Closing the Gap in Surveillance and Audit of Invasive Mold Diseases for Antifungal Stewardship Using Machine Learning
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Clinical Definitions
2.3. Summary of NLP Model Development
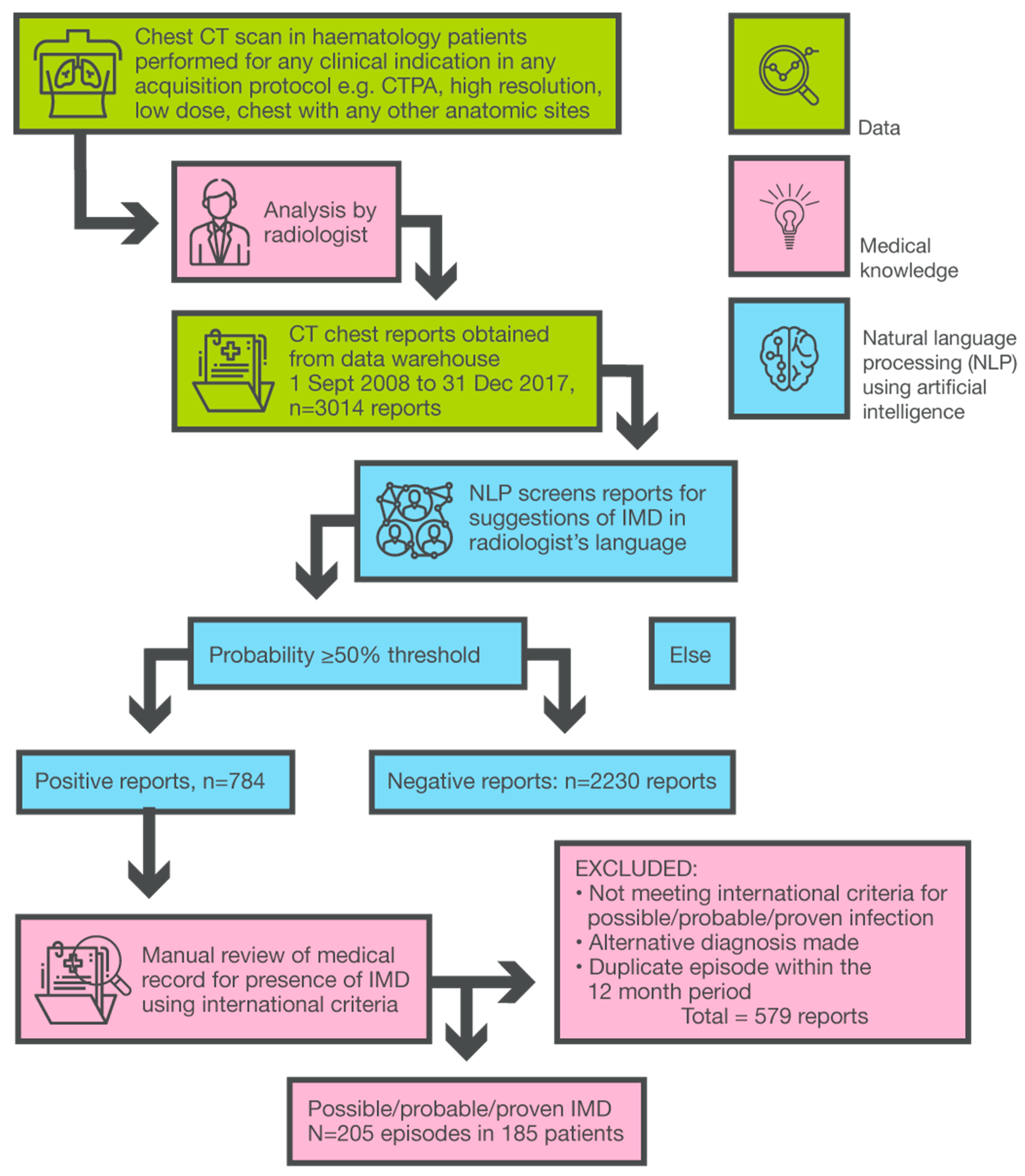
2.4. Clinical Data and Audit Methodology
2.5. Statistical Analysis
3. Results
3.1. An Overview of IMD Epidemiology
3.2. Process and Outcome Measures: Breakthrough-IMD, TDM, Missed Prophylaxis Opportunities
3.3. Effect of Diagnostic Delays on Antifungal Consumption
3.4. Probable/Proven IMD in Patients Who Did Not Receive Prophylaxis
3.5. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enoch, D.; Whitney, L. Antimicrobial Stewardship from Principles to Practice; British Society of Antimicrobial Chemotherapy: Birmingham, UK, 2018. [Google Scholar]
- Wattal, C.; Chakrabarti, A.; Oberoi, J.K.; Donnelly, J.P.; Barnes, R.A.; Sherwal, B.L.; Goel, N.; Saxena, S.; Varghese, G.M.; Soman, R.; et al. Issues in antifungal stewardship: An opportunity that should not be lost. J. Antimicrob. Chemother. 2017, 72, 969–974. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef]
- Arvanitis, M.; Anagnostou, T.; Fuchs, B.B.; Caliendo, A.M.; Mylonakis, E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin. Microbiol. Rev. 2014, 27, 490–526. [Google Scholar] [CrossRef]
- Shannon, V.R.; Andersson, B.S.; Lei, X.; Champlin, R.E.; Kontoyiannis, D.P. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010, 45, 647–655. [Google Scholar] [CrossRef]
- Bienvenu, A.L.; Argaud, L.; Aubrun, F.; Fellahi, J.L.; Guerin, C.; Javouhey, E.; Piriou, V.; Rimmele, T.; Chidiac, C.; Leboucher, G. A systematic review of interventions and performance measures for antifungal stewardship programmes. J. Antimicrob. Chemother. 2018, 73, 297–305. [Google Scholar] [CrossRef]
- Ananda-Rajah, M.R.; Slavin, M.A.; Thursky, T.K. The case for antifungal stewardship. Curr. Opin. Infect. Dis. 2012, 25, 107–115. [Google Scholar] [CrossRef]
- Ananda-Rajah, M.R.; Grigg, A.; Downey, M.T.; Bajel, A.; Spelman, T.; Cheng, A.; Thursky, K.T.; Vincent, J.; Slavin, M.A. Comparative clinical effectiveness of prophylactic voriconazole/posaconazole to fluconazole/itraconazole in patients with acute myeloid leukemia/myelodysplastic syndrome undergoing cytotoxic chemotherapy over a 12-year period. Haematologica 2012, 97, 459–463. [Google Scholar] [CrossRef]
