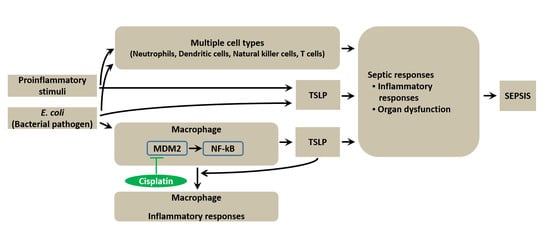
TSLP Exacerbates Septic Inflammation via Murine Double Minute 2 (MDM2) Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Human
2.2. Mice
2.3. Cell Culture
2.4. Assays for Biochemical Markers of Organ Dysfunction and Systemic Inflammation
2.5. PCR
2.6. Transfection with siRNA
2.7. Immunoblot Analysis
2.8. Nuclear Extract
2.9. MTT Assay
2.10. HIF-1α Luciferase Assay
2.11. Statistics
3. Results
3.1. TSLP Is Associated with Sepsis
3.2. Systemic Inflammatory Reaction Is Blunted in the Absence of TSLP during Sepsis
3.3. TSLP Causes Systemic Inflammatory Reaction and Organ Dysfunction in Septic Mice
3.4. TSLP Production Is Mediated by NF-κB and HIF-1α in Macrophages
3.5. TSLP Is Produced via MDM2 Signaling in Macrophages
3.6. Nutlin-3a, an MDM2 Inhibitor, Regulates Inflammatory Responses during Sepsis
3.7. TSLP Upregulates Macrophages-Mediated Inflammatory Responses during Sepsis
3.8. Pharmacological Inhibition of TSLP by Cisplatin Protects Mice against Lethal Sepsis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tiru, B.; DiNino, E.K.; Orenstein, A.; Mailloux, P.T.; Pesaturo, A.; Gupta, A.; McGee, W.T. The Economic and humanistic burden of severe sepsis. Pharmacoeconomics 2015, 33, 925–937. [Google Scholar] [CrossRef]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Shalova, I.N.; Lim, J.Y.; Chittezhath, M.; Zinkernagel, A.S.; Beasley, F.; Hernández-Jiménez, E.; Toledano, V.; Cubillos-Zapata, C.; Rapisarda, A.; Chen, J.; et al. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity 2015, 42, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Scott, M.J.; Loughran, P.; Gibson, G.; Sodhi, C.; Watkins, S.; Hackam, D.; Billiar, T.R. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. J. Immunol. 2013, 190, 5152–5160. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, K.; Peelman, F.; Braun, H.; Lopez, J.; Van Rompaey, D.; Dansercoer, A.; Vandenberghe, I.; Pauwels, K.; Tavernier, J.; Lambrecht, B.N.; et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat. Commun. 2017, 8, 14937. [Google Scholar] [CrossRef] [PubMed]
- Kugelberg, E. Infection: TSLP complements neutrophil killing of bacteria. Nat. Rev. Immunol. 2017, 17, 4–5. [Google Scholar] [CrossRef]
- Borriello, F.; Iannone, R.; Di Somma, S.; Vastolo, V.; Petrosino, G.; Visconte, F.; Raia, M.; Scalia, G.; Loffredo, S.; Varricchi, G.; et al. Lipopolysaccharide-elicited TSLPR expression enriches a functionally discrete subset of human CD14+ CD1c+ Monocytes. J. Immunol. 2017, 198, 3426–3435. [Google Scholar] [CrossRef] [PubMed]
- Kuethe, J.W.; Prakash, P.S.; Midura, E.F.; Johnson, B.L., 3rd; Kasten, K.R.; Caldwell, C.C. Thymic stromal lymphopoietin mediates the host response and increases mortality during sepsis. J. Surg. Res. 2014, 191, 19–24. [Google Scholar] [CrossRef]
- Lee, H.C.; Sung, S.S.; Krueger, P.D.; Jo, Y.A.; Rosen, H.R.; Ziegler, S.F.; Hahn, Y.S. Hepatitis C virus promotes T-helper (Th)17 responses through thymic stromal lymphopoietin production by infected hepatocytes. Hepatology 2013, 57, 1314–1324. [Google Scholar] [CrossRef]
- Piliponsky, A.M.; Lahiri, A.; Truong, P.; Clauson, M.; Shubin, N.J.; Han, H.; Ziegler, S.F. Thymic stromal lymphopoietin improves survival and reduces inflammation in sepsis. Am. J. Respir. Cell Mol. Biol. 2016, 55, 264–274. [Google Scholar] [CrossRef]
- Ebrahim, M.; Mulay, S.R.; Anders, H.J.; Thomasova, D. MDM2 beyond cancer: Podoptosis, development, inflammation, and tissue regeneration. Histol. Histopathol. 2015, 30, 1271–1282. [Google Scholar] [PubMed]
- Gu, L.; Findley, H.W.; Zhou, M. MDM2 induces NF-kappaB/p65 expression transcriptionally through Sp1-binding sites: A novel, p53-independent role of MDM2 in doxorubicin resistance in acute lymphoblastic leukemia. Blood 2002, 99, 3367–3375. [Google Scholar] [CrossRef] [PubMed]
