Longitudinal Molecular Magnetic Resonance Imaging of Endothelial Activation after Severe Traumatic Brain Injury
Abstract
1. Introduction
2. Experimental Section
2.1. Study Approval
2.2. Animals
2.3. Experimental Brain Injury
2.4. MPIOs Preparation
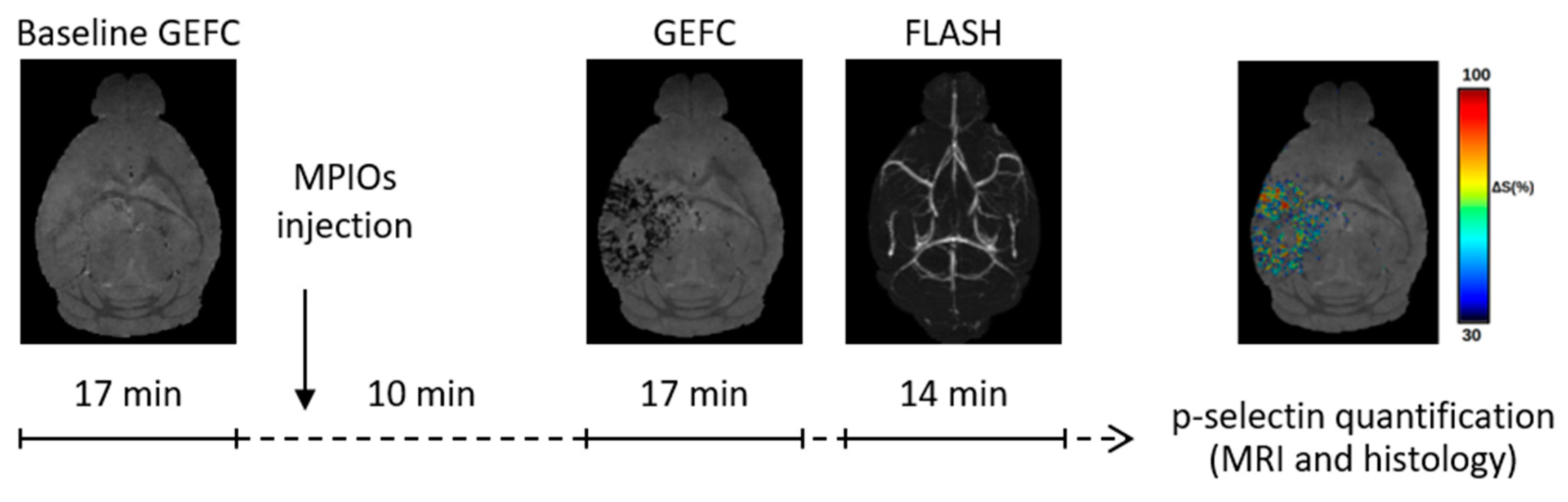
2.5. MRI Acquisition
2.6. MRI Analysis
2.7. Tissue Processing
2.8. P-Selectin
2.9. GFAP and AQP4
2.10. IBA1 and CD68
2.11. Tissue Analysis
2.12. Statistical Methods
3. Results and Discussion
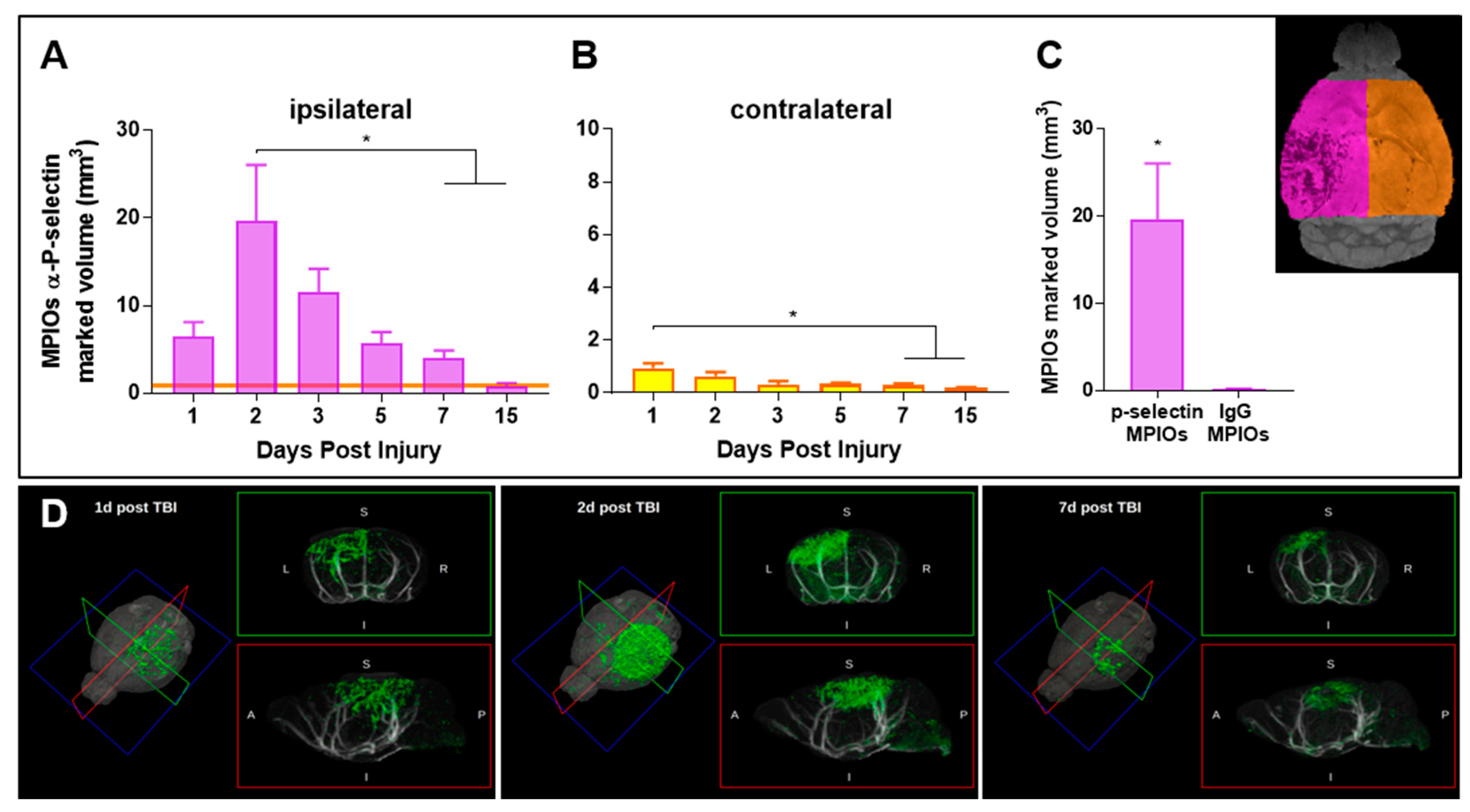
3.1. Endothelial Activation Can Be Detected with Molecular MRI Up to One Week Post-TBI
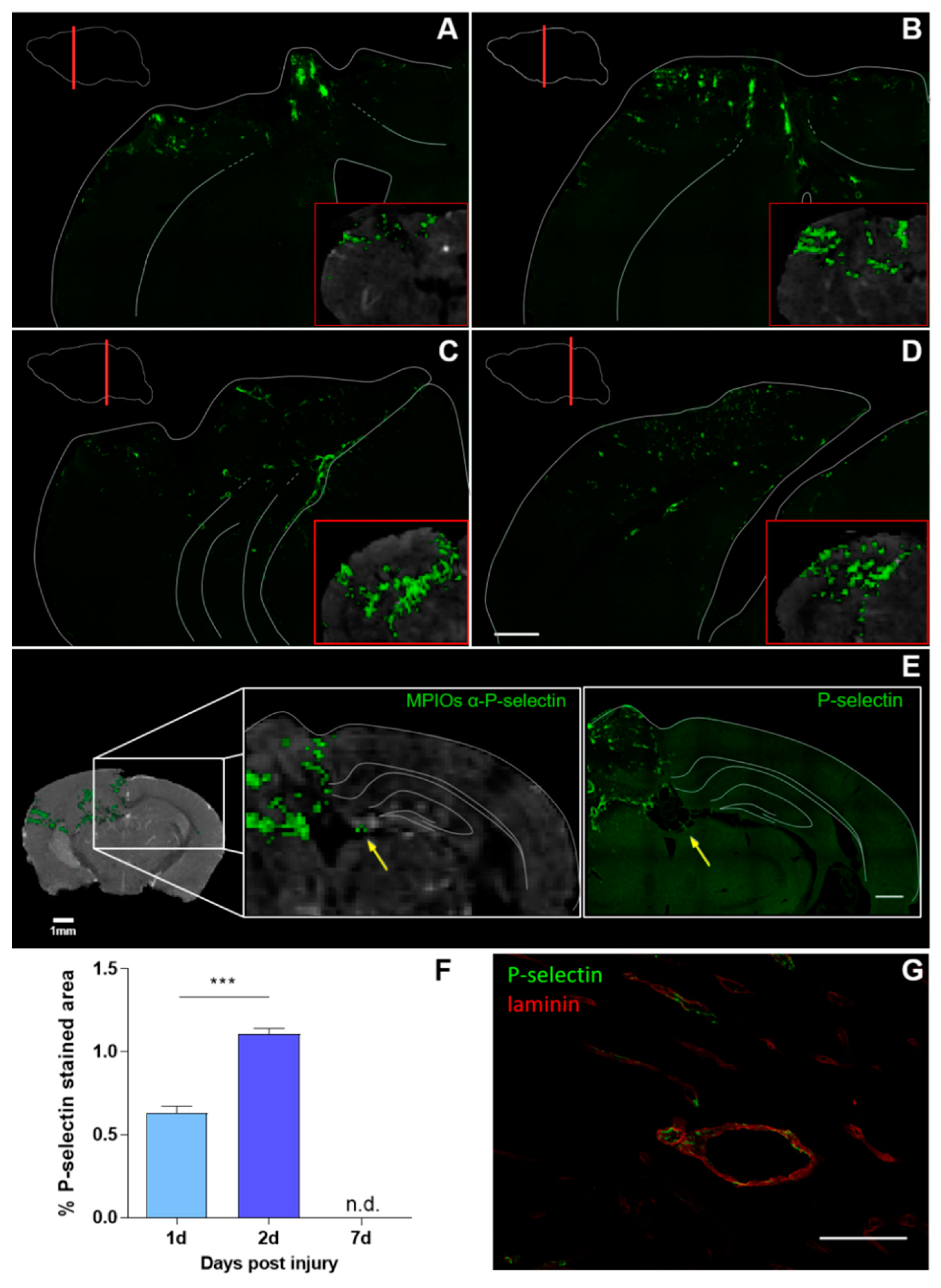
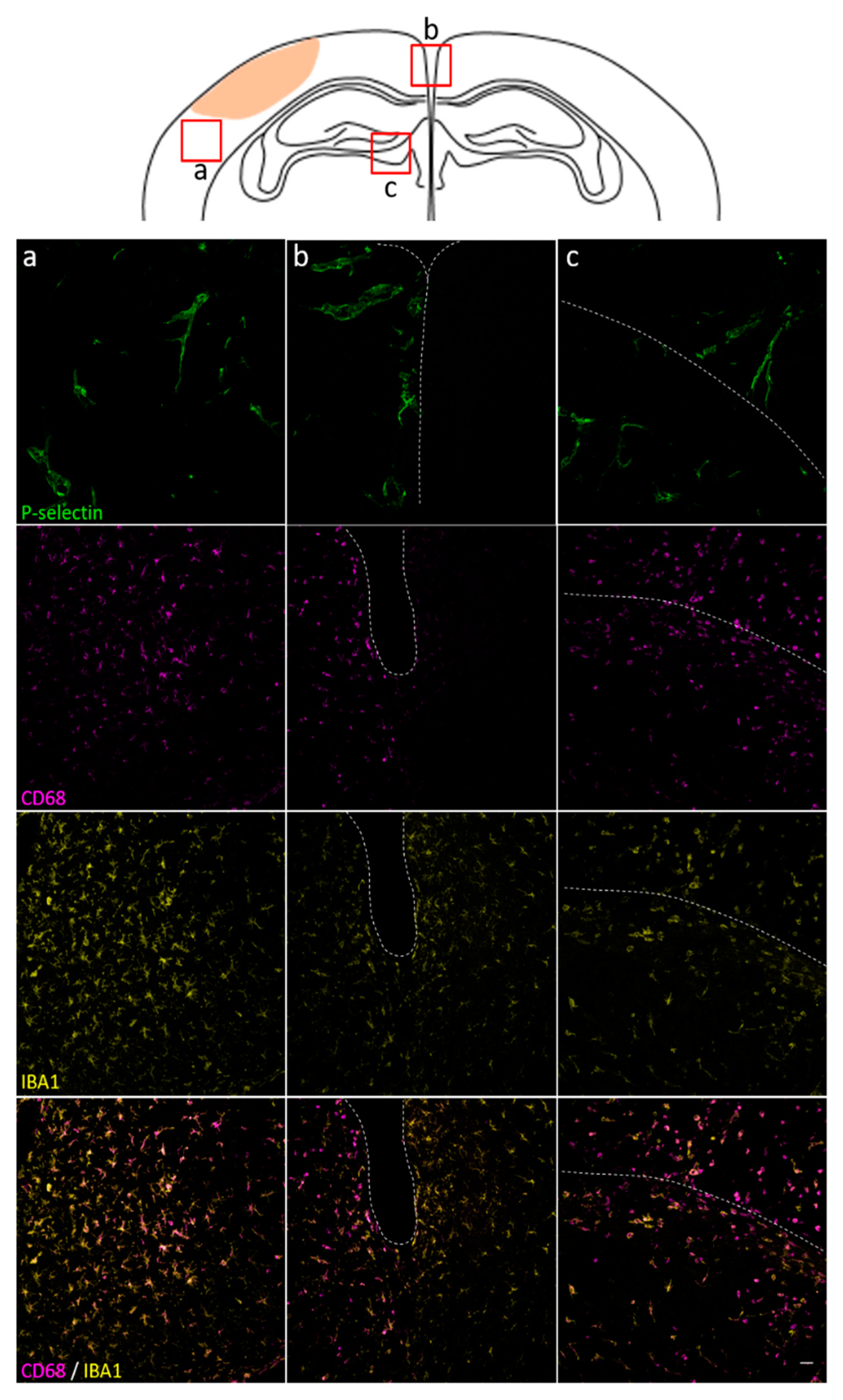
3.2. Histological Analysis Highly Reflects Spatial and Temporal Molecular MRI Findings
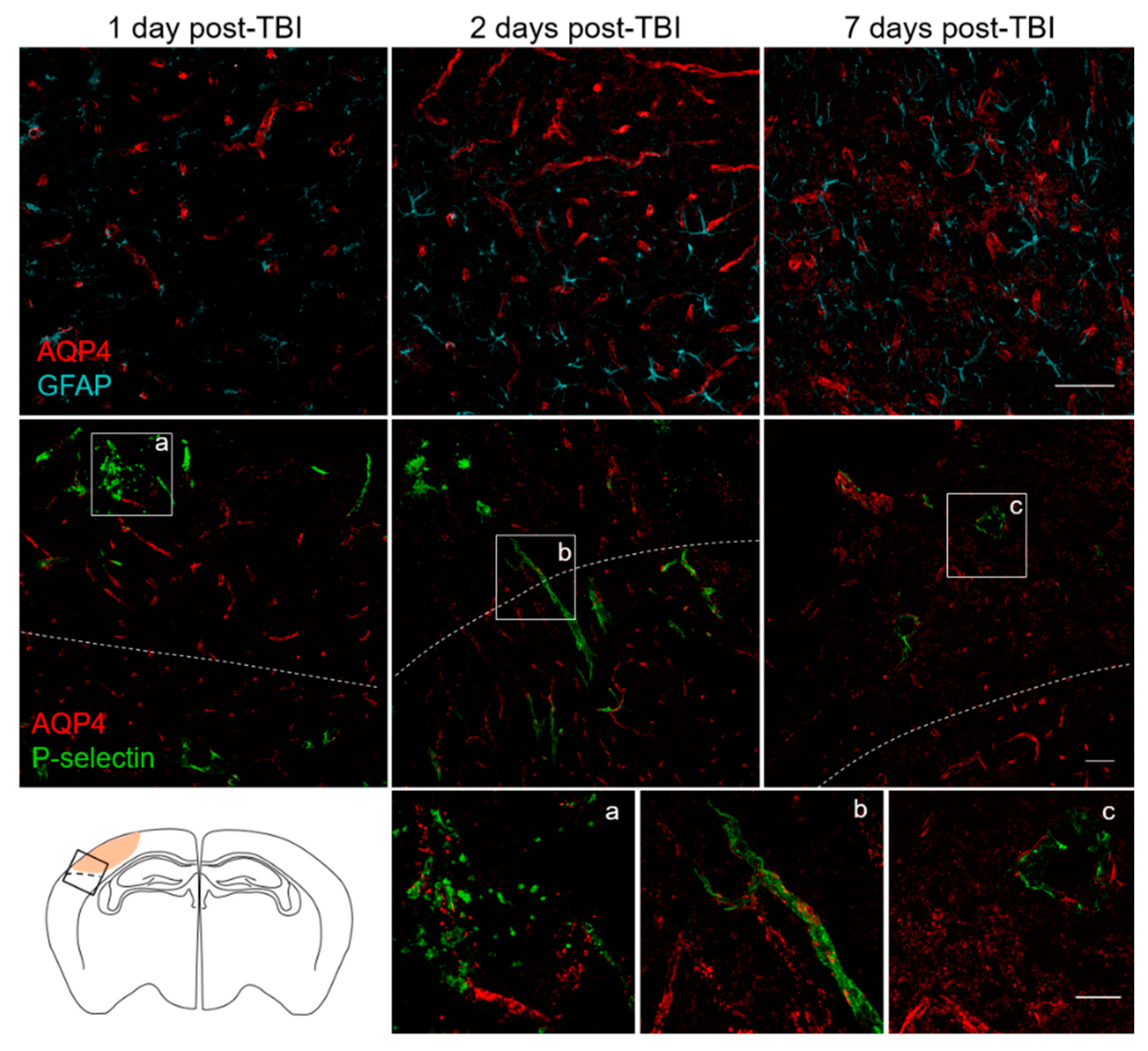
3.3. Spatial and Temporal P-Selectin Expression in Relation to Perivascular Disturbances
3.4. Limitation and Prospects for Molecular In Vivo MRI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [PubMed]
- Stein, S.C.; Georgoff, P.; Meghan, S.; Mizra, K.; Sonnad, S.S. 150 years of treating severe traumatic brain injury: A systematic review of progress in mortality. J. Neurotrauma 2010, 27, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Skolnick, B.; Narayan, R.K. Neuroprotection Trials in Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2016, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Needham, E.J.; Helmy, A.; Zanier, E.R.; Jones, J.L.; Coles, A.J.; Menon, D.K. The immunological response to traumatic brain injury. J. Neuroimmunol. 2019, 332, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Gean, A.D. Imaging for the diagnosis and management of traumatic brain injury. Neurotherapeutics 2011, 8, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Newberg, A. Neuroimaging in traumatic brain imaging. NeuroRx 2005, 2, 372–383. [Google Scholar] [CrossRef]
- Ramlackhansingh, A.F.; Brooks, D.J.; Greenwood, R.J.; Bose, S.K.; Turkheimer, F.E.; Kinnunen, K.M.; Gentleman, S.; Heckemann, R.A.; Gunanayagam, K.; Gelosa, G.; et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011, 70, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Quenault, A.; Martinez de Lizarrondo, S.; Etard, O.; Gauberti, M.; Orset, C.; Haelewyn, B.; Segal, H.C.; Rothwell, P.M.; Vivien, D.; Touzé, E.; et al. Molecular magnetic resonance imaging discloses endothelial activation after transient ischaemic attack. Brain 2017, 140, 146–157. [Google Scholar] [CrossRef]
- McAteer, M.A.; Sibson, N.R.; von Zur Muhlen, C.; Schneider, J.E.; Lowe, A.S.; Warrick, N.; Channon, K.M.; Anthony, D.C.; Choudhury, R.P. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat. Med. 2007, 13, 1253–1258. [Google Scholar] [CrossRef]