- Auberger, J.; Lass-Flörl, C.; Aigner, M.; Clausen, J.; Gastl, G.; Nachbaur, D. Invasive fungal breakthrough infections, fungal colonization and emergence of resistant strains in high-risk patients receiving antifungal prophylaxis with posaconazole: Real-life data from a single-centre institutional retrospective observational study. J. Antimicrob. Chemother. 2012, 67, 2268–2273. [Google Scholar] [CrossRef]
- Australian Commission on Safety and Quality in Health Care. Antimicrobial Stewardship in Australian Health Care; ACSQHC: Sydney, NSW, Australia, 2018. [Google Scholar]
- Teng, J.C.; Slavin, M.A.; Teh, B.W.; Lingaratnam, S.M.; Ananda-Rajah, M.R.; Worth, L.J.; Seymour, J.F.; Thursky, K.A. Epidemiology of invasive fungal disease in lymphoproliferative disorders. Haematologica 2015, 100, e462–e466. [Google Scholar] [CrossRef] [Green Version]
- Teh, B.W.; Teng, J.C.; Urbancic, K.; Grigg, A.; Harrison, S.J.; Worth, L.J.; Slavin, M.A.; Thursky, K.A. Invasive fungal infections in patients with multiple myeloma: A multi-center study in the era of novel myeloma therapies. Haematologica 2015, 100, e28–e31. [Google Scholar] [CrossRef]
- Rausch, C.R.; DiPippo, A.J.; Bose, P.; Kontoyiannis, D.P. Breakthrough fungal infections in leukemia patients receiving isavuconazole. Clin. Infect. Dis. 2018, 67, 1610–1613. [Google Scholar] [CrossRef]
- Lerolle, N.; Raffoux, E.; Socie, G.; Touratier, S.; Sauvageon, H.; Porcher, R.; Bretagne, S.; Bergeron, A.; Azoulay, E.; Molina, J.-M.; et al. Breakthrough invasive fungal disease in patients receiving posaconazole primary prophylaxis: A 4-year study. Clin. Microbiol. Infect. 2014, 20, O952–O959. [Google Scholar] [CrossRef]
- Neofytos, D.; Lu, K.; Hatfield-Seung, A.; Blackford, A.; Marr, K.A.; Treadway, S.; Ostrander, D.; Nussenblatt, V.R.; Karp, J. Epidemiology, outcomes, and risk factors of invasive fungal infections in adult patients with acute myelogenous leukemia after induction chemotherapy. Diagn. Microbiol. Infect. Dis. 2013, 75, 144–149. [Google Scholar] [CrossRef]
- Lortholary, O.; Gangneux, J.P.; Sitbon, K.; Lebeau, B.; De Monbrison, F.; Le Strat, Y.; Coignard, B.; Dromer, F.; Bretagne, S. Epidemiological trends in invasive aspergillosis in France: The SAIF network (2005–2007). Clin. Microbiol. Infect. 2011, 17, 1882–1889. [Google Scholar] [CrossRef]
- Nicolle, M.C.; Bénet, T.; Thiebaut, A.; Bienvenu, A.L.; Voirin, N.; Duclos, A.; Sobh, M.; Cannas, G.; Thomas, X.; Nicolini, F.E.; et al. Invasive aspergillosis in patients with hematologic malignancies: Incidence and description of 127 cases enrolled in a single institution prospective survey from 2004 to 2009. Haematologica 2011, 96, 1685–1691. [Google Scholar] [CrossRef]
