Effects of SHBG rs1799941 Polymorphism on Free Testosterone Levels and Hypogonadism Risk in Young Non-Diabetic Obese Males
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Study Protocol
Polymorphism DNA Analysis
2.3. Statistical Analyses
3. Results
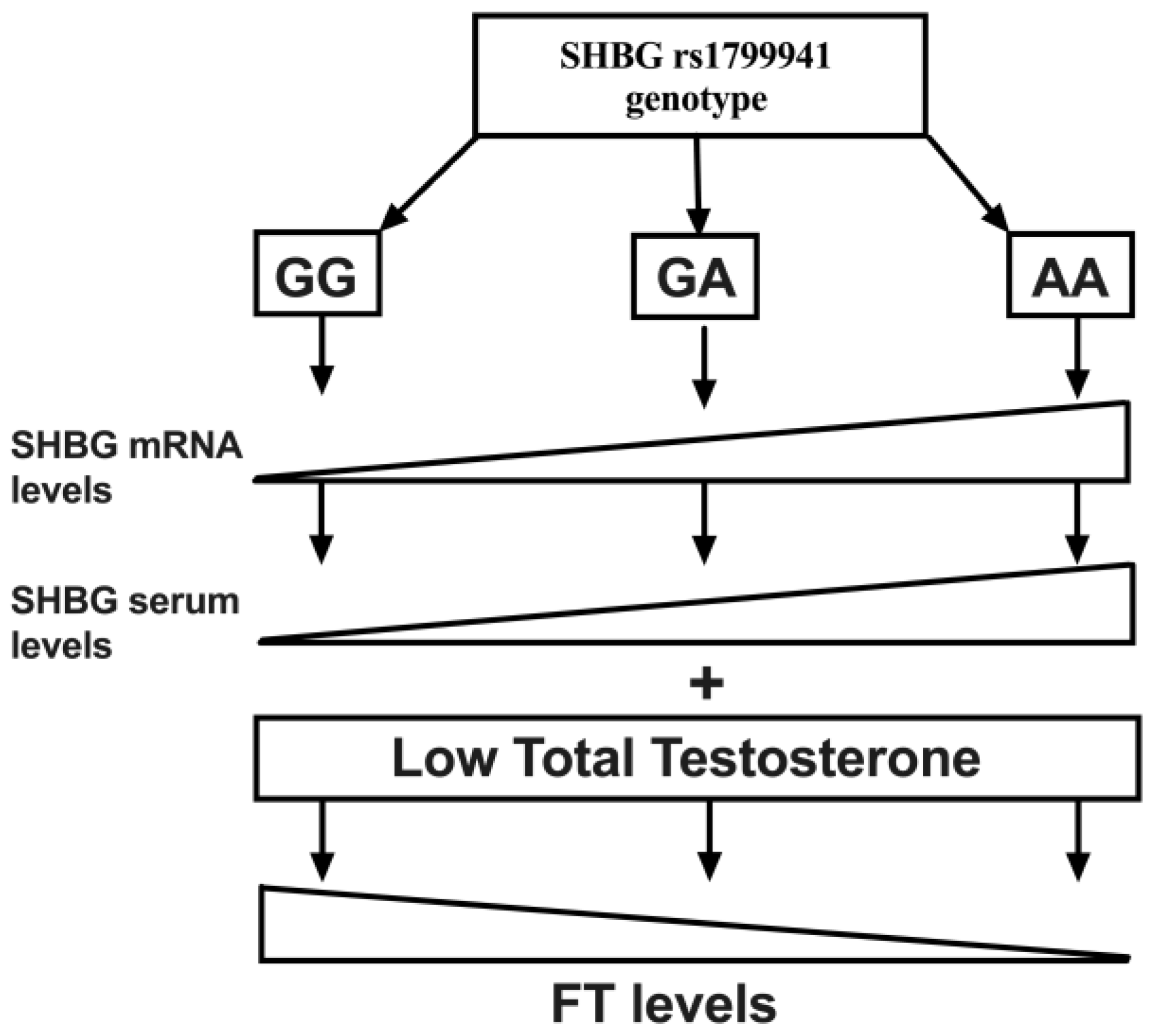
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclosure Statement
References
- Bardou, M.; Barkun, A.N.; Martel, M. Republished: Obesity and Colorectal Cancer. Postgrad. Med. J. 2013, 89, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M. Hypogonadism and Male Obesity: Focus on Unresolved Questions. Clin. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Ventimiglia, E.; Ippolito, S.; Capogrosso, P.; Pederzoli, F.; Cazzaniga, W.; Boeri, L.; Cavarretta, I.; Alfano, M.; Viganò, P.; Montorsi, F.; et al. Primary, Secondary and Compensated Hypogonadism: A Novel Risk Stratification for Infertile Men. Andrology 2017, 5, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Basaria, S. Welcoming Low Testosterone as a Cardiovascular Risk Factor. Int. J. Impot. Res. 2009, 21, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pham, T.; Mcwhinney, B.C.; Ungerer, J.P.; Pretorius, C.J.; Richard, D.J.; Mortimer, R.H.; Emden, M.C.; Richard, K. Sex Hormone Binding Globulin Modifies Testosterone Action and Metabolism in Prostate Cancer Cells. Int. J. Endocrinol. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Tint, A.N.; Hoermann, R.; Wong, H.; Ekinci, E.I.; Macisaac, R.J.; Jerums, G.; Zajac, J.D.; Grossmann, M. Association of Sex Hormone-Binding Globulin and Free Testosterone with Mortality in Men with Type 2 Diabetes Mellitus. Eur. J. Endocrinol. 2016, 174, 59–68. [Google Scholar] [CrossRef]
- Firtser, S.; Juonala, M.; Magnussen, C.G.; Jula, A.; Loo, B.M.; Marniemi, J.; Viikari, J.S.A.; Toppari, J.; Perheentupa, A.; Hutri-Kähönen, N.; et al. Relation of Total and Free Testosterone and Sex Hormone-Binding Globulin with Cardiovascular Risk Factors in Men Aged 24-45 Years. The Cardiovascular Risk in Young Finns Study. Atherosclerosis 2012, 222, 257–262. [Google Scholar] [CrossRef]
