Postoperative Imaging and Tumor Marker Surveillance in Resected Pancreatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Statistical Analysis
3. Results
3.1. Clinicopathological Features and Adjuvant Treatment
3.2. The Follow-Up and the Treatment Outcome of Four Surveillance Groups
3.3. Salvage Treatment for Recurrent Patients
3.4. Prognostic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kalser, M.H.; Ellenberg, S.S. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch. Surg. 1985, 120, 899–903. [Google Scholar] [PubMed]
- Klinkenbijl, J.H.; Jeekel, J.; Sahmoud, T.; van Pel, R.; Couvreur, M.L.; Veenhof, C.H.; Arnaud, J.P.; Gonzalez, D.G.; de Wit, L.T.; Hennipman, A.; et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann. Surg. 1999, 230, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, H.G.; van Eijck, C.H.; Hop, W.C.; Erdmann, J.; Tran, K.C.; Debois, M.; van Cutsem, E.; van Dekken, H.; Klinkenbijl, J.H.; Jeekel, J. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: Long-term results of EORTC trial 40891. Ann. Surg. 2007, 246, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zulke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Abdelghani, M.B.; Wei, A.C.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. Unicancer GI PRODIGE 24/CCTG PA.6 trial: A multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas. J. Clin. Oncol. 2018, 36, LBA4001. [Google Scholar] [CrossRef]
- Seubert, B.; Grunwald, B.; Kobuch, J.; Cui, H.; Schelter, F.; Schaten, S.; Siveke, J.T.; Lim, N.H.; Nagase, H.; Simonavicius, N.; et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 2015, 61, 238–248. [Google Scholar] [CrossRef]
- Grunwald, B.; Harant, V.; Schaten, S.; Fruhschutz, M.; Spallek, R.; Hochst, B.; Stutzer, K.; Berchtold, S.; Erkan, M.; Prokopchuk, O.; et al. Pancreatic Premalignant Lesions Secrete Tissue Inhibitor of Metalloproteinases-1, Which Activates Hepatic Stellate Cells Via CD63 Signaling to Create a Premetastatic Niche in the Liver. Gastroenterology 2016, 151, 1011.e1017–1024.e1017. [Google Scholar] [CrossRef]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McAllister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The pancreas cancer microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 29 April 2019).
- Khorana, A.A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Mohile, S.G.; Mumber, M.; Schulick, R.; Shapiro, M.; et al. Potentially curable pancreatic cancer: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2017, 35, 2324–2328. