In Vivo Endomicroscopy of Lung Injury and Repair in ARDS: Potential Added Value to Current Imaging
Abstract
:1. Introduction
2. Pathophysiological Mechanisms of Lung Repair in ARDS
3. Evaluation of Lung Repair in ARDS: Clinical Settings
4. Recently Developed Imaging Modalities Clinically Available: Moving Towards the Cellular/Molecular Level?
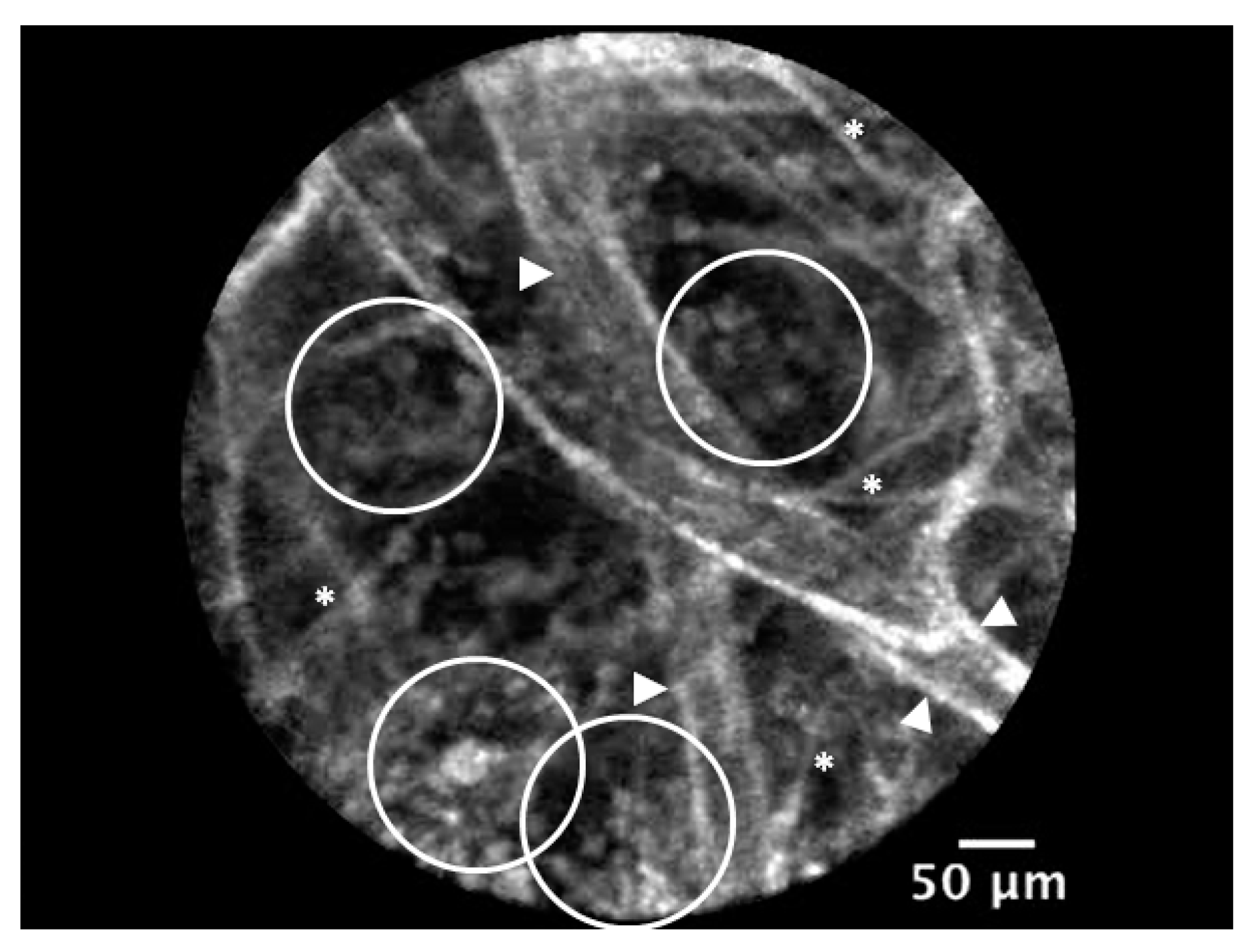
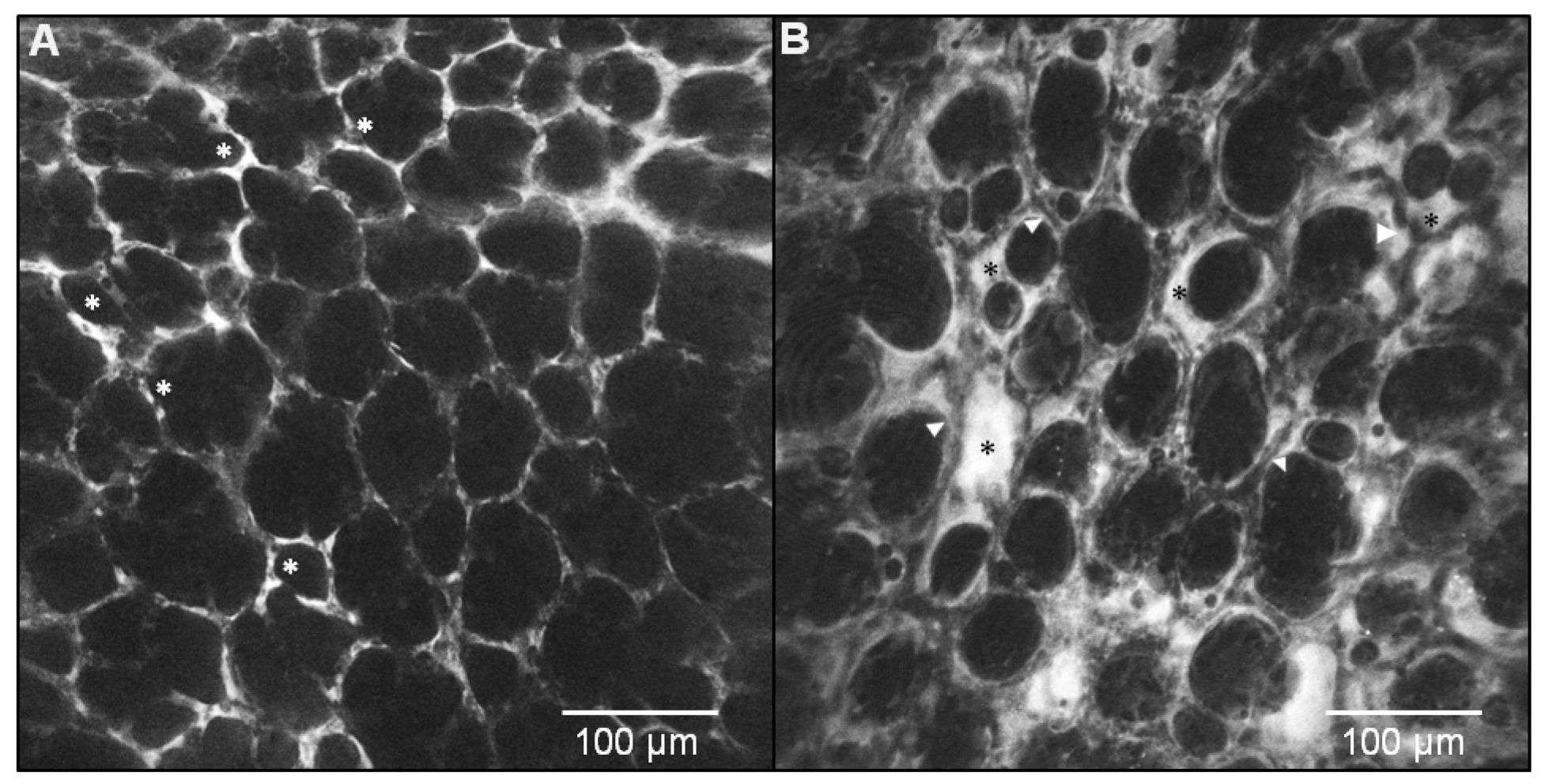
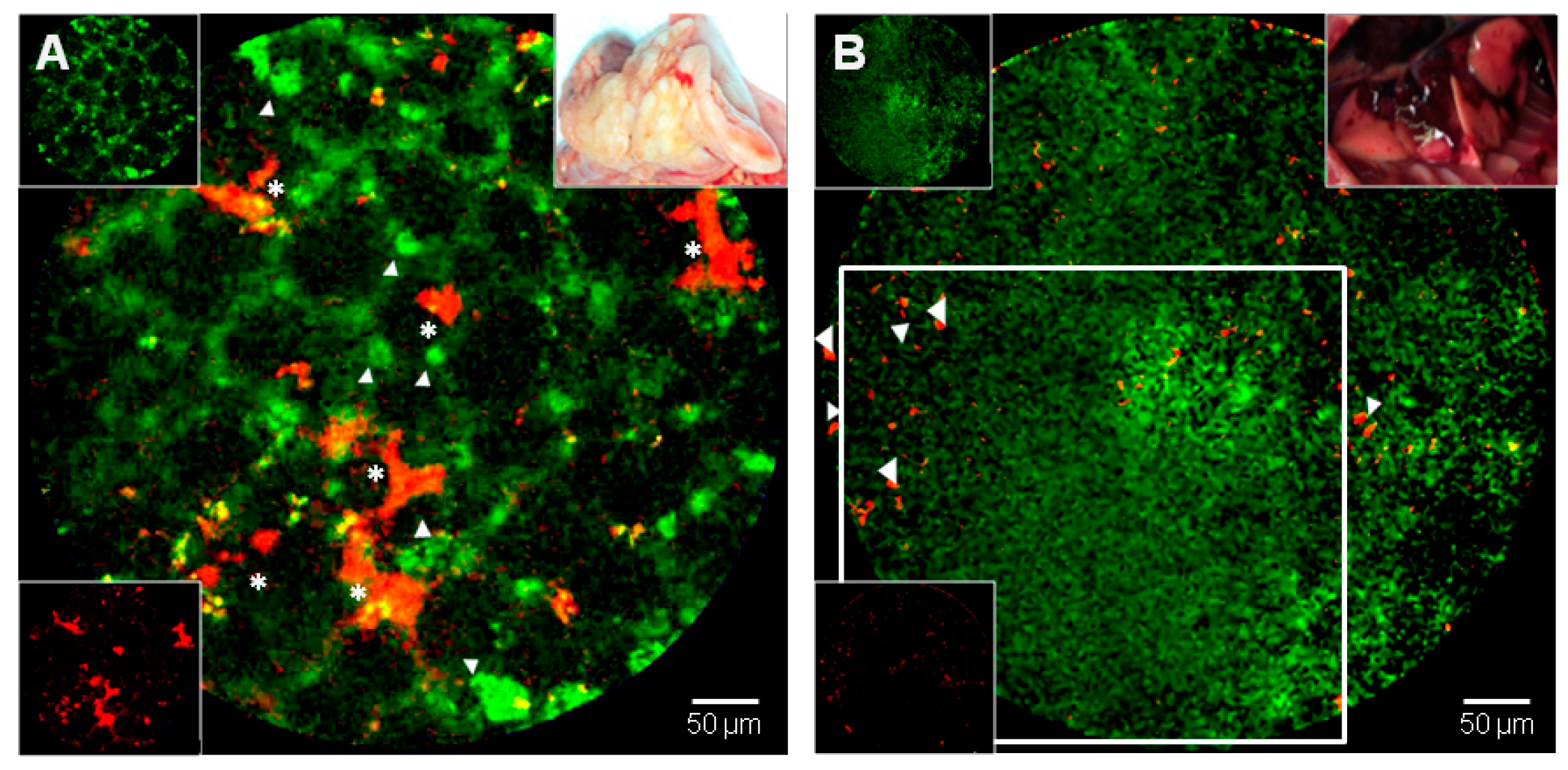
5. New Precision Imaging Modalities for ARDS: In Vivo Endomicroscopy and Targeted Imaging
6. Advantages, Limitations, and Future Directions for in Vivo Microimaging
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Englert, J.A.; Bobba, C.; Baron, R.M. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome. JCI Insight 2019, 4, 124061. [Google Scholar] [CrossRef] [PubMed]
- Cereda, M.; Xin, Y.; Goffi, A.; Herrmann, J.; Kaczka, D.W.; Kavanagh, B.P.; Perchiazzi, G.; Yoshida, T.; Rizi, R.R. Imaging the Injured Lung: Mechanisms of Action and Clinical Use. Anesthesiology 2019. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Vercesi, V.; Costantino, F.; Chandrapatham, K.; Pelosi, P. Lung imaging: How to get better look inside the lung. Ann. Transl. Med. 2017, 5, 294. [Google Scholar] [CrossRef] [PubMed]
- Zompatori, M.; Ciccarese, F.; Fasano, L. Overview of current lung imaging in acute respiratory distress syndrome. Eur. Respir. Rev. 2014, 23, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Rubinowitz, A.N.; Siegel, M.D.; Tocino, I. Thoracic imaging in the ICU. Crit. Care Clin. 2007, 23, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.R.; Horner, P.E.; Primack, S.L. ICU imaging. Clin. Chest Med. 2008, 29, 59–76. [Google Scholar] [CrossRef]
- Brown, R.H.; Irvin, C.G.; Allen, G.B., 3rd; Shapiro, S.D.; Martin, W.J.; Kolb, M.R.; Hyde, D.M.; Nieman, G.F.; Cody, D.D.; Ishii, M.; et al. ATS Small Animal Imaging Subcommittee. An official ATS conference proceedings: Advances in small-animal imaging application to lung pathophysiology. Proc. Am. Thorac. Soc. 2008, 5, 591–600. [Google Scholar] [CrossRef]
- Ingbar, D.H. Mechanisms of repair and remodeling following acute lung injury. Clin. Chest Med. 2000, 21, 589–616. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Taguchi, T. Pulmonary fibrosis: Cellular and molecular events. Pathol. Int. 2003, 53, 133–145. [Google Scholar] [CrossRef]
- Shimabukuro, D.W.; Sawa, T.; Gropper, M.A. Injury and repair in lung and airways. Crit. Care Med. 2003, 31 (Suppl. 8), S524–S531. [Google Scholar] [CrossRef]