- Hammond, G.L. Molecular Properties of Corticosteroid Binding Globulin and the Sex-Steroid Binding Proteins. Endocr. Rev. 1990, 11, 65–79. [Google Scholar] [CrossRef]
- Ahn, J.; Schumacher, F.R.; Berndt, S.I.; Pfeiffer, R.; Albanes, D.; Andriole, G.L.; Ardanaz, E.; Boeing, H.; Bueno-de-Mesquita, B.; Chanock, S.J.; et al. Quantitative Trait Loci Predicting Circulating Sex Steroid Hormones in Men from the NCI-Breast and Prostate Cancer Cohort Consortium (BPC3). Hum. Mol. Genet. 2009, 18, 3749–3757. [Google Scholar] [CrossRef]
- Nenonen, H.A.; Giwercman, A.; Hallengren, E.; Giwercman, Y.L. Non-Linear Association between Androgen Receptor CAG Repeat Length and Risk of Male Subfertility-a Meta-Analysis. Int. J. Androl. 2011, 34, 327–332. [Google Scholar] [CrossRef]
- Nenonen, H.; Björk, C.; Skjaerpe, P.-A.; Giwercman, A.; Rylander, L.; Svartberg, J.; Giwercman, Y.L. CAG Repeat Number Is Not Inversely Associated with Androgen Receptor Activity in Vitro. Mol. Hum. Reprod. 2010, 16, 153–157. [Google Scholar] [CrossRef]
- Ohlsson, C.; Wallaschofski, H.; Lunetta, K.L.; Stolk, L.; Perry, J.R.B.; Koster, A.; Petersen, A.K.; Eriksson, J.; Lehtimäki, T.; Huhtaniemi, I.T.; et al. Genetic Determinants of Serum Testosterone Concentrations in Men. PLoS Genet. 2011, 7. [Google Scholar] [CrossRef] [PubMed]
- Coviello, A.D.; Haring, R.; Wellons, M.; Vaidya, D.; Lehtimäki, T.; Keildson, S.; Lunetta, K.L.; He, C.; Fornage, M.; Lagou, V.; et al. A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone-Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, B.; Duminuco, P.; Vezzoli, V.; Guizzardi, F.; Chiodini, I.; Corona, G.; Maggi, M.; Persani, L.; Bonomi, M. Evidence for a Common Genetic Origin of Classic and Milder Adult-Onset Forms of Isolated Hypogonadotropic Hypogonadism. J. Clin. Med. 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.L.; Lorentzon, M.; Mellström, D.; Vandenput, L.; Swanson, C.; Andersson, N.; Hammond, G.L.; Jakobsson, J.; Rane, A.; Orwoll, E.S.; et al. SHBG Gene Promoter Polymorphisms in Men Are Associated with Serum Sex Hormone-Binding Globulin, Androgen and Androgen Metabolite Levels, and Hip Bone Mineral Density. J. Clin. Endocrinol. Metab. 2006, 91, 5029–5037. [Google Scholar] [CrossRef] [Green Version]