- Thomasova, D.; Mulay, S.R.; Bruns, H.; Anders, H.J. p53-independent roles of MDM2 in NF-κB signaling: Implications for cancer therapy, wound healing, and autoimmune diseases. Neoplasia 2012, 14, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Oh, H.A.; Nam, S.Y.; Moon, P.D.; Kim, D.W.; Kim, H.M.; Jeong, H.J. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. J. Invest. Dermatol. 2014, 134, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.M.; Gao, D.; Trahan, D.N.; Johnson, B.A.; Ludwig, A.; Barbieri, E.; Chen, Z.; Diaz-Miron, J.; Vassilev, L.; Shohet, J.M.; et al. Effect of MDM2 and vascular endothelial growth factor inhibition on tumor angiogenesis and metastasis in neuroblastoma. Angiogenesis 2011, 14, 255–266. [Google Scholar] [CrossRef]
- Frede, S.; Stockmann, C.; Freitag, P.; Fandrey, J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem. J. 2006, 396, 517–527. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, X.; Jiang, X.; Wang, Y.; Miao, Z.; He, W.; Yang, G.; Lv, Z.; Yu, Y.; Zheng, Y. Micheliolide inhibits LPS-induced inflammatory response and protects mice from LPS challenge. Sci. Rep. 2016, 6, 23240. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.Q.; Xu, W.H.; Li, Y.H.; Qi, Y.; Wu, H.Y.; Li, J.Z.; He, Z.G.; Hu, H.G.; Wang, Y.; et al. The matrine derivate MASM prolongs survival, attenuates inflammation, and reduces organ injury in murine established lethal sepsis. J. Infect. Dis. 2016, 214, 1762–1772. [Google Scholar] [CrossRef]
- Li, L.; Ng, D.S.; Mah, W.C.; Almeida, F.F.; Rahmat, S.A.; Rao, V.K.; Leow, S.C.; Laudisi, F.; Peh, M.T.; Goh, A.M.; et al. A unique role for p53 in the regulation of M2 macrophage polarization. Cell Death Differ. 2015, 22, 1081–1093. [Google Scholar] [CrossRef]
- Guo, Y.; Luan, L.; Patil, N.K.; Wang, J.; Bohannon, J.K.; Rabacal, W.; Fensterheim, B.A.; Hernandez, A.; Sherwood, E.R. IL-15 enables septic shock by maintaining NK cell integrity and function. J. Immunol. 2017, 198, 1320–1333. [Google Scholar] [CrossRef]
- Kouser, L.; Paudyal, B.; Kaur, A.; Stenbeck, G.; Jones, L.A.; Abozaid, S.M.; Stover, C.M.; Flahaut, E.; Sim, R.B.; Kishore, U. Human properdin opsonizes nanoparticles and triggers a potent pro-inflammatory response by macrophages without involving complement activation. Front. Immunol. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, L.; Morin, M.D.; Jones, B.T.; Mifune, Y.; Shi, H.; Wang, K.W.; Zhan, X.; Liu, A.; Wang, J.; et al. Adjuvant effect of the novel TLR1/TLR2 agonist Diprovocim synergizes with anti-PD-L1 to eliminate melanoma in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E8698–E8706. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Shah, H.P.; Malu, K.; Berliner, N.; Gaines, P. Differentiation and characterization of myeloid cells. Curr. Protoc. Immunol. 2014, 104. [Google Scholar] [CrossRef]
- Tasaki, M.; Shimada, K.; Kimura, H.; Tsujikawa, K.; Konishi, N. ALKBH3, a human AlkB homologue, contributes to cell survival in human non-small-cell lung cancer. Br. J. Cancer 2011, 104, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, F.; Minakuchi, Y.; Nagahara, S.; Honma, K.; Sasaki, H.; Hirai, K.; Teratani, T.; Namatame, N.; Yamamoto, Y.; Hanai, K.; et al. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 12177–12182. [Google Scholar] [CrossRef]
- Moore, C.C.; Martin, E.N.; Lee, G.; Taylor, C.; Dondero, R.; Reznikov, L.L.; Dinarello, C.; Thompson, J.; Scheld, W.M. Eukaryotic translation initiation factor 5A small interference RNA-liposome complexes reduce inflammation and increase survival in murine models of severe sepsis and acute lung injury. J. Infect. Dis. 2008, 198, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.M.; Oh, S.H.; Bang, H.S.; Kang, N.I.; Cho, B.H.; Im, S.Y.; Lee, H.K. Glutamine protects mice from lethal endotoxic shock via a rapid induction of MAPK phosphatase-1. J. Immunol. 2009, 182, 7957–7962. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hou, J.; Zhou, Y.; Li, Z.; Cao, X. The RNA helicase DDX46 inhibits innate immunity by entrapping m6A-demethylated antiviral transcripts in the nucleus. Nat. Immunol. 2017, 18, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, H.H.; Freysdottir, J.; Hardardottir, I. Dietary fish oil decreases the proportion of classical monocytes in blood in healthy mice but increases their proportion upon induction of inflammation. J. Nutr. 2012, 142, 803–808. [Google Scholar] [CrossRef]