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.; Fou, L.; Hasler, E.; Hawkins, J.; O’Connell, S.; Pelone, F.; Callaway, M.; Campbell, F.; Capel, M.; Charnley, R.; et al. Diagnosis and management of pancreatic cancer in adults: A summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 2018, 18, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Daamen, L.A.; Groot, V.P.; Intven, M.P.W.; Besselink, M.G.; Busch, O.R.; Koerkamp, B.G.; Mohammad, N.H.; Hermans, J.J.; van Laarhoven, H.W.M.; Nuyttens, J.J.; et al. Dutch Pancreatic Cancer Group. Postoperative surveillance of pancreatic cancer patients. Eur. J. Surg. Oncol. 2019, 1. [Google Scholar]
- Sheffield, K.M.; Crowell, K.T.; Lin, Y.L.; Djukom, C.; Goodwin, J.S.; Riall, T.S. Surveillance of pancreatic cancer patients after surgical resection. Ann. Surg. Oncol. 2012, 19, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.W.; Fleming, J.B.; Lee, J.E.; Wang, X.; Pisters, P.W.; Vauthey, J.N.; Varadhachary, G.; Wolff, R.A.; Katz, M.H. Yield of clinical and radiographic surveillance in patients with resected pancreatic adenocarcinoma following multimodal therapy. HPB (Oxford) 2012, 14, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daamen, L.A.; Groot, V.P.; Heerkens, H.D.; Intven, M.P.W.; van Santvoort, H.C.; Molenaar, I.Q. Systematic review on the role of serum tumor markers in the detection of recurrent pancreatic cancer. HPB (Oxford) 2018, 20, 297–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaj, C.; Ayav, A.; Oliver, A.; Jausset, F.; Sellal, C.; Claudon, M.; Laurent, V. CT imaging of early local recurrence of pancreatic adenocarcinoma following pancreaticoduodenectomy. Abdom. Radiol. (NY) 2016, 41, 273–282. [Google Scholar] [CrossRef]
- Tzeng, C.W.; Abbott, D.E.; Cantor, S.B.; Fleming, J.B.; Lee, J.E.; Pisters, P.W.; Varadhachary, G.R.; Abbruzzese, J.L.; Wolff, R.A.; Ahmad, S.A.; et al. Frequency and intensity of postoperative surveillance after curative treatment of pancreatic cancer: A cost-effectiveness analysis. Ann. Surg. Oncol. 2013, 20, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, C.; Michalski, C.W.; Strobel, O.; Giese, N.; Hennche, A.K.; Buchler, M.W.; Hackert, T. Clinical Impact of Structured Follow-up After Pancreatic Surgery. Pancreas 2016, 45, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Ettrich, T.J.; Schulte, L.A.; Eitel, N.; Ettrich, K.; Berger, A.W.; Perkhofer, L.; Seufferlein, T. Surveillance after resection of pancreatic ductal adenocarcinoma with curative intent—A multicenter survey in Germany and review of the literature. Z. Gastroenterol. 2017, 55, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Pietrasz, D.; Pecuchet, N.; Garlan, F.; Didelot, A.; Dubreuil, O.; Doat, S.; Imbert-Bismut, F.; Karoui, M.; Vaillant, J.C.; Taly, V.; et al. Plasma Circulating Tumor DNA in Pancreatic Cancer Patients is a Prognostic Marker. Clin. Cancer Res. 2017, 23, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, S.; Zhang, B.; Ni, Q.; Yu, X.; Xu, J. Circulating biomarkers for early diagnosis of pancreatic cancer: Facts and hopes. Am. J. Cancer Res. 2018, 8, 332–353. [Google Scholar] [PubMed]