- Peter, A.; Kantartzis, K.; Machann, J.; Schick, F.; Staiger, H.; Machicao, F.; Schleicher, E.; Fritsche, A.; Häring, H.U.; Stefan, N. Relationships of Circulating Sex Hormone-Binding Globulin with Metabolic Traits in Humans. Diabetes 2010, 59, 3167–3173. [Google Scholar] [CrossRef]
- Jin, G.; Sun, J.; Kim, S.T.; Feng, J.; Wang, Z.; Tao, S.; Chen, Z.; Purcell, L.; Smith, S.; Isaacs, W.B.; et al. Genome-Wide Association Study Identifies a New Locus JMJD1C at 10q21 That May Influence Serum Androgen Levels in Men. Hum. Mol. Genet. 2012, 21, 5222–5228. [Google Scholar] [CrossRef]
- Svartberg, J.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njølstad, I.; Løchen, M.L.; Jorde, R. Single-Nucleotide Polymorphism, Rs1799941 in the Sex Hormone-Binding Globulin (SHBG) Gene, Related to Both Serum Testosterone and SHBG Levels and the Risk of Myocardial Infarction, Type 2 Diabetes, Cancer and Mortality in Men: The Tromsø Study. Andrology 2014, 2, 212–218. [Google Scholar] [CrossRef]
- Moffat, S.D.; Zondrman, A.B.; Metter, E.J.; Kawas, C.; Blackman, M.R.; Harman, S.M.; Resnick, S.N. Free Testosterone and Risk of Alzheimer’s Disease in Older Men. Neurology 2004, 62, 188–193. [Google Scholar] [CrossRef]
- Hogervorst, E.; Bandelow, S.; Combrinck, M.; Smith, A.D. Low Free Testosterone Is an Independent Risk Factor for Alzheimer’s Disease. Exp. Gerontol. 2004, 39, 1633–1639. [Google Scholar] [CrossRef]
- Gururani, K.; Jose, J.; George, P.V. Testosterone as a Marker of Coronary Artery Disease Severity in Middle Aged Males. Indian Heart J. 2016, 68, S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A Critical Evaluation of Simple Methods for the Estimation of Free Testosterone in Serum. J. Clin. Endocrinol. Metab. 1999, 84, 3666–3672. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Bebb, R.A.; Manjoo, P.; Assimakopoulos, P.; Axler, J.; Collier, C.; Elliott, S.; Goldenberg, L.; Gottesman, I.; Grober, E.D.; et al. Diagnosis and Management of Testosterone Deficiency Syndrome in Men: Clinical Practice Guideline. CMAJ 2015, 187, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Khera, M.; Adaikan, G.; Buvat, J.; Carrier, S.; El-Meliegy, A.; Hatzimouratidis, K.; McCullough, A.; Morgentaler, A.; Torres, L.O.; Salonia, A. Diagnosis and Treatment of Testosterone Deficiency: Recommendations From the Fourth International Consultation for Sexual Medicine (ICSM 2015). J. Sex. Med. 2016, 13, 1787–1804. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-Wauchope, A.; Hammer, G.D.; Husebye, E.S.; Merke, D.P.; Murad, M.H.; Stratakis, C.A.; et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.-Y.; Li, X.-Y.; Li, M.; Zhang, G.-K.; Ma, F.-L.; Liu, Z.-M.; Zhang, N.-Y.; Meng, P. Decline of Serum Levels of Free Testosterone in Aging Healthy Chinese Men. Aging Male 2005, 8, 3–4. [Google Scholar] [CrossRef]
- Yavuz, B.B.; Ozkayar, N.; Halil, M.; Cankurtaran, M.; Ulger, Z.; Tezcan, E.; Gurlek, A.; Ariogul, S. Free Testosterone Levels and Implications on Clinical Outcomes in Elderly Men. Aging Clin. Exp. Res. 2008, 20, 201–206. [Google Scholar] [CrossRef]
- Yeap, B.B. Are Declining Testosterone Levels a Major Risk Factor for Ill-Health in Aging Men? Int. J. Impot. Res. 2009, 21, 24. [Google Scholar] [CrossRef]
- Cooper, L.A.; Page, S.T.; Amory, J.K.; Anawalt, B.D.; Matsumoto, A.M. The Association of Obesity with Sex Hormone-Binding Globulin Is Stronger than the Association with Ageing—Implications for the Interpretation of Total Testosterone Measurements. Clin. Endocrinol. 2015, 83, 828–833. [Google Scholar] [CrossRef]
- Anawalt, B.D.; Hotaling, J.M.; Walsh, T.J.; Matsumoto, A.M. Performance of Total Testosterone Measurement to Predict Free Testosterone for the Biochemical Evaluation of Male Hypogonadism. J. Urol. 2012, 187, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Pye, S.R.; Phil, M.; Silman, A.J.; Finn, J.D.; Sc, B.; Neill, T.W.O.; Bartfai, G.; Casanueva, F.F.; Ph, D.; Forti, G.; et al. Identification of Late-Onset Hypogonadism in Middle-Aged and Elderly Men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar]
