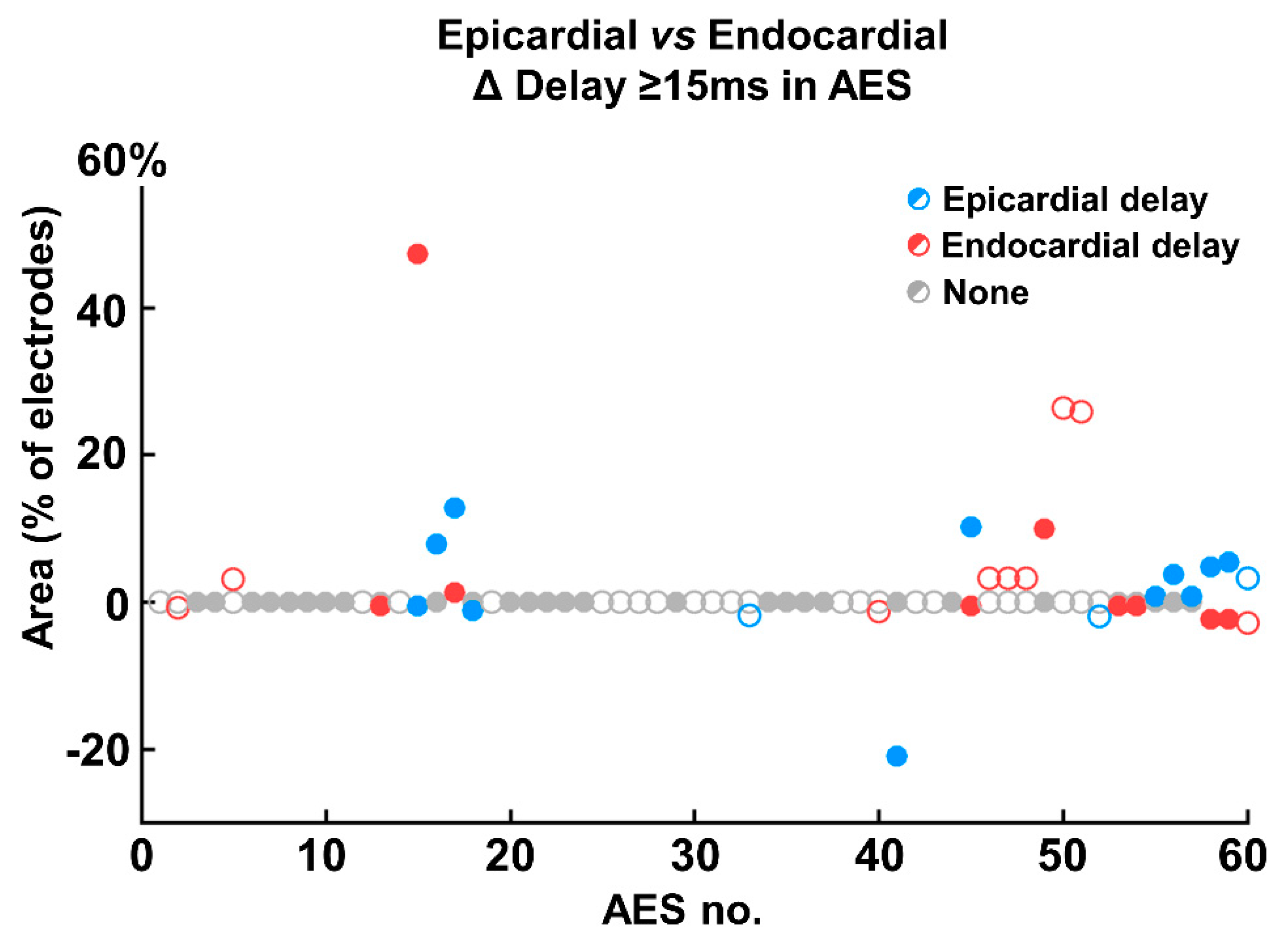
3.3. Endo-Epicardial Asynchrony
3.4. Impact of Prematurity and Aberrancy
4. Discussion
4.1. Main Findings
4.2. Conduction Disorders in AES
4.3. Arrhythmogenic Potential of AES and Effect of EEA
4.4. Multifactorial Conditions for AES to Trigger Tachyarrhythmia
4.5. Clinical Impact
4.6. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, L.S.; Juhlin, T.; Juul-Moller, S.; Hedblad, B.; Nilsson, P.M.; Engstrom, G. A prospective study of supraventricular activity and incidence of atrial fibrillation. Heart Rhythm. 2015, 12, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, A.; Brambilla, R.; Fumagalli, M.G.; Merola, R.; Pedretti, S. Onset mechanism of paroxysmal atrial fibrillation detected by ambulatory Holter monitoring. Europace 2006, 8, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Jais, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Metayer, P.; Clementy, J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teuwen, C.P.; Kik, C.; van der Does, L.J.M.E.; Lanters, E.A.H.; Knops, P.; Mouws, E.J.M.P.; Bogers, A.J.J.C.; de Groot, N.M.S. Quantification of the Arrhythmogenic Effects of Spontaneous Atrial Extrasystole Using High-Resolution Epicardial Mapping. Circ. Arrhythmia Electrophysiol. 2018, 11, e005745. [Google Scholar] [CrossRef] [PubMed]
- de Groot, N.M.S.; van der Does, L.J.M.E.; Yaksh, A.; Lanters, E.A.H.; Teuwen, C.P.; Knops, P.; van de Woestijne, P.; Bekkers, J.; Kik, C.; Bogers, A.J.J.C.; et al. Direct Proof of Endo-Epicardial Asynchrony of the Atrial Wall During Atrial Fibrillation in Humans. Circ. Arrhythmia Electrophysiol. 2016, 9, e003648. [Google Scholar] [CrossRef] [PubMed]
- van der Does, L.J.M.E.; Knops, P.; Teuwen, C.P.; Serban, C.; Starreveld, R.; Lanters, E.A.H.; Mouws, E.J.M.P.; Kik, C.; Bogers, A.J.J.C.; de Groot, N.M.S. Unipolar atrial electrogram morphology from an epicardial and endocardial perspective. Heart Rhythm. 2018, 15, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Knops, P.; Kik, C.; Bogers, A.J.J.C.; de Groot, N.M.S. Simultaneous endocardial and epicardial high-resolution mapping of the human right atrial wall. J. Thorac. Cardiovasc. Surg. 2016, 152, 929–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angel, N.; Li, L.I.; Macleod, R.S.; Marrouche, N.; Ranjan, R.; Dosdall, D.J. Diverse