The Clinical Impact of Flow Titration on Epoprostenol Delivery via High Flow Nasal Cannula for ICU Patients with Pulmonary Hypertension or Right Ventricular Dysfunction: A Retrospective Cohort Comparison Study
Abstract
:1. Introduction
2. Material and Methods
2.1. The Process of iEPO Delivery via HFNC
2.2. Data Collection
2.3. Statistical Analysis
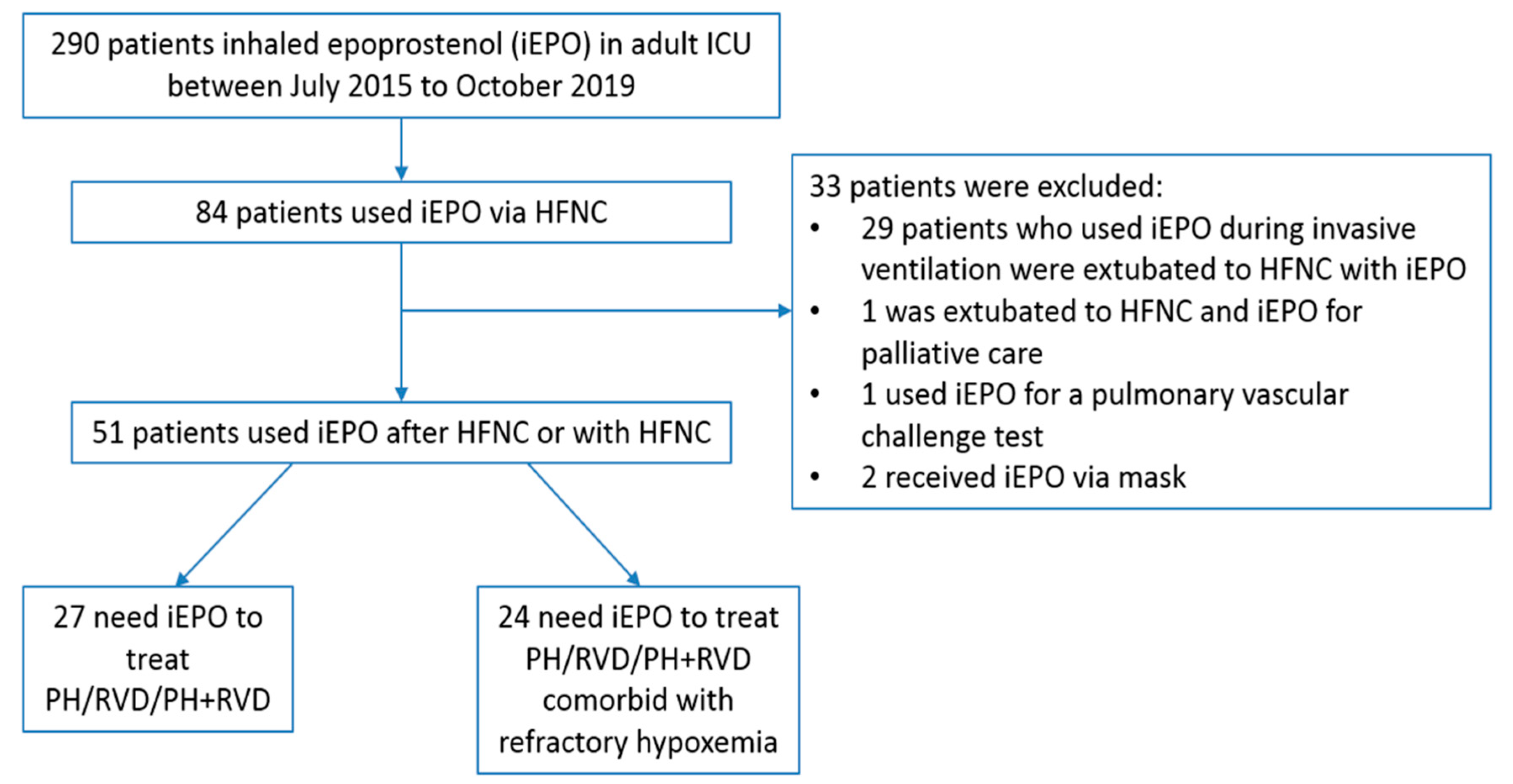
3. Results
3.1. Demographic Information
3.2. Pre- vs. Post- iEPO Hemodynamic Responses
3.3. Pre- vs. Post- iEPO Oxygenation Responses for Patients Comorbid with Refractory Hypoxemia
3.4. Patient Outcomes
4. Discussion
4.1. Clinical Impact of Flow Titration for iEPO Delivery via HFNC
4.2. Clinical Effects of iEPO Delivery via HFNC for Patients with Pulmonary Hypertension and/or Right Ventricular Dysfunction
4.3. Safety
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hill, N.S.; Preston, I.R.; Roberts, K.E. Inhaled Therapies for Pulmonary Hypertension. Respir. Care 2015, 60, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.; Ishida, K.; Masuda, M.; Ueda, H.; Kohno, H.; Matsuura, K.; Tamura, Y.; Watanabe, M.; Matsumiya, G. A Prospective, Randomized Study of Inhaled Prostacyclin Versus Nitric Oxide in Patients with Residual Pulmonary Hypertension after Pulmonary Endarterectomy. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGinn, K.; Reichert, M. A Comparison of Inhaled Nitric Oxide Versus Inhaled Epoprostenol for Acute Pulmonary Hypertension Following Cardiac Surgery. Ann. Pharmacother. 2016, 50, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Preston, I.R.; Sagliani, K.D.; Roberts, K.E.; Shah, A.M.; Desouza, S.A.; Howard, W.; Brennan, J.; Hill, N.S. Comparison of Acute Hemodynamic Effects of Inhaled Nitric Oxide and Inhaled Epoprostenol in Patients with Pulmonary Hypertension. Pulm. Circ. 2013, 3, 68–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, B.M.; Mohr, N.M.; Skrupky, L.; Fowler, S.; Kollef, M.H.; Carpenter, C.R. The Use of Inhaled Prostaglandins in Patients with ARDS: A Systematic Review and Meta-Analysis. Chest 2015, 147, 1510–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, M.A.; Bauer, S.R.; Bass, S.N.; Sasidhar, M.; Mullin, R.; Lam, S.W. Noninferiority of Inhaled Epoprostenol to Inhaled Nitric Oxide for the Treatment of ARDS. Ann. Pharmacother. 