- Rossi, B.; Angiari, S.; Zenaro, E.; Budui, S.L.; Constantin, G. Vascular inflammation in central nervous system diseases: Adhesion receptors controlling leukocyte-endothelial interactions. J. Leukoc. Biol. 2011, 89, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Gauberti, M.; Montagne, A.; Quenault, A.; Vivien, D. Molecular magnetic resonance imaging of brain-immune interactions. Front. Cell Neurosci. 2014, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Gauberti, M.; Montagne, A.; Marcos-Contreras, O.A.; Le Béhot, A.; Maubert, E.; Vivien, D. Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes. Stroke 2013, 44, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Gauberti, M.; Fournier, A.P.; Docagne, F.; Vivien, D.; Martinez de Lizarrondo, S. Molecular Magnetic Resonance Imaging of Endothelial Activation in the Central Nervous System. Theranostics 2018, 8, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Serres, S.; Mardiguian, S.; Campbell, S.J.; McAteer, M.A.; Akhtar, A.; Krapitchev, A.; Choudhury, R.P.; Anthony, D.C.; Sibson, N.R. VCAM-1-targeted magnetic resonance imaging reveals subclinical disease in a mouse model of multiple sclerosis. FASEB J. 2011, 25, 4415–4422. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.P.; Quenault, A.; Martinez de Lizarrondo, S.; Gauberti, M.; Defer, G.; Vivien, D.; Docagne, F.; Macrez, R. Prediction of disease activity in models of multiple sclerosis by molecular magnetic resonance imaging of P-selectin. Proc. Natl. Acad. Sci. USA 2017, 114, 6116–6121. [Google Scholar] [CrossRef] [PubMed]
- Serres, S.; Soto, M.S.; Hamilton, A.; McAteer, M.A.; Carbonell, W.S.; Robson, M.D.; Ansorge, O.; Khrapitchev, A.; Bristow, C.; Balathasan, L.; et al. Molecular MRI enables early and sensitive detection of brain metastases. Proc. Natl. Acad. Sci. USA 2012, 109, 6674–6679. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol Pharmacother 2010, 1, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Brody, D.L.; Mac Donald, C.; Kessens, C.C.; Yuede, C.; Parsadanian, M.; Spinner, M.; Kim, E.; Schwetye, K.E.; Holtzman, D.M.; Bayly, P.V. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J. Neurotrauma 2007, 24, 657–673. [Google Scholar] [CrossRef]
- Avants, B.B.; Tustison, N.J.; Song, G.; Cook, P.A.; Klein, A.; Gee, J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011, 54, 2033–2044. [Google Scholar] [CrossRef]
- Dorr, A.E.; Lerch, J.P.; Spring, S.; Kabani, N.; Henkelman, R.M. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 2008, 42, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Andersson, J.; Ardekani, B.A.; Ashburner, J.; Avants, B.; Chiang, M.