- Berthiaume, Y.; Lesur, O.; Dagenais, A. Treatment of adult respiratory distress syndrome: Plea for rescue therapy of the alveolar epithelium. Thorax 1999, 54, 150–160. [Google Scholar] [CrossRef] [PubMed]
- González-López, A.; Albaiceta, G.M. Repair after acute lung injury: Molecular mechanisms and therapeutic opportunities. Crit. Care Lond. 2012, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.Y. ARDS and diffuse alveolar damage: A pathologist’s perspective. Semin. Thorac. Cardiovasc. Surg. 2006, 18, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Cardinal-Fernández, P.; Lorente, J.A.; Ballén-Barragán, A.; Matute-Bello, G. Acute Respiratory Distress Syndrome and Diffuse Alveolar Damage. New Insights on a Complex Relationship. Ann. Am. Thorac. Soc. 2017, 14, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Cardinal-Fernandez, P.; Ortiz, G.; Chang, C.H.; Kao, K.C.; Bertreau, E.; Philipponnet, C.; Casero-Alonso, V.M.; Souweine, B.; Charbonney, E.; Guérin, C. Predicting the Impact of Diffuse Alveolar Damage through Open Lung Biopsy in Acute Respiratory Distress Syndrome-The PREDATOR Study. J. Clin. Med. 2019, 8, 829. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A. NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]
- Uhal, B.D. Apoptosis in lung fibrosis and repair. Chest 2002, 122 (Suppl. 6), 293S–298S. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Matthay, M.A. Regulation and Repair of the Alveolar-Capillary Barrier in Acute Lung Injury. Annu. Rev. Physiol. 2013, 75, 593–615. [Google Scholar] [CrossRef] [Green Version]
- Potey, P.M.; Rossi, A.G.; Lucas, C.D.; Dorward, D.A. Neutrophils in the initiation and resolution of acute pulmonary inflammation: Understanding biological function and therapeutic potential. J. Pathol. 2019, 247, 672–685. [Google Scholar] [CrossRef]
- Morrell, E.D.; Bhatraju, P.K.; Mikacenic, C.R.; Radella, F., II; Manicone, A.M.; Stapleton, R.D.; Wurfel, M.M.; Gharib, S.A. Alveolar Macrophage Transcriptional Programs are Associated with Outcomes in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2019. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.P.; Bellingan, G.; Webb, S.; Puddicombe, A.; Goldsack, N.; McAnulty, R.J.; Laurent, G.J. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am. J. Respir. Crit. Care Med. 2000, 162, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Gerard, L.; Bidoul, T.; Castanares-Zapatero, D.; Wittebole, X.; Lacroix, V.; Froidure, A.; Hoton, D.; Laterre, P.