- Groot, V.P.; Mosier, S.; Javed, A.A.; Teinor, J.A.; Gemenetzis, G.; Ding, D.; Haley, L.M.; Yu, J.; Burkhart, R.A.; Hasanain, A.; et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin. Cancer Res. 2019, 29. Clincanres.0197.2019. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Dhir, M.; Zenati, M.S.; Hamad, A.; Singhi, A.D.; Bahary, N.; Hogg, M.E.; Zeh, H.J., 3rd; Zureikat, A.H. FOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Neoadjuvant Treatment of Resectable and Borderline Resectable Pancreatic Head Adenocarcinoma. Ann. Surg. Oncol. 2018, 25, 1896–1903. [Google Scholar] [CrossRef]
- Goldstein, D.; El-Maraghi, R.H.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L.; et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase III trial. J. Natl. Cancer Inst. 2015, 107, dju413. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.S.; Jameson, G.; Macarulla, T.; Lee, K.H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 2019, 108, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diab, M.; Azmi, A.; Mohammad, R.; Philip, P.A. Pharmacotherapeutic strategies for treating pancreatic cancer: Advances and challenges. Expert. Opin. Pharmacother. 2019, 20, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Neumann, U.P.; Trautwein, C.; Roderburg, C.; Luedde, T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017, 39, 1010428317692231. [Google Scholar] [CrossRef] [PubMed]
- Gaianigo, N.; Melisi, D.; Carbone, C. EMT and Treatment Resistance in Pancreatic Cancer. Cancers (Basel) 2017, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Hsu, M.C.; Chen, L.T.; Hung, W.C.; Pan, M.R. Alteration of Epigenetic Modifiers in Pancreatic Cancer and Its Clinical Implication. J. Clin. Med. 2019, 8, 903. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Kuo, Y.H.; Tien, Y.W.; Hsu, C.; Hsu, C.H.; Kuo, S.H.; Cheng, A.L. Inferior survival of advanced pancreatic cancer patients who received gemcitabine-based chemotherapy but did not participate in clinical trials. Oncology 2011, 81, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Barhli, A.; Cros, J.; Bartholin, L.; Neuzillet, C. Prognostic stratification of resected pancreatic ductal adenocarcinoma: Past, present, and future. Dig. Liver Dis. 2018, 50, 979–990. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Tullio, K.; Khorana, A.A. Do patients with pancreatic body or tail cancer benefit from adjuvant therapy? A cohort study. Surg. Oncol. 2018, 27, 245–250. [Google Scholar] [CrossRef]
