Total Intravenous Anesthesia Maintained the Degree of Pre-Existing Mitral Regurgitation Better than Isoflurane Anesthesia in Cardiac Surgery: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Randomization and Blinding
2.3. Anesthesia Protocol
2.4. TEE Measurement
2.4.1. Preoperative TEE
2.4.2. Intraoperative TEE
2.5. MR Data Acquisition
2.6. Statistics
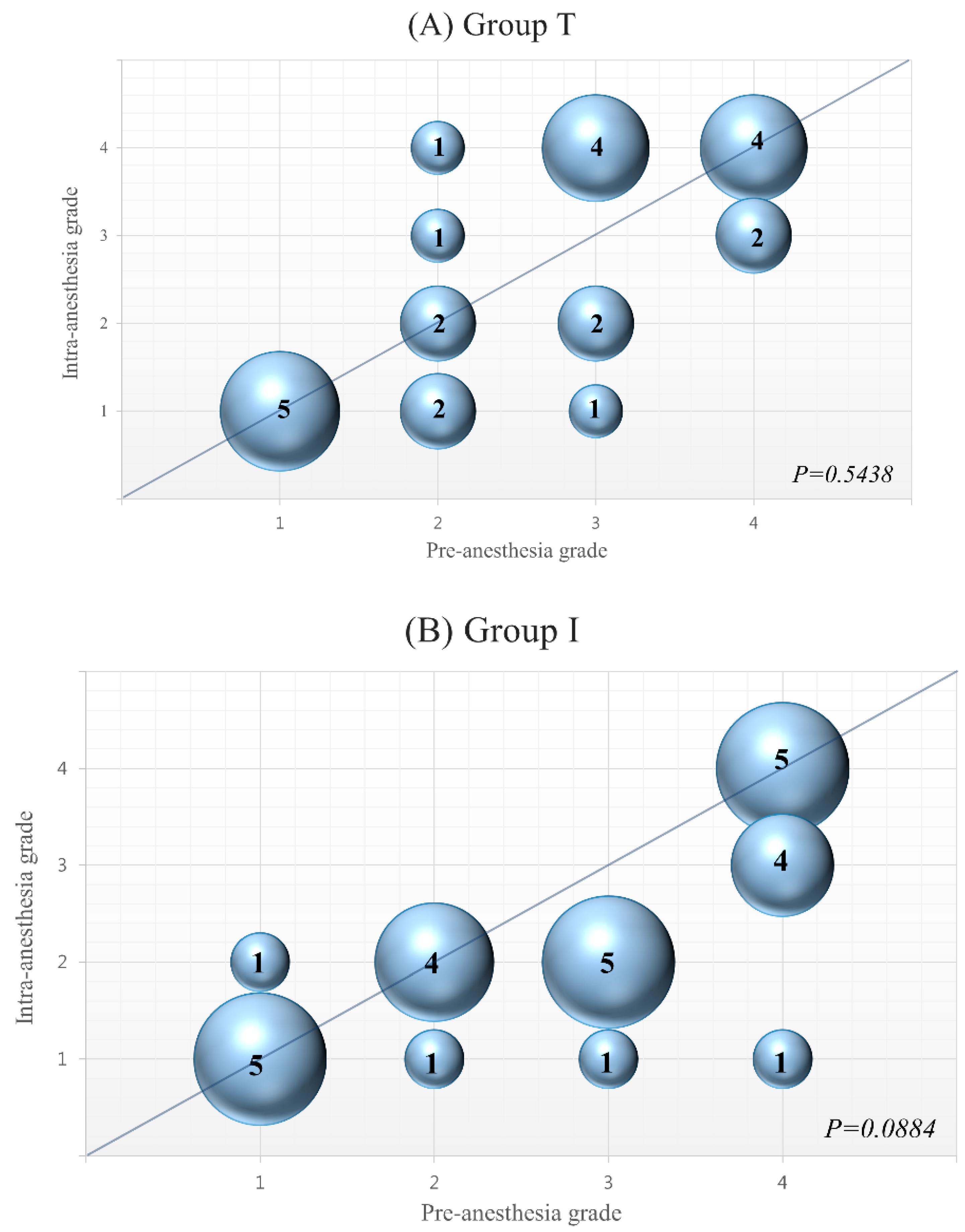
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mallidi, H.R.; Pelletier, M.P.; Lamb, J.; Desai, N.; Sever, J.; Christakis, G.T.; Cohen, G.; Goldman, B.S.; Fremes, S.E. Late outcomes in patients with uncorrected mild to moderate mitral regurgitation at the time of isolated coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2004, 127, 636–644. [Google Scholar] [CrossRef] [PubMed]
- James, K.B.; Marwick, T.; Cosgrove, D.M. Underestimation of mitral regurgitation under general anesthesia. J. Thorac. Cardiovasc. Surg. 1992, 104, 534–535. [Google Scholar] [PubMed]
- Scholte, A.J.; Holman, E.R.; Haverkamp, M.C.; Poldermans, D.; van der Wall, E.E.; Dion, R.A.; Bax, J.J. Underestimation of severity of mitral regurgitation with varying hemodynamics. Eur. J. Echocardiogr. 2005, 6, 297–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kersten, J.R.; Orth, K.G.; Pagel, P.S.; Mei, D.A.; Gross, G.J.; Warltier, D.C. Role of adenosine in isoflurane-induced cardioprotection. Anesthesiology 1997, 86, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Frassdorf, J.; Borowski, A.; Ebel, D.; Feindt, P.; Hermes, M.; Meemann, T.; Weber, R.; Mullenheim, J.; Weber, N.C.; Preckel, B.; et al. Impact of preconditioning protocol on anesthetic-induced cardioprotection in patients having coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 2009, 137, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Olson, J.M.; Paterson, M.; Yan, Y.; Zaja, I.; Liu, Y.; Riess, M.L.; Kersten, J.R.; Liang, M.; Warltier, D.C.; et al. MicroRNA-21 Mediates Isoflurane-induced Cardioprotection against Ischemia-Reperfusion Injury via Akt/Nitric Oxide Synthase/Mitochondrial Permeability Transition Pore Pathway. Anesthesiology 2015, 123, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Piriou, V.; Chiari, P.; Lhuillier, F.; Bastien, O.; Loufoua, J.; Raisky, O.; David, J.S.; Ovize, M.; Lehot, J.J. Pharmacological preconditioning: Comparison of desflurane, sevoflurane, isoflurane and halothane in rabbit myocardium. Br. J. Anaesth. 2002, 89, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Belhomme, D.; Peynet, J.; Louzy, M.; Launay, J.M.; Kitakaze, M.; Menasche, P. Evidence for preconditioning by isoflurane in coronary artery bypass graft surgery. Circulation 1999, 100, II340–II344. [Google Scholar] [CrossRef]