-F. Open Lung Biopsy in Nonresolving Acute Respiratory Distress Syndrome Commonly Identifies Corticosteroid-Sensitive Pathologies, Associated with Better Outcome. Crit. Care Med. 2018, 46, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Philipponnet, C.; Cassagnes, L.; Pereira, B.; Kemeny, J.L.; Devouassoux-Shisheboran, M.; Lautrette, A.; Guerin, C.; Souweine, B. Diagnostic yield and therapeutic impact of open lung biopsy in the critically ill patient. PLoS ONE 2018, 13, e0196795. [Google Scholar] [CrossRef] [PubMed]
- Libby, L.J.; Gelbman, B.D.; Altorki, N.K.; Christos, P.J.; Libby, D.M. Surgical Lung Biopsy in Adult Respiratory Distress Syndrome: A Meta-Analysis. Ann. Thorac. Surg. 2014, 98, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.J.; Kluge, S.; Balke, L.; Yekebas, E.; Izbicki, J.R.; Amthor, M.; Kreymann, G.; Meyer, A. Yield and safety of bedside open lung biopsy in mechanically ventilated patients with acute lung injury or acute respiratory distress syndrome. Surgery 2008, 143, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.C.; Tsai, Y.H.; Wu, Y.K.; Chen, N.H.; Hsieh, M.J.; Huang, S.F.; Huang, C.C. Open lung biopsy in early-stage acute respiratory distress syndrome. Crit. Care Lond. 2006, 10, R106. [Google Scholar] [CrossRef]
- Papazian, L.; Doddoli, C.; Chetaille, B.; Gernez, Y.; Thirion, X.; Roch, A.; Donati, Y.; Bonnety, M.; Zandotti, C.; Thomas, P. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit. Care Med. 2007, 35, 755–762. [Google Scholar] [CrossRef] [Green Version]
- The ARDS Definition Task Force Acute Respiratory Distress Syndrome. The Berlin Definition. JAMA 2012, 307, 2526–2533.
- Van Beek, E.J.; Hoffman, E.A. Functional imaging: CT and MRI. Clin. Chest Med. 2008, 29, 195–216. [Google Scholar] [CrossRef]
- Meduri, G.U.; Kohler, G.; Headley, S.; Tolley, E.; Stentz, F.; Postlethwaite, A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest 1995, 108, 1303–1314. [Google Scholar] [CrossRef]
- Hisanaga, J.; Ichikado, K.; Kawamura, K.; Yoshioka, M. The significance of bronchoalveolar or bronchial lavage in ARDS: Validation in 180 patients. Eur. Respir. J. 2015, 46, PA823. [Google Scholar]
- Steinberg, K.P.; Mitchell, D.R.; Maunder, R.J.; Milberg, J.A.; Whitcomb, M.E.; Hudson, L.D. Safety of bronchoalveolar lavage in patients with adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1993, 148, 556–561. [Google Scholar] [CrossRef]
- Meduri, G.U.; Reddy, R.C.; Stanley, T.; El-Zeky, F. Pneumonia in acute respiratory distress syndrome. A prospective evaluation of bilateral bronchoscopic sampling. Am. J. Respir. Crit. Care Med. 1998, 158, 870–875. [Google Scholar] [CrossRef]