- Bhargava, R.; Altmann, C.J.; Andres-Hernando, A.; Webb, R.G.; Okamura, K.; Yang, Y.; Falk, S.; Schmidt, E.P.; Faubel, S. Acute lung injury and acute kidney injury are established by four hours in experimental sepsis and are improved with pre, but not post, sepsis administration of TNF-α antibodies. PLoS ONE 2013, 8, e79037. [Google Scholar] [CrossRef]
- Teeling, J.L.; Cunningham, C.; Newman, T.A.; Perry, V.H. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: Implications for a role of COX-1. Brain Behav. Immun. 2010, 24, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Faix, J.D. Biomarkers of sepsis. Crit. Rev. Clin. Lab. Sci. 2013, 50, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Riddy, D.M.; Goy, E.; Delerive, P.; Summers, R.J.; Sexton, P.M.; Langmead, C.J. Comparative genotypic and phenotypic analysis of human peripheral blood monocytes and surrogate monocyte-like cell lines commonly used in metabolic disease research. PLoS ONE 2018, 13, e0197177. [Google Scholar] [CrossRef] [PubMed]
- Chinetti, G.; Lestavel, S.; Bocher, V.; Remaley, A.T.; Neve, B.; Torra, I.P.; Teissier, E.; Minnich, A.; Jaye, M.; Duverger, N.; et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 2001, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.H.; Angelin-Duclos, C.; Calame, K. BLIMP-1: Trigger for differentiation of myeloid lineage. Nat. Immunol. 2000, 1, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Iskander, K.N.; Osuchowski, M.F.; Stearns-Kurosawa, D.J.; Kurosawa, S.; Stepien, D.; Valentine, C.; Remick, D.G. Sepsis: Multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol. Rev. 2013, 93, 1247–1288. [Google Scholar] [CrossRef] [PubMed]
- Carcillo, J.A.; Podd, B.; Aneja, R.; Weiss, S.L.; Hall, M.W.; Cornell, T.T.; Shanley, T.P.; Doughty, L.A.; Nguyen, T.C. Pathophysiology of pediatric multiple organ dysfunction syndrome. Pediatr. Crit. Care Med. 2017, 18, S32–S45. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Wang, W.; Guo, D.; Huang, S.; Jie, S. The clinical characteristics and outcomes of patients with human granulocytic anaplasmosis in China. Int. J. Infect. Dis. 2011, 15, e859–e866. [Google Scholar] [CrossRef][Green Version]
- Frink, M.; van Griensven, M.; Kobbe, P.; Brin, T.; Zeckey, C.; Vaske, B.; Krettek, C.; Hildebrand, F. IL-6 predicts organ dysfunction and mortality in patients with multiple injuries. Scand. J. Trauma Resusc. Emerg. Med. 2009, 17, 49. [Google Scholar] [CrossRef]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.M.; Karin, M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007, 317, 121–124. [Google Scholar] [CrossRef]
- Vernet, M.A.; Reynard, S.; Fizet, A.; Schaeffer, J.; Pannetier, D.; Guedj, J.; Rives, M.; Georges, N.; Garcia-Bonnet, N.; Sylla, A.I.; et al. Clinical, virological, and biological parameters associated with outcomes of Ebola virus infection in Macenta, Guinea. JCI Insight 2017, 2, e88864. [Google Scholar] [CrossRef] [PubMed]
- Ashrin, M.N.; Arakaki, R.; Yamada, A.; Kondo, T.; Kurosawa, M.; Kudo, Y.; Watanabe, M.; Ichikawa, T.; Hayashi, Y.; Ishimaru, N. A critical role for thymic stromal lymphopoietin in nickel-induced allergy in mice. J. Immunol. 2014, 192, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Al-Shami, A.; Spolski, R.; Kelly, J.; Keane-Myers, A.; Leonard, W.J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005, 202, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P. Animal models of sepsis. Virulence 2014, 5, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ye, X.; Steinberg, H.; Liu, S.F. Selective blockade of endothelial NF-kappaB pathway differentially affects systemic inflammation and multiple organ dysfunction and injury in septic mice. J. Pathol. 2010, 220, 490–498. [Google Scholar] [PubMed]