- Ramasamy, R.; Golan, R.; Wilken, N.; Scovell, J.M.; Lipshultz, L.I. Association of Free Testosterone with Hypogonadal Symptoms in Men with Near-Normal Total Testosterone Levels HHS Public Access. Urology 2015, 86, 287–290. [Google Scholar] [CrossRef] [PubMed]
| Eugonadal (n = 101) | Normal FT HG (n = 60) | HG (n = 51) | |
|---|---|---|---|
| Age (years) | 37.62 ± 7.65 | 36.12 ± 7.35 | 38.41 ± 7.59 |
| Smokers (%) * | 31 | 19 | 15 |
| BMI (kg/m2) | 36.76 ± 5.29a | 38.63 ± 5.76b | 44.49 ± 8.42c |
| Waist (cm) | 119.55 ± 13.11a | 123.84 ± 13.33b | 136.11 ± 17.83c |
| Glucose (mg/dl) | 91.39 ± 9.95 | 93.12 ± 11.33 | 93.25 ± 10.38 |
| Insulin (μU/mL) | 16.36 ± 8.05a | 22.45 ± 11.65b | 25.58 ± 18.70b |
| HOMA-IR | 3.74 ± 2.04a | 5.41 ± 6.48b | 5.95 ± 4.40b |
| Triglycerides (mg/dl) | 151.26 ± 81.68 | 165.12 ± 72.19 | 151.12 ± 81.93 |
| Chol (mg/dl) | 191.75 ± 34.68 | 185.73 ± 32.83 | 179.75 ± 29.46 |
| HDL (mg/dl) | 42.94 ± 10.47 | 39.93 ± 7.04 | 41.02 ± 9.59 |
| LDL (mg/dl) | 119.70 ± 29.57 | 114.20 ± 28.35 | 110.10 ± 24.81 |
| CRP (mg/L) | 5.12 ± 3.63a | 6.74 ± 5.77b | 8.50 ± 6.09b |
| HbA1c (%) | 5.32 ± 0.36a | 5.46 ± 0.32b | 5.52 ± 0.35b |
| Hematocrit (%) | 46.50 ± 2.74 | 45.66 ± 3.02 | 45.65 ± 3.09 |
| TSH (μU/mL) | 1.79 ± 0.97 | 1.81 ± 0.81 | 1.89 ± 0.93 |
| FSH (mUI/mL) | 4.18 ± 2.43 | 3.89 ± 2.31 | 3.52 ± 2.09 |
| LH (mUI/mL) | 4.10 ± 1.62a | 3.79 ± 1.62a,b | 3.21 ± 1.62b |
| Estradiol (pg/mL) | 33.77 ± 12.58 | 31.56 ± 14.76 | 34.10 ± 13.39 |
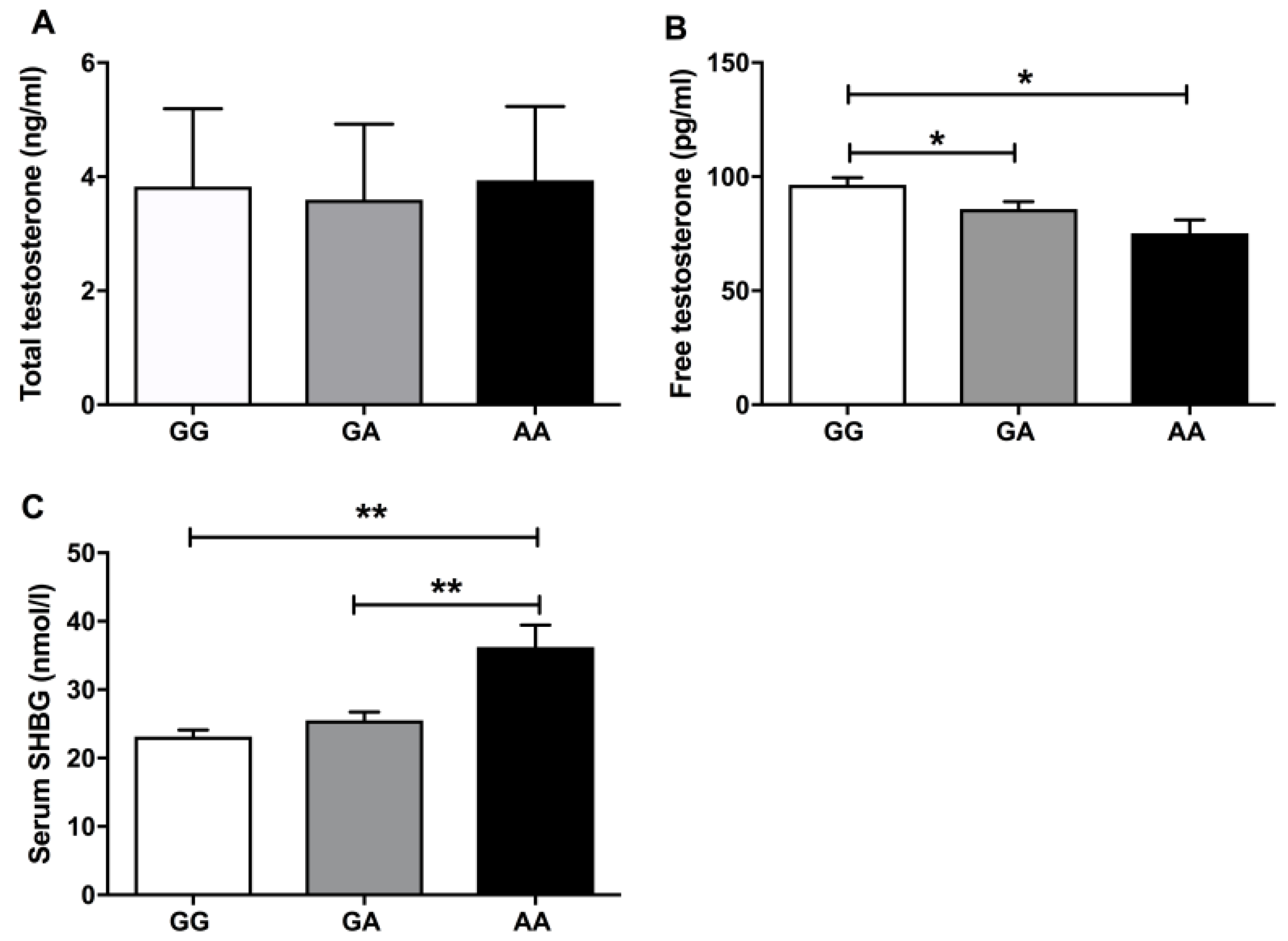
| Testosterone (ng/mL) | 4.83 ± 1.10a | 3.04 ± 0.32b | 2.41 ± 0.54c |
| FT (pg/mL) | 111.33 ± 30.99a | 84.22 ± 9.54b | 56.90 ± 9.85c |
| SHBG (nmol/L) | 30.00 ± 11.60a | 17.48 ± 5.47b | 24.82 ± 10.30c |
| SHBG rs1799941 Polymorphism (%) | |||
|---|---|---|---|
| GG | GA | AA | |
| Eugonadal | 52.5 | 36.6 | 10.9 |
| Normal FT HG | 58.3 | 41.7 | 0.0 |
| HG | 41.2 | 43.1 | 15.7 |
| Free Testosterone (R = 0.442. R2 = 0.195) | |||
|---|---|---|---|
| β | p | 95% CI | |
| Age (years) | −0.440 | 0.099 | −0.964–0.083 |
| BMI (kg/m2) | −1.448 | 0.000 | −2.057–(−0.839) |
| HOMA-IR | −0.186 | 0.704 | −1.146–0.775 |
| LH (mUI/mL) | 3.305 | 0.007 | 0.925–5.685 |