- Obadina, E.T.; Torrealba, J.M.; Kanne, J.P. Acute pulmonary injury: High-resolution CT and histopathological spectrum. Br. J. Radiol. 2013, 86, 20120614. [Google Scholar] [CrossRef]
- Eichinger, M.; Tetzlaff, R.; Puderbach, M.; Woodhouse, N.; Kauczor, H.U. Proton magnetic resonance imaging for assessment of lung function and respiratory dynamics. Eur. J. Radiol. 2007, 64, 329–334. [Google Scholar] [CrossRef]
- Hopkins, S.R.; Levin, D.L.; Emami, K.; Kadlecek, S.; Yu, J.; Ishii, M.; Rizi, R.R. Advances in magnetic resonance imaging of lung physiology. J. Appl. Physiol. 2007, 102, 1244–1254. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Cereda, M.; Kadlecek, S.; Emamai, K.; Hamedani, H.; Duncan, I.; Rajaei, J.; Hugues, L.; Meeder, N.; Naji, J.; et al. Hyperpolarized gas diffusion MRI of biphasic lung inflation in short- and long-term emphysema models. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L305–L312. [Google Scholar] [CrossRef]
- Harris, R.S.; Schuster, D.P. Visualizing lung function with positron emission tomography. J. Appl. Physiol. 2007, 102, 448–458. [Google Scholar] [CrossRef]
- Montesi, S.B.; Désogère, P.; Fuchs, B.C.; Caravan, P. Molecular imaging of fibrosis: Recent advances and future directions. J. Clin. Investig. 2019, 129, 24–33. [Google Scholar] [CrossRef]
- Chaari, A.; Bousselmi, K.; Assar, W.; Kumar, V.; Khalil, E.; Kauts, V.; Abdelhakim, K. Usefulness of ultrasound in the management of acute respiratory distress syndrome. Int. J. Crit. Illn. Inj. Sci. 2019, 9, 11–15. [Google Scholar] [CrossRef]
- Ma, H.; Huang, D.; Guo, L.; Chen, Q.; Zhong, W.; Geng, Q.; Zhang, M. Strong correlation between lung ultrasound and chest computerized tomography imaging for the detection of acute lung injury/acute respiratory distress syndrome in rats. J. Thorac. Dis. 2016, 8, 1443–1448. [Google Scholar] [CrossRef] [Green Version]
- Gargani, L.; Lionetti, V.; Di Cristofano, C.; Bevilacqua, G.; Recchia, F.A.; Picano, E. Early detection of acute lung injury uncoupled to hypoxemia in pigs using ultrasound lung comets. Crit. Care Med. 2007, 35, 2769–2774. [Google Scholar]
- See, K.C.; Ong, V.; Tan, Y.L.; Sahagun, J.; Taculod, J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: A retrospective observational study. Crit. Care 2018, 22, 203. [Google Scholar] [CrossRef]