- Ananda-Rajah, M.R.; Martinez, D.; Slavin, M.A.; Cavedon, L.; Dooley, M.; Cheng, A.; Thursky, K.A. Facilitating surveillance of pulmonary invasive mold diseases in patients with haematological malignancies by screening computed tomography reports using natural language processing. PLoS ONE 2014, 9, e107797. [Google Scholar] [CrossRef]
- Martinez, D.; Ananda-Rajah, M.R.; Suominen, H.; Slavin, M.A.; Thursky, K.A.; Cavedon, L. Automatic detection of patients with invasive fungal disease from free-text computed tomography (CT) scans. J. Biomed. Inform. 2015, 53, 251–260. [Google Scholar] [CrossRef]
- Liu, M.; Haffari, G.; Buntine, W.; Ananda-Rajah, M. Leveraging linguistic resources for improving neural text classification. In Proceedings of the Australasian Language Technology Association Workshop 2017, Brisbane, Australia, December 2017; pp. 34–42. Available online: https://www.aclweb.org/anthology/U17-1004 (accessed on 5 September 2019).
- Marom, E.M.; Kontoyiannis, D.P. Imaging studies for diagnosing invasive fungal pneumonia in immunocompromised patients. Curr. Opin. Infect. Dis. 2011, 24, 309–314. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar]
- Ananda-Rajah, M.; Tang, T.; Josh, H.; Ellis, S.; Kam, A.; Varma, D.; Haffari, G.; Liu, M.; Seah, J.; Bergmeir, C.; et al. Deep learning based image analysis of invasive mold diseases on chest computed tomography in haematology-oncology patients. In Proceedings of the International Immunocompromised Host Society Symposium, Athens, Greece, 17–19 June 2018. [Google Scholar]
- Ananda-Rajah, M.R.; Bergmeir, C.; Petitjean, F.; Slavin, M.A.; Thursky, K.A.; Webb, G.I. Toward electronic surveillance of invasive mold diseases in hematology-oncology patients: An expert system combining natural language processing of chest computed tomography reports, microbiology, and antifungal drug data. JCO Clin. Cancer Inf. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, P.D. Breakthrough Invasive Mold Infections in the Hematology Patient: Current Concepts and Future Directions. Clin. Infect. Dis. 2018, 67, 1621–1630. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. S1), e1–e38. [Google Scholar] [CrossRef]
- Liu, M.; Haffari, G.; Buntine, W. Learning cascaded latent variable models for biomedical text classification. Proceedings of the Australasian Language Technology Association Workshop (ALTA), Caulfield, Australia, 5–7 December 2016, 139–143.