- Birnbaum, D.J.; Bertucci, F.; Finetti, P.; Birnbaum, D.; Mamessier, E. Head and Body/Tail Pancreatic Carcinomas Are Not the Same Tumors. Cancers (Basel) 2019, 11, 497. [Google Scholar] [CrossRef]
- Motoi, F.; Murakami, Y.; Okada, K.I.; Matsumoto, I.; Uemura, K.; Satoi, S.; Sho, M.; Honda, G.; Fukumoto, T.; Yanagimoto, H.; et al. Sustained Elevation of Postoperative Serum Level of Carbohydrate Antigen 19-9 is High-Risk Stigmata for Primary Hepatic Recurrence in Patients with Curatively Resected Pancreatic Adenocarcinoma. World J. Surg. 2019, 43, 634–641. [Google Scholar] [CrossRef]
- Rieser, C.J.; Zenati, M.; Hamad, A.; Al Abbas, A.I.; Bahary, N.; Zureikat, A.H.; Zeh, H.J., 3rd; Hogg, M.E. CA19-9 on Postoperative Surveillance in Pancreatic Ductal Adenocarcinoma: Predicting Recurrence and Changing Prognosis over Time. Ann. Surg. Oncol. 2018, 25, 3483–3491. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Pasquali, C.; Bissoli, S.; Chierichetti, F.; Liessi, G.; Pedrazzoli, S. Tumor relapse after pancreatic cancer resection is detected earlier by 18-FDG PET than by CT. J. Gastrointest. Surg. 2010, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Reitz, D.; Gerger, A.; Seidel, J.; Kornprat, P.; Samonigg, H.; Stotz, M.; Szkandera, J.; Pichler, M. Combination of tumour markers CEA and CA19-9 improves the prognostic prediction in patients with pancreatic cancer. J. Clin. Pathol. 2015, 68, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Liu, L.; Xiang, J.F.; Wang, W.Q.; Qi, Z.H.; Wu, C.T.; Liu, C.; Long, J.; Xu, J.; Ni, Q.X.; et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery 2017, 161, 373–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simeone, D.M.; Ji, B.; Banerjee, M.; Arumugam, T.; Li, D.; Anderson, M.A.; Bamberger, A.M.; Greenson, J.; Brand, R.E.; Ramachandran, V.; et al. CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas 2007, 34, 436–443. [Google Scholar] [CrossRef]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, F.; Wicklein, D.; Horst, J.; Sundermann, P.; Maar, H.; Streichert, T.; Tachezy, M.; Izbicki, J.R.; Bockhorn, M.; Schumacher, U. Carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 1, 5 and 6 as biomarkers in pancreatic cancer. PLoS ONE 2014, 9, e113023. [Google Scholar] [CrossRef]
- Koopmann, J.; Fedarko, N.S.; Jain, A.; Maitra, A.; Iacobuzio-Donahue, C.; Rahman, A.; Hruban, R.H.; Yeo, C.J.; Goggins, M. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 487–491. [Google Scholar] [CrossRef]