- McGregor, H.C.; Short, M.A.; McWilliams, A.; Shaipanich, T.; Ionescu, D.N.; Zhao, J.; Wang, W.; Chen, G.; Lam, S.; Zeng, H. Real-time endoscopic Raman spectroscopy for in vivo early lung cancer detection. J. Biophotonics 2017, 10, 98–110. [Google Scholar] [CrossRef]
- Ding, M.; Chen, Y.; Guan, W.-J.; Zhong, C.-H.; Jiang, M.; Luo, W.-Z.; Chen, X.-B.; Tang, C.-L.; Tang, Y.; Jian, Q.-M.; et al. Measuring Airway Remodeling in Patients with Different COPD Staging Using Endobronchial Optical Coherence Tomography. Chest 2016, 150, 1281–1290. [Google Scholar] [CrossRef]
- Kim, D.Y.; Hwang, K.; Ahn, J.; Seo, Y.-H.; Kim, J.-B.; Lee, S.; Yoon, J.-H.; Kong, E.; Jeong, Y.; Jon, S.; et al. Lissajous Scanning Two-photon Endomicroscope for In vivo Tissue Imaging. Sci. Rep. 2019, 9, 3560. [Google Scholar] [CrossRef] [Green Version]
- Perchant, A.; Le Goualher, G.; Genet, M.; Viellerobe, B.; Berier, F. An integrated fibered confocal microscopy system for in vivo and in situ fluorescence imaging—Applications to endoscopy in small animal imaging, in Biomedical Imaging: Nano to Macro, 2004. IEEE Int. Symp. Biomed. Imaging 2004, 1, 692–695. [Google Scholar]
- Parker, H.E.; Stone, J.M.; Marshall, A.D.L.; Choudhary, T.R.; Thomson, R.R.; Dhaliwal, K.; Tanner, M.G. Fibre-based spectral ratio endomicroscopy for contrast enhancement of bacterial imaging and pulmonary autofluorescence. Biomed. Opt. Express 2019, 10, 1856–1869. [Google Scholar] [CrossRef]
- Thiberville, L.; Moreno-Swirc, S.; Vercauteren, T.; Peltier, E.; Cavé, C.; Bourg Heckly, G. In vivo imaging of the bronchial wall microstructure using fibered confocal fluorescence microscopy. Am. J. Respir. Crit. Care Med. 2007, 175, 22–31. [Google Scholar] [CrossRef]
- Salaün, M.; Guisier, F.; Dominique, S.; Genevois, A.; Jounieaux, V.; Bergto, E.; Thill, C.; Piton, N.; Thiberville, L. In vivo probe-based confocal laser endomicroscopy in chronic interstitial lung diseases: Specific descriptors and correlation with chest CT. Respirology 2019, 24, 783–791. [Google Scholar] [CrossRef]
- Chagnon, F.; Fournier, C.; Charette, P.G.; Moleski, L.; Payet, M.D.; Dobbs, L.G.; Lesur, O. In vivo intravital endoscopic confocal fluorescence microscopy of normal and acutely injured rat lungs. Lab. Investig. 2010, 90, 824–834. [Google Scholar] [CrossRef] [Green Version]
- Jozwiak, M.; Silva, S.; Persichini, R.; Anguel, N.; Osman, D.; Richard, C.; Teboul, J.L.; Monnet, X. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit. Care Med. 2013, 41, 472–480. [Google Scholar] [CrossRef]