- Eskandari, M.K.; Bolgos, G.; Miller, C.; Nguyen, D.T.; DeForge, L.E.; Remick, D.G. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J. Immunol. 1992, 148, 2724–2730. [Google Scholar] [PubMed]
- Remick, D.; Manohar, P.; Bolgos, G.; Rodriguez, J.; Moldawer, L.; Wollenberg, G. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock 1995, 4, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Kim, H.M. Thymic stromal lymphopoietin is expressed and produced by caspase-1/NF-κB pathway in mast cells. Cytokine 2011, 54, 239–243. [Google Scholar] [CrossRef]
- Jang, Y.; Jeong, S.H.; Park, Y.H.; Bae, H.C.; Lee, H.; Ryu, W.I.; Park, G.H.; Son, S.W. UVB induces HIF-1α-dependent TSLP expression via the JNK and ERK pathways. J. Invest. Dermatol. 2013, 133, 2601–2608. [Google Scholar] [CrossRef]
- Abraham, E. Nuclear factor-kappaB and its role in sepsis-associated organ failure. J. Infect. Dis. 2003, 187, S364–S369. [Google Scholar] [CrossRef]
- Peyssonnaux, C.; Cejudo-Martin, P.; Doedens, A.; Zinkernagel, A.S.; Johnson, R.S.; Nizet, V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J. Immunol. 2007, 178, 7516–7519. [Google Scholar] [CrossRef] [PubMed]
- Barabutis, N.; Dimitropoulou, C.; Birmpas, C.; Joshi, A.; Thangjam, G.; Catravas, J.D. p53 protects against LPS-induced lung endothelial barrier dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L776–L787. [Google Scholar] [CrossRef] [PubMed]
- Odkhuu, E.; Mendjargal, A.; Koide, N.; Naiki, Y.; Komatsu, T.; Yokochi, T. Lipopolysaccharide downregulates the expression of p53 through activation of MDM2 and enhances activation of nuclear factor-kappa B. Immunobiology 2015, 220, 136–141. [Google Scholar] [CrossRef]
- Ishikawa, M.; Takayanagi, Y.; Sasaki, K. Drug interaction effects on antitumour drugs (X): exacerbation of cisplatin lethality by bacterial lipopolysaccharide in mice. Pharmacol. Toxicol. 1991, 68, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Ma, X.; Shao, B.; Gao, X.; Zhang, B.; Xu, G.; Wei, Y. Low-dose cisplatin administration to septic mice improves bacterial clearance and programs peritoneal macrophage polarization to M1 phenotype. Pathog. Dis. 2014, 72, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Cardinal, J.; Dhupar, R.; Rosengart, M.R.; Lotze, M.T.; Geller, D.A.; Billiar, T.R.; Tsung, A. Low-dose cisplatin administration in murine cecal ligation and puncture prevents the systemic release of HMGB1 and attenuates lethality. J. Leukoc. Biol. 2009, 86, 625–632. [Google Scholar] [CrossRef]
- Figueiredo, N.; Chora, A.; Raquel, H.; Pejanovic, N.; Pereira, P.; Hartleben, B.; Neves-Costa, A.; Moita, C.; Pedroso, D.; Pinto, A.; et al. Anthracyclines induce DNA damage response-mediated protection against severe sepsis. Immunity 2013, 39, 874–884. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, N.-R.; Moon, P.-D.; Kim, H.-M.; Jeong, H.-J. TSLP Exacerbates Septic Inflammation via Murine Double Minute 2 (MDM2) Signaling Pathway. J. Clin. Med. 2019, 8, 1350. https://doi.org/10.3390/jcm8091350
Han N-R, Moon P-D, Kim H-M, Jeong H-J. TSLP Exacerbates Septic Inflammation via Murine Double Minute 2 (MDM2) Signaling Pathway. Journal of Clinical Medicine. 2019; 8(9):1350. https://doi.org/10.3390/jcm8091350
Chicago/Turabian StyleHan, Na-Ra, Phil-Dong Moon, Hyung-Min Kim, and Hyun-Ja Jeong. 2019. "TSLP Exacerbates Septic Inflammation via Murine Double Minute 2 (MDM2) Signaling Pathway" Journal of Clinical Medicine 8, no. 9: 1350. https://doi.org/10.3390/jcm8091350
APA StyleHan, N.-R., Moon, P.-D., Kim, H.-M., & Jeong, H.-J. (2019). TSLP Exacerbates Septic Inflammation via Murine Double Minute 2 (MDM2) Signaling Pathway. Journal of Clinical Medicine, 8(9), 1350. https://doi.org/10.3390/jcm8091350