- Fleming, S.; Yannakou, C.K.; Haeusler, G.M.; Clark, J.; Grigg, A.; Heath, C.H.; Bajel, A.; van Hal, S.J.; Chen, S.C.; Milliken, S.T.; et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern. Med. J. 2014, 44, 1283–1297. [Google Scholar] [CrossRef]
- Gangneux, J.P.; El Cheikh, J.; Herbrecht, R.; Yakoub-Agha, I.; Quiniou, J.B.; Caillot, D.; Michallet, M. Systemic antifungal prophylaxis in patients hospitalized in hematology units in France: The AFHEM cross-sectional observational study. Infect. Dis. Ther. 2018, 7, 309–325. [Google Scholar] [CrossRef]
- Cornely, O.A.; Leguay, T.; Maertens, J.; Vehreschild, M.J.; Anagnostopoulos, A.; Castagnola, C.; Verga, L.; Rieger, C.; Kondakci, M.; Härter, G.; et al. Randomized comparison of liposomal amphotericin B versus placebo to prevent invasive mycoses in acute lymphoblastic leukaemia. J. Antimicrob. Chemother. 2017, 72, 2359–2367. [Google Scholar] [CrossRef] [Green Version]
- Ananda-Rajah, M.R.; Grigg, A.; Slavin, A.M. Making sense of posaconazole therapeutic drug monitoring: A practical approach. Curr. Opin. Infect. Dis. 2012, 25, 605–611. [Google Scholar] [CrossRef]
- Topol, E.J. High–performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Valerio, M.; Munoz, P.; Rodriguez, C.G.; Caliz, B.; Padilla, B.; Fernandez-Cruz, A.; Sánchez-Somolinos, M.; Gijón, P.; Peral, J.; Gayoso, J.; et al. Antifungal stewardship in a tertiary–care institution: A bedside intervention. Clin. Microbiol. Infect. 2015, 21, 492.e1–492.e9. [Google Scholar] [CrossRef]
| Characteristics | N (%) |
|---|---|
| Number of patients | 185 |
| Age, years | |
| Median [IQR] | 58 (44–67) |
| Elderly (≥65 years) | 56 (30) |
| Male gender | 125 (68) |
| Number of IMD episodes | 205 |
| Outpatients | 14 (6.8) |
| Length of hospitalization, days | |
| Median [IQR] | 29 (15–43) |
| Underlying hematologic disease, n = 138 non-HSCT episodes | |
| Acute myeloid leukemia | 93 (67) |
| Acute lymphoblastic leukemia | 17 (12) |
| Non-Hodgkin lymphoma | 10 (7.3) |
| Myelodysplastic syndrome | 5 (3.6) |
| Multiple myeloma | 3 (2.2) |
| Chronic lymphocytic leukemia | 3 (2.2) |
| Hodgkin lymphoma | 2 (1.5) |
| Acute promyelocytic leukemia | 1 (0.8) |
| Other a | 4 (2.9) |
| HSCT recipients | 67 (33) |
| Type of HSCT | |
| Autologous | 15 (22) |
| Allogeneic | 52 (78) |
| Matched related | 35 (52) |