- Tinker, J.H.; Covino, B.G.; Longnecker, D.E. Pharmacology of inhalational anesthetics. In Principles and Practice of Anesthesiology, 2nd ed.; Mosby Year Book: St. Louis, MO, USA, 1998; Volume 11232. [Google Scholar]
- Meng, T.; Bu, W.; Ren, X.; Chen, X.; Yu, J.; Eckenhoff, R.G.; Gao, W.D. Molecular mechanism of anesthetic-induced depression of myocardial contraction. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 2915–2925. [Google Scholar] [CrossRef]
- Royse, C.F.; Liew, D.F.; Wright, C.E.; Royse, A.G.; Angus, J.A. Persistent depression of contractility and vasodilation with propofol but not with sevoflurane or desflurane in rabbits. Anesthesiology 2008, 108, 87–93. [Google Scholar] [CrossRef]
- Morison, D.H. New iv induction anaesthetics. Can. J. Anaesth. J. Canadien D’anesthesie 1993, 40, R9–R18. [Google Scholar] [CrossRef]
- Stewart, W.J.; Currie, P.J.; Salcedo, E.E.; Klein, A.L.; Marwick, T.; Agler, D.A.; Homa, D.; Cosgrove, D.M. Evaluation of mitral leaflet motion by echocardiography and jet direction by Doppler color flow mapping to determine the mechanisms of mitral regurgitation. J. Am. Coll. Cardiol. 1992, 20, 1353–1361. [Google Scholar] [CrossRef]
- Carpentier, A. Cardiac valve surgery—The “French correction”. J. Thorac. Cardiovasc. Surg. 1983, 86, 323–337. [Google Scholar] [PubMed]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; Nihoyannopoulos, P.; Otto, C.M.; Quinones, M.A.; Rakowski, H.; et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2003, 16, 777–802. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Miller, F.A., Jr.; Hayes, S.N.; Bailey, K.R.; Tajik, A.J.; Seward, J.B. Effective mitral regurgitant orifice area: Clinical use and pitfalls of the proximal isovelocity surface area method. J. Am. Coll. Cardiol. 1995, 25, 703–709. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Ogawa, T.; Doshi, R.; Patel, D.; Quan, M.; Henry, W.L.; Gardin, J.M. Doppler color flow “proximal isovelocity surface area” method for estimating volume flow rate: Effects of orifice shape and machine factors. J. Am. Coll. Cardiol. 1991, 17, 1103–1111. [Google Scholar] [CrossRef]
- Grewal, K.S.; Malkowski, M.J.; Piracha, A.R.; Astbury, J.C.; Kramer, C.M.; Dianzumba, S.; Reichek, N. Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am. J. Cardiol. 2000, 85, 199–203. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Johnson, C.; Bellavia, D.; Morsolini, M.; Romano, G.; Santonocito, C.; Centineo, L.; Pastore, F.; Pilato, M.; Arcadipane, A. Mitral Regurgitation Grading in the Operating Room: A Systematic Review and Meta-analysis Comparing Preoperative and Intraoperative Assessments During Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1681–1691. [Google Scholar] [CrossRef]