- Freise, A.C.; Wu, A.M. In vivo imaging with antibodies and engineered fragments. Mol. Immunol. 2015, 67 Pt 2, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Goetz, M.; Ziebart, A.; Foersch, S.; Vieth, M.; Waldner, M.J.; Delaney, P.; Galle, P.R.; Neurath, M.F.; Kiesslich, R. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology 2010, 138, 435–446. [Google Scholar] [CrossRef]
- Atreya, R.; Neumann, H.; Neufert, C.; Waldner, M.J.; Billmeier, U.; Zopf, Y.; Willma, M.; App, C.; Münster, T.; Kessler, H.; et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat. Med. 2014, 20, 313–318. [Google Scholar] [CrossRef]
- Staderini, M.; Megia-Fernandez, A.; Dhaliwal, K.; Bradley, M. Peptides for optical medical imaging and steps towards therapy. Bioorg. Med. Chem. 2018, 26, 2816–2826. [Google Scholar] [CrossRef]
- Henderson, N.C.; Sheppard, D. Integrin-mediated regulation of TGFβ in fibrosis. Biochim. Biophys. Acta 2013, 1832, 891–896. [Google Scholar] [CrossRef]
- Fiore, V.F.; Wong, S.S.; Tran, C.; Tan, C.; Xu, W.; Sulchek, T.; White, E.S.; Hagood, J.S.; Barker, T.H. αvβ3 Integrin drives fibroblast contraction and strain stiffening of soft provisional matrix during progressive fibrosis. JCI Insight 2018, 3, e97597. [Google Scholar] [CrossRef]
- Bendib, I.; de Chaisemartin, L.; Granger, V.; Schlemmer, F.; Maitre, B.; Hüe, S.; Surenaud, M.; Beldi-Ferchiou, A.; Carteaux, G.; Razazi, K.; et al. Neutrophil Extracellular Traps Are Elevated in Patients with Pneumonia-related Acute Respiratory Distress Syndrome. Anesthesiology 2019, 130, 581–591. [Google Scholar] [CrossRef]
- Mikacenic, C.; Moore, R.; Dmyterko, V.; West, T.E.; Altemeier, W.A.; Liles, W.C.; Lood, C. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit. Care Lond. 2018, 22, 358. [Google Scholar] [CrossRef]
- Garland, M.; Yim, J.J.; Bogyo, M. A Bright Future for Precision Medicine: Advances in Fluorescent Chemical Probe Design and Their Clinical Application. Cell Chem. Biol. 2016, 23, 122–136. [Google Scholar] [CrossRef] [Green Version]
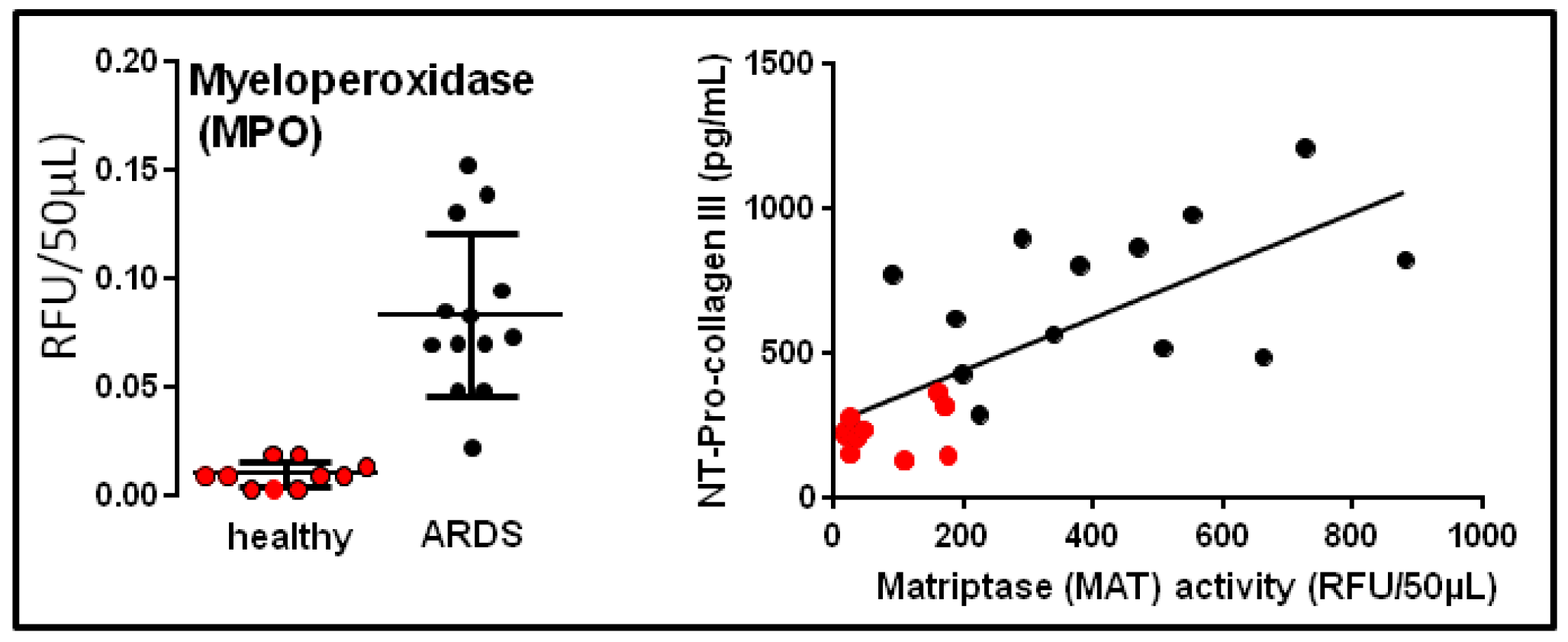
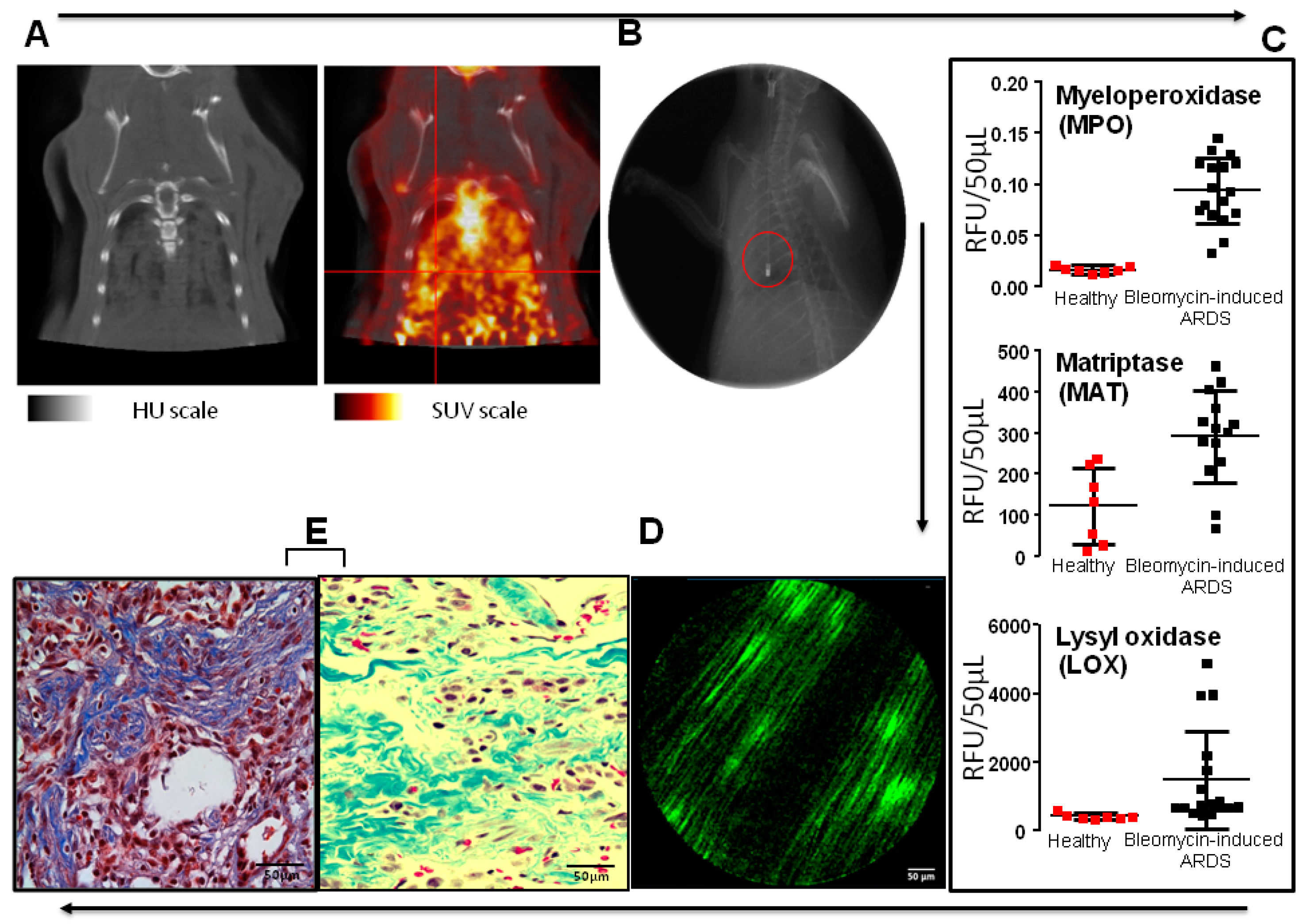
- Chagnon, F.; Bourgouin, A.; Lebel, R.; Bonin, M.A.; Marsault, E.; Lepage, M.; Lesur, O. Smart imaging of acute lung injury: Exploration of myeloperoxidase activity using in vivo endoscopic confocal fluorescence microscopy. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L543–L551. [Google Scholar] [CrossRef]
- Aslam, T.; Miele, A.; Chankeshwara, S.V.; Megia-Fernandez, A.; Michels, C.; Akram, A.R.; McDonald, N.; Hirani, N.; Haslett, C.; Bradley, M.; et al. Optical molecular imaging of lysyl oxidase activity—Detection of active fibrogenesis in human lung tissue. Chem. Sci. 2015, 6, 4946–4953. [Google Scholar] [CrossRef]