- Kuhlmann, K.F.; van Till, J.W.; Boermeester, M.A.; de Reuver, P.R.; Tzvetanova, I.D.; Offerhaus, G.J.; Ten Kate, F.J.; Busch, O.R.; van Gulik, T.M.; Gouma, D.J.; et al. Evaluation of matrix metalloproteinase 7 in plasma and pancreatic juice as a biomarker for pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 886–891. [Google Scholar] [CrossRef]
- Zhou, W.; Sokoll, L.J.; Bruzek, D.J.; Zhang, L.; Velculescu, V.E.; Goldin, S.B.; Hruban, R.H.; Kern, S.E.; Hamilton, S.R.; Chan, D.W.; et al. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 109–112. [Google Scholar]
- Firpo, M.A.; Gay, D.Z.; Granger, S.R.; Scaife, C.L.; DiSario, J.A.; Boucher, K.M.; Mulvihill, S.J. Improved diagnosis of pancreatic adenocarcinoma using haptoglobin and serum amyloid A in a panel screen. World J. Surg. 2009, 33, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Firpo, M.A.; Adler, D.G.; Mulvihill, S.J. Screening for pancreatic cancer: Why, how, and who? Ann. Surg. 2013, 257, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nordby, T.; Hugenschmidt, H.; Fagerland, M.W.; Ikdahl, T.; Buanes, T.; Labori, K.J. Follow-up after curative surgery for pancreatic ductal adenocarcinoma: Asymptomatic recurrence is associated with improved survival. Eur. J. Surg. Oncol. 2013, 39, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Guo, J.C.; Yeh, K.H.; Tien, Y.W.; Cheng, A.L.; Kuo, S.H. Association of radiotherapy with favorable prognosis in daily clinical practice for treatment of locally advanced and metastatic pancreatic cancer. J. Gastroenterol. Hepatol. 2016, 31, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Ahn, S.; Cho, I.K.; Lee, J.; Kim, J.; Hwang, J.H. Management of recurrent pancreatic cancer after surgical resection: A protocol for systematic review, evidence mapping and meta-analysis. BMJ Open 2018, 8, e017249. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; van Santvoort, H.C.; Rombouts, S.J.; Hagendoorn, J.; Borel Rinkes, I.H.; van Vulpen, M.; Herman, J.M.; Wolfgang, C.L.; Besselink, M.G.; Molenaar, I.Q. Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; re-resection, chemoradiotherapy and SBRT. HPB (Oxford) 2017, 19, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.; Jiang, R.; Liang, F.; Yu, G.; Long, J.; Zhao, J. Definitive chemoradiotherapy and salvage chemotherapy for patients with isolated locoregional recurrence after radical resection of primary pancreatic cancer. Cancer Manag. Res. 2019, 11, 5065–5073. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Kennedy, E.B.; Khorana, A.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Krishnamurthi, S.; Moravek, C.; O’Reilly, E.M.; Philip, P.A.; et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2545–2556. [Google Scholar] [CrossRef]