- Pagel, P.S.; Warltier, D.C. Negative inotropic effects of propofol as evaluated by the regional preload recruitable stroke work relationship in chronically instrumented dogs. Anesthesiology 1993, 78, 100–108. [Google Scholar] [CrossRef]
- Muzi, M.; Berens, R.A.; Kampine, J.P.; Ebert, T.J. Venodilation contributes to propofol-mediated hypotension in humans. Anesth. Analg. 1992, 74, 877–883. [Google Scholar] [CrossRef]
- Goodchild, C.S.; Serrao, J.M. Cardiovascular effects of propofol in the anaesthetized dog. Br. J. Anaesth. 1989, 63, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.A.; Gibson, C.N.; Boyett, M.R.; Hopkins, P.M.; Harrison, S.M. Effects of isoflurane, sevoflurane, and halothane on myofilament Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release in rat ventricular myocytes. Anesthesiology 2000, 93, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, B.W.; Mehner, R.W.; Stowe, D.F.; Bosnjak, Z.J.; Kampine, J.P. Direct myocardial effects of halothane and isoflurane. Comparison between adult and infant rabbits. Anesthesiology 1994, 81, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Deryck, Y.L.; Brimioulle, S.; Maggiorini, M.; de Canniere, D.; Naeije, R. Systemic vascular effects of isoflurane versus propofol anesthesia in dogs. Anesth. Analg. 1996, 83, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Caines, D.; Sinclair, M.; Valverde, A.; Dyson, D.; Gaitero, L.; Wood, D. Comparison of isoflurane and propofol for maintenance of anesthesia in dogs with intracranial disease undergoing magnetic resonance imaging. Vet. Anaesth. Analg. 2014, 41, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Shiran, A.; Merdler, A.; Ismir, E.; Ammar, R.; Zlotnick, A.Y.; Aravot, D.; Lazarovici, H.; Zisman, E.; Pizov, R.; Lewis, B.S. Intraoperative transesophageal echocardiography using a quantitative dynamic loading test for the evaluation of ischemic mitral regurgitation. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2007, 20, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, A.; Souliere, V.; Denault, A.Y.; Bouchard, D.; Couture, P.; Pellerin, M.; Carrier, M.; Levesque, S.; Ducharme, A.; Basmadjian, A.J. Dynamic quantitative echocardiographic evaluation of mitral regurgitation in the operating department. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2006, 19, 140–146. [Google Scholar] [CrossRef]