Fibrosis Architecture and Premature Stimulation Facilitate Initiation of Reentrant Activity Following Chronic Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2015, 26, 1352–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawara, T.; Derksen, R.; de Groot, J.R.; Coronel, R.; Tasseron, S.; Linnenbank, A.C.; Hauer, R.N.; Kirkels, H.; Janse, M.J.; de Bakker, J.M. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation 2001, 104, 3069–3075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spach, M.S.; Heidlage, J.F.; Dolber, P.C.; Barr, R.C. Mechanism of origin of conduction disturbances in aging human atrial bundles: Experimental and model study. Heart Rhythm. 2007, 4, 175–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spach, M.S.; Dolber, P.C.; Heidlage, J.F. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of re-entry based on anisotropic discontinuous propagation. Circ. Res. 1988, 62, 811–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spach, M.S.; Dolber, P.C.; Heidlage, J.F. Interaction of inhomogeneities of repolarization with anisotropic propagation in dog atria. A mechanism for both preventing and initiating re-entry. Circ. Res. 1989, 65, 1612–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuessler, R.B.; Kawamoto, T.; Hand, D.E.; Mitsuno, M.; Bromberg, B.I.; Cox, J.L.; Boineau, J.P. Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation 1993, 88, 250–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sisti, A.; Attuel, P.; Manot, S.; Fiorello, P.; Halimi, F.; Leclercq, J.F. Electrophysiological determinants of atrial fibrillation in sinus node dysfunction despite atrial pacing. Europace 2000, 2, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.J.; Zhao, J.; Csepe, T.A.; Moore, B.T.; Li, N.; Jayne, L.A.; Kalyanasundaram, A.; Lim, P.; Bratasz, A.; Powell, K.A.; et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur. Heart J. 2015, 36, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, J.; Zeemering, S.; Linz, D.; Maesen, B.; Verheule, S.; van Hunnik, A.; Crijns, H.; Allessie, M.A.; Schotten, U. Transmural conduction is the predominant mechanism of breakthrough during atrial fibrillation: Evidence from simultaneous endo-epicardial high-density activation mapping. Circ. Arrhythmia Electrophysiol. 2013, 6, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spach, M.S.; Miller, W.T.; Dolber, P.C.; Kootsey, J.M.; Sommer, J.R.; Mosher, C.E., Jr. The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circ. Res. 1982, 50, 175–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allessie, M.; Ausma, J.; Schotten, U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef]