| Unrelated | 16 (24) |
| Mismatched | 1 (1.5) |
| Early post allo-HSCT (≤100 days) | 18 (35%) |
| Late post allo-HSCT (>100 days) | 34 (65%) |
| Stem cell source in allogeneic-HSCT recipients, n = 52 episodes | |
| Peripheral blood | 48 (94) |
| Cord blood | 2 (3.9) |
| Marrow | 1 (2.0) |
| Status of hematological disease, n = 203 episodes b | |
| Newly diagnosed or receiving first induction therapy | 49 (24) |
| Complete remission | 55 (27) |
| Active hematologic disease (partial remission, progression, relapsed/refractory) | 99 (49) |
| Enrolled in clinical trial | 42 (20) |
| Neutropenia ≤0.5 × 109/L lasting ≥10 days in 30 days prior to IMD diagnosis | 108 (53) |
| Duration of neutropenia ≤0.5 × 109/L, median [IQR] | 22 (15–37) |
| IMD Characteristics, n = 205 Episodes | N (%) |
|---|---|
| EORTC/MSG classification | |
| Proven/probable | 90 (44) |
| Possible | 115 (56) |
| Site of infection | |
| Localized | 190 (93) |
| Disseminated | 15 (7.3) |
| Site of involvement | |
| Lung only | 198 (97) |
| Sinus | 5 (2.4) |
| Bloodstream | 4 (2.0) |
| Liver | 2 (1.0) |
| Spleen | 2 (1.0) |
| CNS | 2 (1.0) |
| Other a | 4 (2.0) |
| Organism in probable/proven IMD episodes, n = 90 episodes b | |
| Aspergillus species | 66 (73) |
| Aspergillus fumigatus | 20 (30) |
| Non-fumigatus Aspergillus species | 9 (14) |
| Aspergillus species not otherwise specified | 25 (38) |
| Positive galactomannan from BAL | 24 (27) |
| Non-aspergillus molds | 31 (34) |
| Mucorales | 16 (18) |
| Lomentospora prolificans | 5 (5.6) |
| Other rare molds c | 10 (11) |
| Mixed fungal infections | 8 (8.9) |
| Aspergillus PCR positive d | 13 (14) |
| Panfungal PCR positive d | 5 (5.6) |
| Possible IMD episodes | 115 |
| Aspergillus PCR positive | 12 (10) |
| Panfungal PCR positive | 5 (4.3) |
| Clinical outcomes | |
| ICU admission | 53 (26) |
| Invasive ventilation | 17 (32) |
| All-cause mortality, n = 130 evaluable episodes | |
| 6 week | 52 (40) |
| 12 week | 68 (52) |
| Characteristics | N (%) |
|---|---|
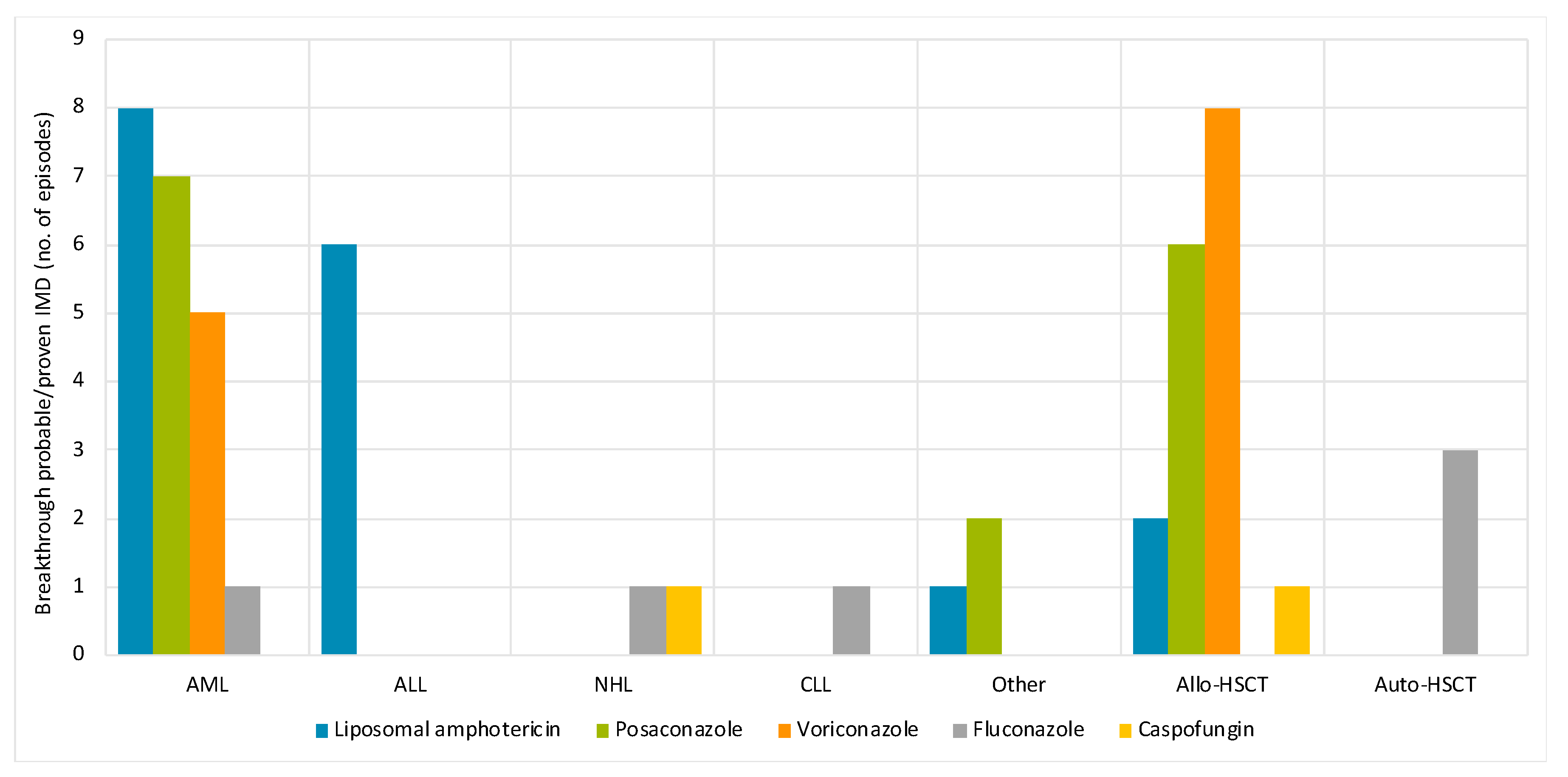
| Breakthrough probable/proven IMD a | 53/88 (60) |
| Posaconazole | 15 (28) |
| Liposomal amphotericin B | 17 (32) |
| Voriconazole | 13 (25) |
| Fluconazole | 6 (11) |
| Caspofungin | 2 (3.8) |
| TDM performed in 2 weeks prior to breakthrough probable/proven IMD diagnosis b | |