-C.; Christensen, G.E.; Collins, D.L.; Gee, J.; Hellier, P.; et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 2009, 46, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Meeker, R.B.; Williams, K.; Killebrew, D.A.; Hudson, L.C. Cell trafficking through the choroid plexus. Cell Adh. Migr. 2012, 6, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Merten, M.; Thiagarajan, P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation 2000, 102, 1931–1936. [Google Scholar] [CrossRef]
- Martin, G.E.; Xia, B.; Kim, Y.; Johnson, M.D.; Veile, R.; Friend, L.A.; Makley, A.T.; Caldwell, C.C.; Goodman, M.D. Platelet Function Changes in a Time-Dependent Manner Following Traumatic Brain Injury in a Murine Model. Shock 2018, 50, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Iliff, J.J.; Liao, Y.; Chen, M.J.; Shinseki, M.S.; Venkataraman, A.; Cheung, J.; Wang, W.; Nedergaard, M. Cognitive deficits and delayed neuronal loss in a mouse model of multiple microinfarcts. J. Neurosci. 2012, 32, 17948–17960. [Google Scholar] [CrossRef]
- Monai, H.; Wang, X.; Yahagi, K.; Lou, N.; Mestre, H.; Xu, Q.; Abe, Y.; Yasui, M.; Iwai, Y.; Nedergaard, M.; et al. Adrenergic receptor antagonism induces neuroprotection and facilitates recovery from acute ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 11010–11019. [Google Scholar] [CrossRef]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef]