- Pathik, B.; Lee, G.; Sacher, F.; Haïssaguerre, M.; Jaïs, P.; Massoullié, G.; Derval, N.; Sanders, P.; Kistler, P.; Kalman, J.M. Epicardial-endocardial breakthrough during stable atrial macrore-entry: Evidence from ultra-high-resolution 3-dimensional mapping. Heart Rhythm. 2017, 8, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
| Sinus Rhythm | Atrial Extrasystole | p-Value | |||
|---|---|---|---|---|---|
| Overall | |||||
| CB (mm) | 8 | (0–48) | 20 | (6–41) | 0.020 |
| CD (mm) | 24 | (12.5–36) | 38 | (18.5–50) | 0.014 |
| Epicardium | |||||
| CB (mm) | 2 | (0–16) | 4 | (0–15.5) | 0.639 |
| CD (mm) | 11 | (4–19.5) | 16 | (8–27.5) | 0.030 |
| Endocardium | |||||
| CB (mm) | 4 | (0–26) | 14 | (5–23.5) | 0.113 |
| CD (mm) | 12 | (6–22) | 16 | (8–26) | 0.276 |
| Endo- vs. Epicardium | |||||
| ∆CB (mm) | 2 | (0–8) | 10 | (2–17.5) | 0.037 |
| ∆CD (mm) | 6 | (2–11.5) | 7 | (4–14) | 0.102 |
| Endo-Epicardial Asynchrony | |||||
| EEA ≥ 10 ms (%) | 0 | (0–2.3) | 0.7 | (0–6.0) | 0.023 |
| EEA ≥ 15 ms (%) | 0 | (0–0.6) | 0 | (0–3.0) | 0.053 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Does, L.J.M.E.; Kharbanda, R.K.; Teuwen, C.P.; Knops, P.; Kik, C.; Bogers, A.J.J.C.; de Groot, N.M.S. Atrial Ectopy Increases Asynchronous Activation of the Endo- and Epicardium at the Right Atrium. J. Clin. Med. 2020, 9, 558. https://doi.org/10.3390/jcm9020558
van der Does LJME, Kharbanda RK, Teuwen CP, Knops P, Kik C, Bogers AJJC, de Groot NMS. Atrial Ectopy Increases Asynchronous Activation of the Endo- and Epicardium at the Right Atrium. Journal of Clinical Medicine. 2020; 9(2):558. https://doi.org/10.3390/jcm9020558
Chicago/Turabian Stylevan der Does, Lisette J.M.E., Rohit K. Kharbanda, Christophe P. Teuwen, Paul Knops, Charles Kik, Ad J.J.C. Bogers, and Natasja M.S. de Groot. 2020. "Atrial Ectopy Increases Asynchronous Activation of the Endo- and Epicardium at the Right Atrium" Journal of Clinical Medicine 9, no. 2: 558. https://doi.org/10.3390/jcm9020558