2015, 49, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Torbic, H.; Szumita, P.M.; Anger, K.E.; Nuccio, P.; LaGambina, S.; Weinhouse, G. Inhaled Epoprostenol Vs Inhaled Nitric Oxide for Refractory Hypoxemia in Critically Ill Patients. J. Crit. Care 2013, 28, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Harnois, L.J.; Markos, B.; Roberts, K.M.; Homoud, S.A.; Liu, J.; Mirza, S.; Vines, D. Epoprostenol Delivered via High Flow Nasal Cannula for ICU Subjects with Severe Hypoxemia Comorbid with Pulmonary Hypertension or Right Heart Dysfunction. Pharmaceutics 2019, 11, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, M.A.; Sasidhar, M.; Lam, S.W. Inhaled Epoprostenol through Noninvasive Routes of Ventilator Support Systems. Ann. Pharmacother. 2018, 52, 1173–1181. [Google Scholar] [CrossRef]
- Dugernier, J.; Reychler, G.; Vecellio, L.; Ehrmann, S. Nasal High-Flow Nebulization for Lung Drug Delivery: Theoretical, Experimental, and Clinical Application. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 341–351. [Google Scholar] [CrossRef]
- Ari, A. Aerosol Drug Delivery through High Flow Nasal Cannula. Curr. Pharm. Biotechnol. 2017, 18, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, L.; Fink, J.B. The Ratio of Nasal Cannula Gas Flow to Patient Inspiratory Flow on Trans-nasal Pulmonary Aerosol Delivery for Adults: An in Vitro Study. Pharmaceutics 2019, 11, 225. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gong, L.; Ari, A.; Fink, J.B. Decrease the Flow Setting to Improve Trans-Nasal Pulmonary Aerosol Delivery Via “High-Flow Nasal Cannula” to Infants and Toddlers. Pediatr. Pulmonol. 2019, 54, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Alcoforado, L.; Ari, A.; Barcelar, J.M.; Brandao, S.C.S.; Fink, J.B.; Andrade, A.D. Impact of Gas Flow and Humidity on Trans-Nasal Aerosol Deposition via Nasal Cannula in Adults: A Randomized Cross-Over Study. Pharmaceutics 2019, 11, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, T.E.; Saville, A.; Adams, P.S.; Johnston, D.J.; Czachowski, M.R.; Domnina, Y.A.; Lin, J.H.; Weiner, D.J.; Huber, A.S.; Sanchez De Toledo, J.; et al. Deposition Studies of Aerosol Delivery by Nasal Cannula to Infants. Pediatr. Pulmonol. 2019, 54, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Hayden, D.L.; Schoenfeld, D.A.; Ware, L.B.; National Institutes of Health, N.H.L.; Blood Institute, A.N. Comparison of the SpO2/FIO2 Ratio and the PaO2/FIO2 Ratio in Patients with Acute Lung Injury or ARDS. Chest 2007, 132, 410–417. [Google Scholar] [CrossRef]