- Ren, Z.; Iliff, J.J.; Yang, L.; Yang, J.; Chen, X.; Chen, M.J.; Giese, R.N.; Wang, B.; Shi, X.; Nedergaard, M. “Hit & Run” model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 2013, 33, 834–845. [Google Scholar] [PubMed]
- Foley, L.M.; Hitchens, T.K.; Ho, C.; Janesko-Feldman, K.L.; Melick, J.A.; Bayır, H.; Kochanek, P.M. Magnetic Resonance Imaging Assessment of Macrophage Accumulation in Mouse Brain after Experimental Traumatic Brain Injury. J. Neurotrauma 2009, 26, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Lutton, E.M.; Farney, S.K.; Andrews, A.M.; Shuvaev, V.V.; Chuang, G.-Y.; Muzykantov, V.R.; Ramirez, S.H. Endothelial Targeted Strategies to Combat Oxidative Stress: Improving Outcomes in Traumatic Brain Injury. Front. Neurol. 2019, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Zweckberger, K.; Hackenberg, K.; Jung, C.S.; Hertle, D.N.; Kiening, K.L.; Unterberg, A.W.; Sakowitz, O.W. Glibenclamide reduces secondary brain damage after experimental traumatic brain injury. Neuroscience 2014, 272, 199–206. [Google Scholar] [CrossRef]