| SHBG rs1799941_GA | −9.950 | 0.020 | −18.335–(−1.564) |
| SHBG rs1799941_AA | −17.994 | 0.016 | −32.664–(−3.324) |
| Serum SHBG (R = 0.640. R2 = 0.410) | |||
|---|---|---|---|
| β | p | 95% CI | |
| Age (years) | 0.391 | 0.000 | 0.232–0.551 |
| BMI (kg/m2) | 0.155 | 0.121 | −0.041–0.351 |
| HOMA-IR | −0.46 | 0.326 | −0.440–0.147 |
| LH (mUI/mL) | −0.032 | 0.931 | −0.771–0.706 |
| Testosterone (ng/mL) | 4.128 | 0.000 | 3.147–5.110 |
| SHBG rs1799941_GA | 3.103 | 0.018 | 0.542–5.664 |
| SHBG rs1799941_AA | 11.695 | 0.000 | 7.230–16.161 |
| Normal FT HG/HG R2 = 0.197–0.264 | ||
|---|---|---|
| OR (95% CI) | p | |
| Age | 1.05 (0.99–1.11) | 0.082 |
| BMI | 1.13 (1.06–1.21) | 0.000 |
| HOMA-IR | 0.95 (0.88–1.04) | 0.306 |
| SHBG rs1799941 | ||
| Absence A | 1 (reference) | |
| Presence A | 2.54 (1.05–6.12) | 0.037 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellano-Castillo, D.; Royo, J.L.; Martínez-Escribano, A.; Sánchez-Alcoholado, L.; Molina-Vega, M.; Queipo-Ortuño, M.I.; Ruiz-Galdon, M.; J. Álvarez-Millán, J.; Cabezas-Sanchez, P.; Reyes-Engel, A.; et al. Effects of SHBG rs1799941 Polymorphism on Free Testosterone Levels and Hypogonadism Risk in Young Non-Diabetic Obese Males. J. Clin. Med. 2019, 8, 1136. https://doi.org/10.3390/jcm8081136
Castellano-Castillo D, Royo JL, Martínez-Escribano A, Sánchez-Alcoholado L, Molina-Vega M, Queipo-Ortuño MI, Ruiz-Galdon M, J. Álvarez-Millán J, Cabezas-Sanchez P, Reyes-Engel A, et al. Effects of SHBG rs1799941 Polymorphism on Free Testosterone Levels and Hypogonadism Risk in Young Non-Diabetic Obese Males. Journal of Clinical Medicine. 2019; 8(8):1136. https://doi.org/10.3390/jcm8081136
Chicago/Turabian StyleCastellano-Castillo, Daniel, José Luis Royo, Ana Martínez-Escribano, Lidia Sánchez-Alcoholado, María Molina-Vega, María Isabel Queipo-Ortuño, Maximiliano Ruiz-Galdon, Juan J. Álvarez-Millán, Pablo Cabezas-Sanchez, Armando Reyes-Engel, and et al. 2019. "Effects of SHBG rs1799941 Polymorphism on Free Testosterone Levels and Hypogonadism Risk in Young Non-Diabetic Obese Males" Journal of Clinical Medicine 8, no. 8: 1136. https://doi.org/10.3390/jcm8081136