- Kjellström, B.; Nisell, M.; Kylhammar, D.; Bartfay, S.E.; Ivarsson, B.; Rådegran, G.; Hjalmarsson, C. Sex-Specific Differences and Survival in Patients with Idiopathic Pulmonary Arterial Hypertension 2008–2016. ERJ Open Res. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, J.E.; Williams, A.B.; Gerard, C.; Hockey, H. Evaluation of A Humidified Nasal High-Flow Oxygen System, Using Oxygraphy, Capnography and Measurement of Upper Airway Pressures. Anaesth. Intensive Care 2011, 39, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Levy, S.D.; Alladina, J.W.; Hibbert, K.A.; Harris, R.S.; Bajwa, E.K.; Hess, D.R. High-Flow Oxygen Therapy and Other Inhaled Therapies in Intensive Care Units. Lancet 2016, 387, 1867–1878. [Google Scholar] [CrossRef]
- Li, J.; Zhao, M.; Hadeer, M.; Luo, J.; Fink, J.B. Dose Response to Transnasal Pulmonary Administration of Bronchodilator Aerosols via Nasal High-Flow Therapy in Adults with Stable Chronic Obstructive Pulmonary Disease and Asthma. Respiration 2019, 98, 401–409. [Google Scholar] [CrossRef]
- Chikata, Y.; Onodera, M.; Oto, J.; Nishimura, M. FiO2 in An Adult Model Simulating High-Flow Nasal Cannula Therapy. Respir. Care 2017, 62, 193–198. [Google Scholar] [CrossRef] [PubMed]
| Overall | Flow Titration Group | Constant Flow Group | p | |
|---|---|---|---|---|
| No. of patients | 51 | 25 | 26 | |
| Age, years | 61.9 ± 16.7 | 63.4 ± 15.3 | 60.4 ± 18.0 | 0.529 |
| Gender (Female) (%) | 29 (56.9%) | 13 (52 %) | 16 (61.5%) | 0.577 |
| Race (%) | ||||
| African American | 26 (51.0%) | 12 (48.0%) | 14 (53.8%) | 0.716 |
| Caucasian | 18 (35.3%) | 10 (40.0%) | 8 (30.8%) | |
| Hispanic or Latino | 6 (11.8%) | 3 (12.0%) | 3 (11.5%) | |
| Asian | 1 (2.0%) | 0 | 1 (3.8%) | |
| SICU (%) | 23 (45.1%) | 13 (52%) | 10 (38.5%) | 0.592 |
| MICU (%) | 15 (29.4%) | 6 (24%) | 9 (34.6%) | |
| Diagnosis (%) | 0.112 | |||
| PH | 16 (31.4%) | 10 (40%) | 6 (23.1%) | |
| PH + Hypoxemia | 14 (27.5%) | 9 (36%) | 5 (19.2%) | |
| PH + RVD | 7 (13.7%) | 3 (12%) | 4 (15.4%) | |
| RVD | 4 (7.8%) | 0 | 4 (15.4%) | |
| RVD + Hypoxemia | 4 (7.8%) | 2 (8.0%) | 2 (7.7%) | |
| PH + RVD + Hypoxemia | 6 (11.8%) | 1 (4%) | 5 (19.2%) | |
| PH WHO classification | ||||
| Class I | 21 (41.2%) | 8 (32.0%) | 13 (50.0%) | 0.258 |
| Class II | 22 (43.1%) | 14 (56.0%) | 8 (30.8%) | 0.093 |