- Puntambekar, S.S.; Saber, M.; Lamb, B.T.; Kokiko-Cochran, O.N. Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury. Brain Behav. Immun. 2018, 71, 9–17. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vegliante, G.; Tolomeo, D.; Drieu, A.; Rubio, M.; Micotti, E.; Moro, F.; Vivien, D.; Forloni, G.; Ali, C.; Zanier, E.R. Longitudinal Molecular Magnetic Resonance Imaging of Endothelial Activation after Severe Traumatic Brain Injury. J. Clin. Med. 2019, 8, 1134. https://doi.org/10.3390/jcm8081134
Vegliante G, Tolomeo D, Drieu A, Rubio M, Micotti E, Moro F, Vivien D, Forloni G, Ali C, Zanier ER. Longitudinal Molecular Magnetic Resonance Imaging of Endothelial Activation after Severe Traumatic Brain Injury. Journal of Clinical Medicine. 2019; 8(8):1134. https://doi.org/10.3390/jcm8081134
Chicago/Turabian StyleVegliante, Gloria, Daniele Tolomeo, Antoine Drieu, Marina Rubio, Edoardo Micotti, Federico Moro, Denis Vivien, Gianluigi Forloni, Carine Ali, and Elisa R. Zanier. 2019. "Longitudinal Molecular Magnetic Resonance Imaging of Endothelial Activation after Severe Traumatic Brain Injury" Journal of Clinical Medicine 8, no. 8: 1134. https://doi.org/10.3390/jcm8081134
APA StyleVegliante, G., Tolomeo, D., Drieu, A., Rubio, M., Micotti, E., Moro, F., Vivien, D., Forloni, G., Ali, C., & Zanier, E. R. (2019). Longitudinal Molecular Magnetic Resonance Imaging of Endothelial Activation after Severe Traumatic Brain Injury. Journal of Clinical Medicine, 8(8), 1134. https://doi.org/10.3390/jcm8081134