- Khorana, A.A.; McKernin, S.E.; Berlin, J.; Hong, T.S.; Maitra, A.; Moravek, C.; Mumber, M.; Schulick, R.; Zeh, H.J.; Katz, M.H.G. Potentially Curable Pancreatic Adenocarcinoma: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2019, JCO1900946. [Google Scholar] [CrossRef]
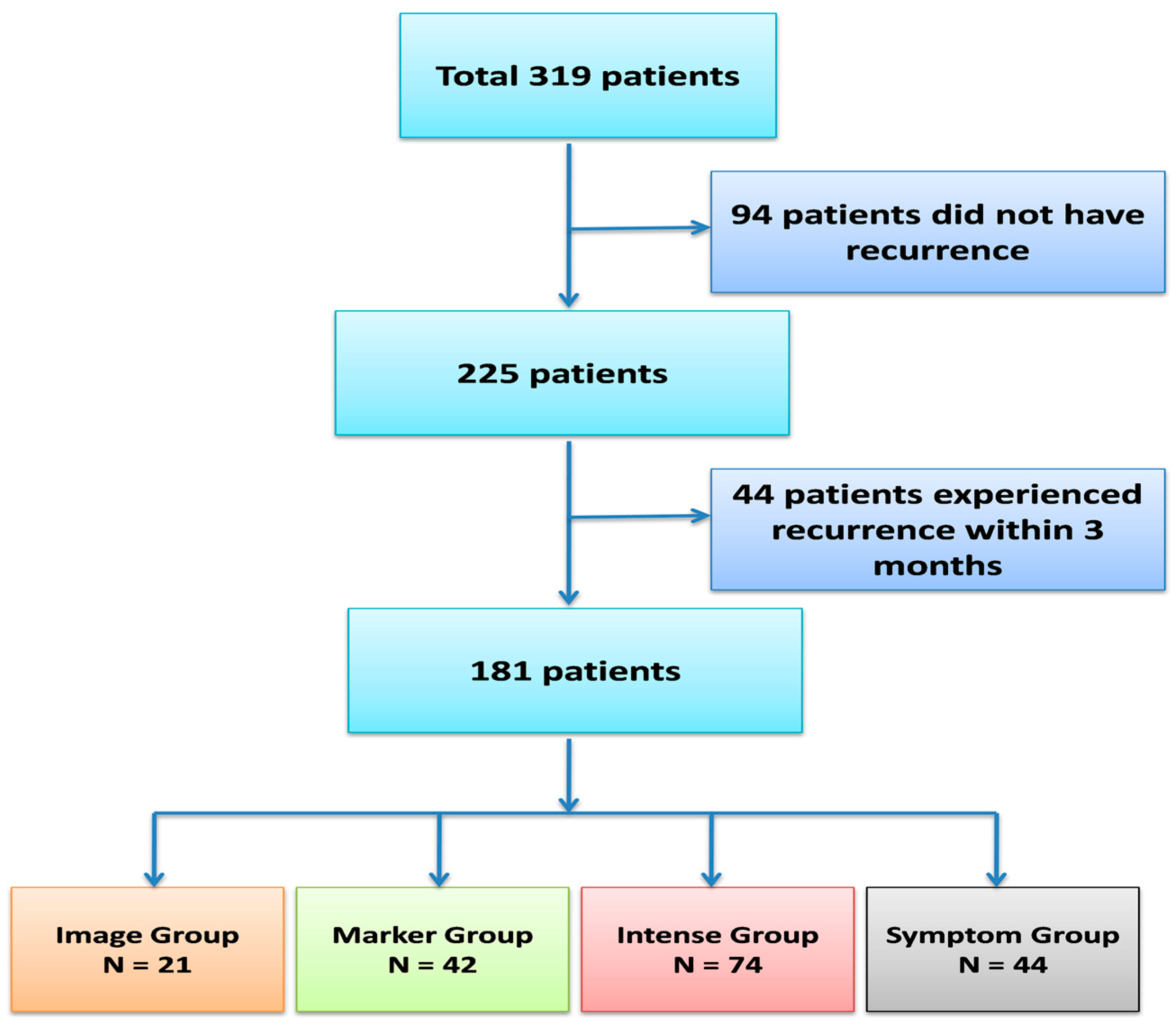



| 8 | Symptom Group (n = 44) | Imaging Group (n = 21) | Marker Group (n = 42) | Intense Group (n = 74) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |||
| Age | Median | 72.5 | 70.3 | 65.1 | 66.0 | 0.069 ¶ | ||||
| Gender | Male | 24 | 54.5% | 14 | 66.7% | 29 | 69.0% | 44 | 59.5% | 0.518 |
| Female | 20 | 45.5% | 7 | 33.3% | 13 | 31.0% | 30 | 40.5% | ||
| ECOG PS | 0 | 3 | 6.8% | 3 | 14.3% | 7 | 16.7% | 13 | 17.6% | 0.511 |
| 1 | 34 | 77.3% | 14 | 66.7% | 32 | 76.2% | 55 | 74.3% | ||
| 2 | 7 | 15.9% | 4 | 19.0% | 3 | 7.1% | 5 | 6.8% | ||
| 3 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.4% | ||
| Primary site | Head | 37 | 84.1% | 15 | 71.4% | 33 | 78.6% | 55 | 74.3% | 0.900 |
| Body | 2 | 4.5% | 2 | 9.5% | 3 | 7.1% | 5 | 6.8% | ||
| Tail | 5 | 11.4% | 4 | 19.5% | 6 | 14.3% | 14 | 18.9% | ||
| Section margin | Negative | 33 | 75.0% | 15 | 71.4% | 36 | 85.7% | 59 | 79.7% | 0.510 |
| Positive | 11 | 25.0% | 6 | 28.6% | 6 | 14.3% | 15 | 20.3% | ||
| pT | T1 | 4 | 9.1% | 2 | 9.5% | 1 | 2.4% | 0 | 0.0% | 0.198 |
| T2 | 6 | 13.6% | 3 | 14.3% | 5 | 11.9% | 9 | 12.2% | ||
| T3 | 34 | 77.3% | 16 | 76.2% | 36 | 85.7% | 65 | 87.8% | ||
| T4 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | ||
| pN | N0 | 24 | 54.5% | 10 | 47.6% | 23 | 54.8% | 34 | 45.9% | 0.736 |
| N1 | 20 | 45.5% | 11 | 52.4% | 19 | 45.2% | 40 | 54.1% | ||
| Differentiation | Grade 1 | 9 | 20.5% | 4 | 19.0% | 10 | 23.8% | 10 | 13.5% | 0.764 |
| Grade 2 | 30 | 68.2% | 16 | 76.2% | 29 | 69.0% | 55 | 74.3% | ||
| Grade 3 | 5 | 11.4% | 1 | 4.8% | 3 | 7.1% | 9 | 12.2% | ||
| Adjuvant C/T | No | 40 | 90.9% | 18 | 85.7% | 27 | 64.3% | 41 | 55.4% | <0.001 * |
| Yes | 4 | 9.1% | 3 | 14.3% | 15 | 35.7% | 33 | 44.6% | ||
| Pattern of first R | Distant | 27 | 61.4% | 14 | 66.7% | 34 | 81.0% | 56 | 75.7% | 0.175 |
| Local | 17 | 38.6% | 7 | 33.3% | 8 | 19.0% | 18 | 24.3% | ||
| Treatment after R | No | 24 | 54.5% | 8 | 38.1% | 17 | 40.5% | 24 | 32.4% | 0.130 |
| Yes | 20 | 45.5% | 13 | 61.9% | 25 | 59.5% | 50 | 67.6% | ||
| CA199 post OP (U/mL) | Median | 19.5 | 37.85 | 24.8 | 38.4 | 0.149 ¶ | ||||
| Elevated | 7 | 41.2% | 5 | 55.6% | 17 | 44.7% | 37 | 50.7% | 0.827 | |
| Normal | 10 | 58.8% | 4 | 44.4% | 21 | 55.3% | 36 | 49.3% | ||
| CEA post OP (ng/mL) | Median | 2.45 | 0.91 | 1.26 | 1.52 | 0.042 ¶ * | ||||
| Elevated | 2 | 11.1% | 0 | 0.0% | 1 | 2.9% | 6 | 9.2% | 0.539 | |
| Normal | 16 | 88.9% | 6 | 100.0% | 33 | 97.1% | 59 | 90.8% | ||