- Mihalatos, D.G.; Gopal, A.S.; Kates, R.; Toole, R.S.; Bercow, N.R.; Lamendola, C.; Berkay, S.H.; Damus, P.; Robinson, N.; Grimson, R.; et al. Intraoperative assessment of mitral regurgitation: Role of phenylephrine challenge. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2006, 19, 1158–1164. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Doshi, R.; Patel, D.; Mehta, K.; Nguyen, D.; Henry, W.L.; Gardin, J.M. Calculation of volume flow rate by the proximal isovelocity surface area method: Simplified approach using color Doppler zero baseline shift. J. Am. Coll. Cardiol. 1993, 22, 277–282. [Google Scholar] [CrossRef]
- Yasuda, N.; Targ, A.G.; Eger, E.I., 2nd; Johnson, B.H.; Weiskopf, R.B. Pharmacokinetics of desflurane, sevoflurane, isoflurane, and halothane in pigs. Anesth. Analg. 1990, 71, 340–348. [Google Scholar] [CrossRef]
- Hemmerling, T.; Olivier, J.F.; Le, N.; Prieto, I.; Bracco, D. Myocardial protection by isoflurane vs. sevoflurane in ultra-fast-track anaesthesia for off-pump aortocoronary bypass grafting. Eur. J. Anaesthesiol. 2008, 25, 230–236. [Google Scholar] [CrossRef] [PubMed]


| TIVA (N = 27) | Isoflurane (N = 27) | |
|---|---|---|
| Gender (Female/Male) | 14/13 | 10/17 |
| Age, years | 61 (11) | 62 (10) |
| Weight, kg | 61 (11) | 67 (13) |
| Height, cm | 161 (8) | 164 (11) |
| ASA PS (III/IV) | 13/14 | 14/13 |
| Carpentier classification (I/II/IIIa/IIIb) | 0/14/9/4 | 0/13/9/5 |
| Operation | ||
| MVR/CABG | 23/4 | 22/5 |
| Underlying disease | 16 | 16 |
| Diabetes mellitus | 7 | 7 |
| Hypertension | 8 | 11 |
| Atrial fibrillation | 17 | 16 |
| Others | 14 | 11 |
| Smoking | 9 | 6 |
| Alcohol | 12 | 11 |
| Beta blocker | 7 | 9 |
| ACEi/ARB | 7 | 5 |
| Digoxin | 6 | 2 |
| Pre-Anesthesia | Intra-Anesthesia | |||||
|---|---|---|---|---|---|---|
| TIVA (N = 27) | Isoflurane (N = 27) | p | TIVA (N = 27) | Isoflurane (N = 27) | p | |
| HR (beat/min) | 70 (54 to 81) | 76 (65 to 88) | 0.05 | 63 (59 to 86) | 70 (65 to 84) | 0.50 |
| SBP (mmHg) | 112 (103 to126) | 112 (104 to 129) | 0.62 | 113 (16) | 102 (18) | 0.023 |
| MBP (mmHg) | 80 (12) | 83 (13) | 0.448 | 80 (14) | 71 (13) | 0.0304 |
| DBP (mmHg) | 65 (13) | 70 (13) | 0.126 | 62 (16) | 62 (13) | 0.85 |
| EF (%) | 62 (59 to 64) | 57 (52 to 64) | 0.15 | 53 (52 to 60) | 49 (44 to 55) | 0.007 |
| RVSP (mmHg) | 43 (15) | 45 (17) | 0.76 | 39 (12) | 39 (14) | 0.99 |
| RWMA | 1 | 5 | 0.08 | 1 | 5 | 0.08 |
| LAE/RAE | 27/10 | 27/8 | -/0.85 | 27/10 | 27/8 | -/0.85 |
| PISA (cm) | 0.85 (0.17) | 0.85 (0.26) | 1 | 0.77 (0.2) | 0.72(0.22) | 0.39 |
| EROA () | 34 (25 to 41) | 35 (22 to 51) | 0.65 | 25 (13 to 42) | 26 (15 to 42) | 0.67 |
| RV (mL/beat) | 52 (22) | 54 (30) | 0.76 | 53 (29 to 73) | 36 (27 to 57) | 0.25 |
| Total | TIVA (N = 27) | Isoflurane (N = 27) | p |
| ΔHR (beat/min) | 4 (−8, 13) | −3 (−11, 0) | 0.10 |
| ΔSBP (mmHg) | −1 (−8, 6) | −14 (−23, −6) | 0.017 |