- Edgington, L.E.; Berger, A.B.; Blum, G.; Albrow, V.E.; Paulick, M.G.; Lineberry, N.; Bogyo, M. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat. Med. 2009, 15, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Krstajic, N.; Akram, A.R.; Choudhary, T.R.; McDonald, N.; Tanner, M.G.; Pedretti, E.; Dalgarno, P.A.; Scholefield, E.; Girkin, J.M.; Moore, A.; et al. Two-color widefield fluorescence microendoscopy enables multiplexed molecular imaging in the alveolar space of human lung tissue. J. Biomed. Opt. 2016, 21, 046009. [Google Scholar] [CrossRef]
- Forel, J.M.; Guervilly, C.; Hraiech, S.; Voillet, F.; Thomas, G.; Somma, C.; Secq, V.; Farnarier, C.; Payan, M.J.; Donati, S.Y.; et al. Type III procollagen is a reliable marker of ARDS-associated lung fibroproliferation. Intensive Care Med. 2015, 41, 1–11. [Google Scholar] [CrossRef]
- Hamon, A.; Scemama, U.; Bourenne, J.; Daviet, F.; Coiffard, B.; Persico, N.; Adda, M.; Guervilly, C.; Hraiech, S.; Chaumoitre, K.; et al. Chest CT scan and alveolar procollagen III to predict lung fibroproliferation in acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 42. [Google Scholar] [CrossRef]
- Bardou, O.; Menou, A.; François, C.; Duitman, J.W.; von der Thüsen, J.H.; Borie, R.; Sales, K.U.; Mutze, K.; Castier, Y.; Sage, E.; et al. Membrane-anchored Serine Protease Matriptase Is a Trigger of Pulmonary Fibrogenesis. Am. J. Respir. Crit. Care Med. 2016, 193, 847–860. [Google Scholar] [CrossRef]
- Bauer, T.T.; Ewig, S.; Rodloff, A.C.; Müller, E.E. Acute Respiratory Distress Syndrome and Pneumonia: A Comprehensive Review of Clinical Data. Clin. Infect. Dis. 2006, 43, 748–756. [Google Scholar] [CrossRef]
- Akram, A.R.; Chankeshwara, S.V.; Scholefield, E.; Aslam, T.; McDonald, N.; Megia-Fernandez, A.; Marshall, A.; Mills, B.; Avlonitis, N.; Craven, T.H.; et al. In situ identification of Gram-negative bacteria in human lungs using a topical fluorescent peptide targeting lipid A. Sci. Transl. Med. 2018, 10, eaal0033. [Google Scholar] [CrossRef] [Green Version]
- Pedretti, E.; Tanner, M.G.; Choudhary, T.R.; Krstajić, N.; Megia-Fernandez, A.; Henderson, R.K.; Bradley, M.; Thomson, R.R.; Girkin, J.M.; Dhaliwal, K.; et al. High-speed dual color fluorescence lifetime endomicroscopy for highly-multiplexed pulmonary diagnostic applications and detection of labeled bacteria. Biomed. Opt. Express 2018, 10, 181–195. [Google Scholar] [CrossRef]