| Class III | 16 (31.4%) | 7 (28.0%) | 9 (34.6%) | 0.764 |
| Class IV | 3 (5.9%) | 2 (8.0%) | 1 (3.8%) | 0.610 |
| Class V | 2 (3.9%) | 2 (8.0%) | 0 | 0.235 |
| iEPO indication (%) | ||||
| PH | 43 (84.3%) | 23 (92%) | 20 (76.9%) | 0.248 |
| RVD | 21 (41.2%) | 6 (24%) | 15 (57.7%) | 0.023 |
| Hypoxemia | 24 (47.1%) | 12 (48%) | 12 (46.2%) | 1.0 |
| iEPO initiated after HFNC (%) | 34 (66.7%) | 20 (80%) | 14 (53.8%) | 0.075 |
| ECMO while iEPO was initiated (%) | 4 (7.8%) | 1 (4%) | 3 (11.5%) | 0.061 |
| Chronic pulmonary disease (%) | 22 (43.1%) | 12 (48%) | 10 (38.5%) | 0.577 |
| Home oxygen use (%) | 22 (43.1%) | 8 (32%) | 14 (53.8%) | 0.072 |
| Baseline sPAP by Echo, mmHg a | 64 (50, 82.5) | 61.8 ± 20.8 | 77.7 ± 28.7 | 0.058 |
| Baseline mPAP, mmHg b | 45.7 ± 12.4 | 42.4 ± 6.8 | 51.7 ± 18.2 | 0.404 |
| Baseline CO, L/min c | 4.93 ± 2.02 | 4.53 ± 1.25 | 5.53 ± 2.87 | 0.607 |
| Baseline CI, L/min/m2 c | 2.48 ± 1.04 | 2.20 ± 60 | 2.91 ± 1.43 | 0.328 |
| Code status of do-not-intubate (%) | 7 (13.7%) | 4 (16%) | 3 (11.5%) | 0.703 |
| Invasive hemodynamic monitoring available (%) | 21 (41.2%) | 13 (52.0%) | 8 (30.8%) | 0.124 |
| Patient No. | iEPO Indication | FIO2 Prior to iEPO | Flow Prior to iEPO | mPAP (mmHg) at Different Gas Flow | Final Flow | Final FIO2 | HFNC hours Prior to iEPO | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior to iEPO | 50 L/min | 40 L/min | 30 L/min | 20 L/min | 10 L/min | 5 L/min | At final Flow | |||||||
| 1 | PH + hypoxemia | 1.0 | 40 | 64.3 | 53 | 53 | 54.7 | 56.3 | 53 | 40 | 1.0 | 90 | ||
| 2 | PH | 0.3 | 30 | 56.7 | 50.7 | 47.7 | 42.7 | 42.7 | 10 | 0.3 | 0.33 | |||
| 3 | PH | 0.4 | 50 | 54.3 | 49.3 | 50.3 | 50.0 | 44.7 | 46.7 | 44.7 | 20 | 0.4 | 0.42 | |
| 4 | PH + hypoxemia | 0.95 | 40 | 53.3 | 41.7 | 40.7 | 37 | 38.3 | 37 | 30 | 0.47 | 18.42 | ||
| 5 a | PH | 0.21 | 60 | 45.7 | 44.3 | 43.7 | 44.3 | 43 | 39.3 | 38.7 | 38.7 | 5 | 0.21 | 0.17 |
| 6 | PH | 0.4 | 50 | 44 | 39 | 36 | 35 | 40 | 34 | 30 | 0.4 | 1.85 | ||
| 7 | PH + hypoxemia | 0.5 | 50 | 41.3 | 41.3 | 37 | 33.3 | 30.3 | 32 | 20 | 0.5 | 6.33 | ||
| 8 | RVD | 0.4 | 30 | 22.3 | 21 | 20.3 | 20 | 19.3 | 17.7 | 17.7 | 10 | 0.4 | 1.08 | |
| 9 | PH | 0.21 | NA | 65.3 | 56 | 55.3 | 55.3 | 55.3 | 20 | 0.3 | 0 | |||
| 10 | PH | 0.29 | NA | 42.7 | 21.7 | 20.0 | 20.3 | 20.0 | 10 | 0.3 | 0 | |||
| 11 | PH | 0.37 | NA | 43.0 | 38.7 | 40.7 | 38.7 | 40 | 0.4 | 0 | ||||