| CA199 when R (U/mL) | Median | 209.71 | 1011.95 | 501 | 374.4 | 0.521¶ | ||||
| Elevated | 27 | 81.8% | 9 | 75.0% | 30 | 81.1% | 55 | 78.6% | 0.950 | |
| Normal | 6 | 18.2% | 3 | 25.0% | 7 | 18.9% | 15 | 21.4% | ||
| CEA when R (ng/mL) | Median | 3.0 | 2.89 | 2.45 | 2.3 | 0.435 ¶ | ||||
| Elevated | 12 | 40.0% | 2 | 22.2% | 10 | 33.3% | 15 | 23.4% | 0.366 | |
| Normal | 18 | 60.0% | 7 | 77.8% | 20 | 66.7% | 49 | 76.6% | ||
| Symptom Group | Imaging Group | Marker Group | Intense Group | p-Value | |
|---|---|---|---|---|---|
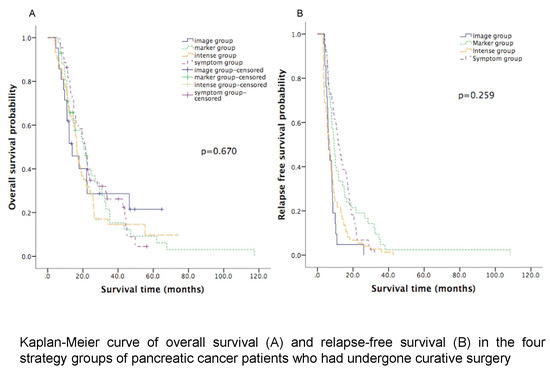
| Median OS (months) | 21.4 (95% CI: 17.9 to 25.0) | 13.9 (95% CI: 6.5 to 21.4) | 20.5 (95% CI: 13.5 to 27.5) | 16.5 (95% CI: 14.9 to 18.1) | 0.670 |
| Median RFS (months) | 11.7 (95% CI: 8.9 to 14.5) | 6.3 (95% CI: 4.6 to 7.9) | 9.3 (95% CI: 7.5 to 11.1) | 6.9 (95% CI: 5.2 to 8.6) | 0.259 |
| Median PROS (months) | 6.9 (95% CI: 2.9 to 10.9) | 7.5 (95% CI: 1.2 to 13.9) | 5.0 (95% CI: 3.0 to 7.0) | 7.8 (95% CI: 5.6 to 10.0) | 0.953 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio (HR) | 95.0% CI for HR | P-Value | Hazard Ratio (HR) | 95.0% CI for HR | P-Value | |
| Adjuvant chemotherapy Yes vs. No | 1.003 | 0.696–1.445 | 0.988 | 0.925 | 0.596–1.436 | 0.728 |
| ECOG PS 0 vs. ≥1 | 0.626 | 0.381–1.028 | 0.064 | 0.516 | 0.295–0.903 | 0.020 * |
| Primary site Non-tail vs. tail | 0.930 | 0.599–1.445 | 0.747 | 0.599 | 0.360–0.997 | 0.049 * |
| Section margin Positive vs. Negative | 1.230 | 0.817–1.852 | 0.322 | 1.128 | 0.736–1.729 | 0.581 |
| T stage T1/T2 vs. T3 | 0.732 | 0.471–1.139 | 0.167 | 0.623 | 0.387–1.002 | 0.051 |
| N stage N1 vs. N0 | 1.406 | 1.010–1.956 | 0.043 * | 1.368 | 0.961–1.947 | 0.082 |
| Differentiation Grade 1/2 vs. Grade 3 | 0.595 | 0.353–1.002 | 0.051 | 0.553 | 0.322–0.950 | 0.032 * |
| Treatment after recurrence Yes vs. No | 0.958 | 0.682–1.347 | 0.806 | 0.996 | 0.675–1.469 | 0.983 |
| Follow up strategy | 0.985 | 0.831–1.167 | 0.862 | 0.996 | 0.837–1.184 | 0.960 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Guo, J.-C.; Yang, S.-H.; Tien, Y.-W.; Kuo, S.-H. Postoperative Imaging and Tumor Marker Surveillance in Resected Pancreatic Cancer. J. Clin. Med. 2019, 8, 1115. https://doi.org/10.3390/jcm8081115
Wu H, Guo J-C, Yang S-H, Tien Y-W, Kuo S-H. Postoperative Imaging and Tumor Marker Surveillance in Resected Pancreatic Cancer. Journal of Clinical Medicine. 2019; 8(8):1115. https://doi.org/10.3390/jcm8081115
Chicago/Turabian StyleWu, Hsu, Jhe-Cyuan Guo, Shih-Hung Yang, Yu-Wen Tien, and Sung-Hsin Kuo. 2019. "Postoperative Imaging and Tumor Marker Surveillance in Resected Pancreatic Cancer" Journal of Clinical Medicine 8, no. 8: 1115. https://doi.org/10.3390/jcm8081115