- Steinberg, K.P.; Hudson, L.D.; Goodman, R.B.; Hough, C.L.; Lanken, P.N.; Hyzy, R.; Thompson, B.T.; Ancukiewicz, M. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 2006, 354, 1671–1684. [Google Scholar]
- Perez, J.R.; Ybarra, N.; Chagnon, F.; Serban, M.; Pare, G.; Lesur, O.; Seuntjens, J.; Naqa, I.E. Image-Guided Fluorescence Endomicroscopy: From Macro- to Micro-Imaging of Radiation-Induced Pulmonary Fibrosis. Sci. Rep. 2017, 7, 17829. [Google Scholar] [CrossRef] [Green Version]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Guillamat-Prats, R.; Camprubí-Rimblas, M.; Bringué, J.; Tantinyà, N.; Artigas, A. Cell therapy for the treatment of sepsis and acute respiratory distress syndrome. Ann. Transl. Med. 2017, 5, 446. [Google Scholar] [CrossRef]
- Cardenes, N.; Aranda-Valderrama, P.; Carney, J.P.; Sellares Torres, J.; Alvarez, D.; Kocydirim, E.; Wolfram Smith, J.A.; Ting, A.E.; Lagazzi, L.; Yu, Z.; et al. Cell therapy for ARDS: Efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respir. Res. 2019, 6, e000308. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Antebi, B.; Batchinsky, A.I.; Cancio, L.C. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir. Res. 2018, 19, 218. [Google Scholar] [CrossRef]
- Perez, J.R.; Ybarra, N.; Chagnon, F.; Lesur, O.; Seuntjens, J.; Naqa, I.E. Fluorescence Endomicroscopy Imaging of Mesenchymal Stem Cells in the Rat Lung. Curr. Protoc. Stem Cell Biol. 2018, 45, e52. [Google Scholar] [CrossRef]
- Perez, J.R.; Ybarra, N.; Chagnon, F.; Serban, M.; Lee, S.; Seuntjens, J.; Lesur, O.; El Naqa, I. Tracking of Mesenchymal Stem Cells with Fluorescence Endomicroscopy Imaging in Radiotherapy-Induced Lung Injury. Sci. Rep. 2017, 7, 40748. [Google Scholar] [CrossRef] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesur, O.; Chagnon, F.; Lebel, R.; Lepage, M. In Vivo Endomicroscopy of Lung Injury and Repair in ARDS: Potential Added Value to Current Imaging. J. Clin. Med. 2019, 8, 1197. https://doi.org/10.3390/jcm8081197
Lesur O, Chagnon F, Lebel R, Lepage M. In Vivo Endomicroscopy of Lung Injury and Repair in ARDS: Potential Added Value to Current Imaging. Journal of Clinical Medicine. 2019; 8(8):1197. https://doi.org/10.3390/jcm8081197
Chicago/Turabian StyleLesur, Olivier, Frédéric Chagnon, Réjean Lebel, and Martin Lepage. 2019. "In Vivo Endomicroscopy of Lung Injury and Repair in ARDS: Potential Added Value to Current Imaging" Journal of Clinical Medicine 8, no. 8: 1197. https://doi.org/10.3390/jcm8081197