| 12 | PH | 0.25 | NA | 44.7 | 40.7 | 43.7 | 40.7 | 30 | 0.25 | 0 | ||||
| 13 | RVD | 0.29 | NA | NA | 22.3 | 21.3 | 21 | 20.7 | 19.7 | 19.7 | 10 | 0.35 | 0 | |
| No. of Patients | Prior to iEPO | Post iEPO | p | Power | |
|---|---|---|---|---|---|
| mPAP, mmHg | 21 | 43.6 ± 11.7 | 36.3 ± 9.7 | <0.001 | 0.823 |
| Flow titration group | 13 | 46.9 ± 12.0 | 37.7 ± 10.8 | 0.002 | 0.741 |
| Constant flow group | 8 | 38.2 ± 9.4 | 34.0 ± 7.6 | 0.030 | 0.215 |
| CO, L/min | 14 | 5.12 ± 1.81 | 6.11 ± 2.21 | 0.024 | 0.380 |
| Flow titration group | 8 | 5.06 ± 1.79 | 6.55 ± 1.68 | 0.050 | 0.526 |
| Constant flow group | 6 | 5.20 ± 1.99 | 5.52 ± 2.83 | 0.462 | 0.057 |
| CI, L/min/m2 | 15 | 2.65 ± 1.81 | 3.13 ± 0.98 | 0.011 | 0.213 |
| Flow titration group | 9 | 2.61 ± 0.78 | 3.29 ± 0.75 | 0.021 | 0.623 |
| Constant flow group | 6 | 2.71 ± 0.85 | 2.88 ± 1.30 | 0.416 | 0.061 |
| HR, beats/min | 51 | 97.2 ± 25.1 | 93.4 ± 19.7 | 0.002 | 0.210 |
| Flow titration group | 25 | 97.8 ± 22.2 | 93.2 ± 17.4 | 0.045 | 0.191 |
| Constant flow group | 26 | 96.7 ± 28.0 | 93.6 ± 22.0 | 0.198 | 0.091 |
| mBP, mmHg | 51 | 82.5 ± 15.4 | 81.7 ± 14.4 | 0.612 | |
| RR, breaths/min | 51 | 20.9 ± 5.8 | 20.4 ± 5.6 | 0.305 | |
| FIO2 | 51 | 0.5 (0.33, 0.8) | 0.45 (0.35, 0.6) | 0.065 | |
| SpO2, % | 51 | 95 (91, 98) | 97 (94, 99) | 0.030 | |
| SpO2/FIO2 | 51 | 212.8 ± 99.9 | 218.2 ± 85.4 | 0.345 |
| No. of Patients | Prior to iEPO | Post iEPO | p | Power | |
|---|---|---|---|---|---|
| SpO2/FIO2 | 24 | 127.8 ± 45.7 | 157.6 ± 62.2 | <0.001 | 0.705 |
| Flow titration group | 12 | 133.4 ± 52.5 | 173.6 ± 73.1 | 0.003 | 0.493 |
| Constant flow group | 12 | 122.2 ± 39.1 | 141.6 ± 46.7 | 0.034 | 0.283 |
| FIO2 | 24 | 0.8 (0.53, 1.0) | 0.65 (0.5, 0.89) | 0.006 | |
| Flow titration group | 12 | 0.79 ± 0.23 | 0.64 ± 0.25 | 0.016 | 0.483 |
| Constant flow group | 12 | 0.79 ± 0.20 | 0.72 ± 0.20 | 0.140 | 0.190 |
| SpO2, % | 24 | 92.5 (89, 96) | 95.5 (93, 97.8) | 0.008 | |
| Flow titration group | 12 | 92.5 ± 4.3 | 95.9 ± 2.5 | 0.037 | 0.844 |
| Constant flow group | 12 | 89.9 ± 8.8 | 94.0 ± 3.9 | 0.098 | 0.454 |
| HFNC flow, L/min | 23 | 40 (40, 50) | 40 (30, 40) | 0.007 | |
| Flow titration group | 11 | 50 (40, 50) | 30 (20, 40) | 0.007 | |
| Constant flow group | 12 | 40 (30, 45) | 40 (30, 45) | NA | |
| HFNC duration, hours | 24 | 15.5 (2.5, 46.9) | NA | ||
| HR, beats/min | 24 | 102.0 ± 28.3 | 94.2 ± 19.8 | 0.018 | |