| Posaconazole prophylaxis | 8/15 (53) |
| Therapeutic | 5 (63) |
| Voriconazole prophylaxis | 9/13 (69) |
| Therapeutic | 7 (78) |
| Invasive diagnostic tests, (n = 205 episodes) | 170 (83) |
| Fiberoptic bronchoscopy | 123 (60) |
| Lung | 27 (13) |
| Sinus | 8 (3.9) |
| Other site | 9 (4.3) |
| Lung resection | 3 (1.4) |
| Interval from CT to fiberoptic bronchoscopy, n = 117 episodes c | |
| Median time [IQR], days | 2 (1–5) |
| ≤2 days | 63 (54) |
| 3–5 days | 38 (32) |
| >5 days | 16 (14) |
| Time from bronchoalveolar lavage galactomannan request to result, n = 24 episodes, mean (range), days | 12 (7–22) |
| Potential missed opportunities for antifungal prophylaxis in probable/proven IMD episodes d | |
| Early post allogeneic-HSCT (≤100 days) | 3/18 (17) |
| Chronic GVHD e in 60 days prior to IMD diagnosis | 6/13 (46) |
| Neutrophils ≤0.5 × 109/L for >3 weeks and <5 weeks | 9/27 (33) |
| Neutrophils ≤0.5 × 109/L for >5 weeks | 9/28 (32) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baggio, D.; Peel, T.; Peleg, A.Y.; Avery, S.; Prayaga, M.; Foo, M.; Haffari, G.; Liu, M.; Bergmeir, C.; Ananda-Rajah, M. Closing the Gap in Surveillance and Audit of Invasive Mold Diseases for Antifungal Stewardship Using Machine Learning. J. Clin. Med. 2019, 8, 1390. https://doi.org/10.3390/jcm8091390
Baggio D, Peel T, Peleg AY, Avery S, Prayaga M, Foo M, Haffari G, Liu M, Bergmeir C, Ananda-Rajah M. Closing the Gap in Surveillance and Audit of Invasive Mold Diseases for Antifungal Stewardship Using Machine Learning. Journal of Clinical Medicine. 2019; 8(9):1390. https://doi.org/10.3390/jcm8091390
Chicago/Turabian StyleBaggio, Diva, Trisha Peel, Anton Y. Peleg, Sharon Avery, Madhurima Prayaga, Michelle Foo, Gholamreza Haffari, Ming Liu, Christoph Bergmeir, and Michelle Ananda-Rajah. 2019. "Closing the Gap in Surveillance and Audit of Invasive Mold Diseases for Antifungal Stewardship Using Machine Learning" Journal of Clinical Medicine 8, no. 9: 1390. https://doi.org/10.3390/jcm8091390
APA StyleBaggio, D., Peel, T., Peleg, A. Y., Avery, S., Prayaga, M., Foo, M., Haffari, G., Liu, M., Bergmeir, C., & Ananda-Rajah, M. (2019). Closing the Gap in Surveillance and Audit of Invasive Mold Diseases for Antifungal Stewardship Using Machine Learning. Journal of Clinical Medicine, 8(9), 1390. https://doi.org/10.3390/jcm8091390