| mBP, mmHg | 24 | 85.3 ± 13.9 | 83.8 ± 13.9 | 0.549 | |
| mPAP, mmHg | 6 | 47.5 ± 11.3 | 40.4 ± 7.5 | 0.116 | |
| RR, breaths/min | 24 | 23.0 ± 6.2 | 22.6 ± 6.2 | 0.542 |
| Overall | Flow Titration | Constant Flow | p | |
|---|---|---|---|---|
| No. of patients | 51 | 25 | 26 | |
| Responders in total a | 69.2% (27/39) | 85.7% (18/21) | 50% (9/18) | 0.035 |
| Responders of hypoxemic patients using criteria of SpO2/FIO2 improvement > 20% | 50% (12/24) | 63.6% (7/11) | 38.5% (5/13) | 0.414 |
| Responders using criteria of mPAP reduction > 10% | 76.2% (16/21) | 84.6% (11/13) | 62.5% (5/8) | 0.325 |
| Responders with mPAP reduction > 20% | 28.6% (6/21) | 46.2% (6/13) | 0 | 0.046 |
| iEPO duration, hours | 64.5 (34.8,107.3) | 55 (37, 101.7) | 69.7 (22.9, 165.5) | 0.843 |
| Intubation (n,%) | 8 (15.7%) | 2 (8%) | 6 (23.1%) | 0.248 |
| iEPO complications (n,%) | 11 (21.6%) | 4 (16%) | 7 (26.9%) | 0.214 |
| Bleeding | 3 (5.9%) | 0 | 3 (11.5%) | |
| Hemodynamic instability | 8 (15.7%) | 4 (16%) | 4 (15.4%) | |
| ICU alive (n,%) | 40 (78.4%) | 21 (84%) | 19 (73.1%) | 0.499 |
| ICU stay, days | 11 (6, 18) | 10 (6, 14) | 11.5 (6.8, 19.5) | 0.257 |
| Hospital alive (n,%) | 37 (72.5%) | 19 (76%) | 18 (69.2%) | 0.755 |
| Hospital stay, days | 13 (8, 20) | 12 (7, 18.5) | 14 (9.3, 21.5) | 0.509 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Gurnani, P.K.; Roberts, K.M.; Fink, J.B.; Vines, D. The Clinical Impact of Flow Titration on Epoprostenol Delivery via High Flow Nasal Cannula for ICU Patients with Pulmonary Hypertension or Right Ventricular Dysfunction: A Retrospective Cohort Comparison Study. J. Clin. Med. 2020, 9, 464. https://doi.org/10.3390/jcm9020464
Li J, Gurnani PK, Roberts KM, Fink JB, Vines D. The Clinical Impact of Flow Titration on Epoprostenol Delivery via High Flow Nasal Cannula for ICU Patients with Pulmonary Hypertension or Right Ventricular Dysfunction: A Retrospective Cohort Comparison Study. Journal of Clinical Medicine. 2020; 9(2):464. https://doi.org/10.3390/jcm9020464
Chicago/Turabian StyleLi, Jie, Payal K. Gurnani, Keith M. Roberts, James B. Fink, and David Vines. 2020. "The Clinical Impact of Flow Titration on Epoprostenol Delivery via High Flow Nasal Cannula for ICU Patients with Pulmonary Hypertension or Right Ventricular Dysfunction: A Retrospective Cohort Comparison Study" Journal of Clinical Medicine 9, no. 2: 464. https://doi.org/10.3390/jcm9020464