| ΔMBP (mmHg) | 0 (−6, 6) | −11 (−19, −4) | 0.0192 |
| ΔDBP (mmHg) | −2 (−9, 4) | −8 (−15, −2) | 0.17 |
| ΔEF (%) | −6 (−7, −4) | −7 (−9, −6) | 0.18 |
| ΔRVSP (mmHg) | −5 (−7 to −2) | −6 (−9 to −2) | 0.56 |
| ΔPISA (cm) | −0.08 (−0.14, −0.02) | −0.13 (−0.19, −0.08) | 0.21 |
| ΔEROA () | −4.73 (−9.61, 0.15) | −9.07 (−14.64, −3.50) | 0.23 |
| ΔRV (mL/beat) | −0.20 (−6.15, 5.75) | −9.66 (−15.77, −3.56) | 0.0266 |
| MR grade 1,2 | TIVA (N = 11) | Isoflurane (N = 11) | p |
| ΔHR (beat/min) | 3 (−8, 13) | 3 (−12, 19) | 0.95 |
| ΔSBP (mmHg) | 4 (−9, 17) | −15 (−30, 0) | 0.0410 |
| ΔMBP (mmHg) | −1 (−13, 13) | −6 (−28, 3) | 0.3091 |
| ΔDBP (mmHg) | −5 (−18, 7) | −4 (−14, 6) | 0.86 |
| ΔEF (%) | −5 (−7, −4) | −6 (−8, −3) | 0.69 |
| ΔRVSP (mmHg) | −6 (−9, −3) | −5 (−9, −1) | 0.75 |
| ΔPISA (cm) | −0.08 (−0.17, 0.01) | −0.065 (−0.15, 0.02) | 0.75 |
| ΔEROA () | −4.38 (−12.26, 3.50) | −0.48 (−4.78, 3.82) | 0.34 |
| ΔRV (mL/beat) | −2.24 (−7.80, 2.74) | −2.58 (−9.60, 12.20) | 0.92 |
| MR grade 3, 4 | TIVA (N = 16) | Isoflurane (N = 16) | p |
| ΔHR (beat/min) | 1 (−7, 9) | −7 (−14, 0) | 0.13 |
| ΔSBP (mmHg) | −4 (−14, 5) | −14 (−26, −2) | 0.18 |
| ΔMBP (mmHg) | −1 (11, 15) | −15 (−27, −5) | 0.0373 |
| ΔDBP (mmHg) | 0 (−8, 8) | −11 (−21, −2) | 0.06 |
| ΔEF (%) | −6 (−9, −4) | −8 (−10, −6) | 0.19 |
| ΔRVSP (mmHg) | −4 (−7, 0) | −6 (−12, 0) | 0.42 |
| ΔPISA (cm) | −0.13 (−0.19, −0.04) | −0.19 (−0.24, −0.11) | 0.14 |
| ΔEROA () | −4.98 (−11.95, 1.20) | −13.47 (−21.95, −4.98) | 0.10 |
| ΔRV (mL/beat) | −0.33 (−9.10, 8.44) | −16.20 (−24.22, −8.18) † | 0.0079 |
| Carpentier classification (Ⅱ) | TIVA (N = 14) | Isoflurane (N = 13) | |
| ΔHR (beat/min) | 1.5 (−11, 9) | −7 (−13, 3) | 0.47 |
| ΔSBP (mmHg) | −6 (−17, 1) | −9 (−20, 4) | 0.94 |
| ΔMBP (mmHg) | −4 (−13. 3) | −3 (16, 4) | 0.84 |
| ΔDBP (mmHg) | −4 (−14, 3) | −1 (−14, 6) | 0.78 |
| ΔEF (%) | −4 (−8, 0) | −10 (−11, −6) | 0.12 |
| ΔRVSP (mmHg) | −4 (−9, 0) | −5 (−10, −1) | 0.69 |
| ΔPISA (cm) | −0.11 (−0.20, 0) | −0.12 (0.18, −0.06) | 0.71 |
| ΔEROA () | −4.75 (−12.60, 3.76) | −9.00 (−20.23, −3.24) | 0.19 |
| ΔRV (mL/beat) | −2.95 (−9.04, 5.65) | −11.00 (−14.71, −3.50) | 0.10 |
| Carpentier classification (Ⅲa/Ⅲb) | TIVA (N = 13) | Isoflurane (N = 14) | |
| ΔHR (beat/min) | 6 (−4, 12) | −1.5 (−13, 12) | 0.50 |
| ΔSBP (mmHg) | 2 (−4, 18) | −14 (−34, −8) | 0.0021 |
| ΔMBP (mmHg) | 3 (−8, 12) | −12 (−23, −7) | 0.0124 |
| ΔDBP (mmHg) | 6 (−9, 12) | −12 (−22, −3) | 0.0375 |
| ΔEF (%) | −6 (−8, −4) | −7 (−8, −4) | 0.87 |
| ΔRVSP (mmHg) | −5 (−8, −1) | −6 (−12, 1) | 0.70 |
| ΔPISA (cm) | −0.05 (−0.15, 0.03) | −0.15 (−0.23, −0.04) | 0.20 |
| ΔEROA () | −1.60 (11.44, 1.30) | −1.90 (−13.07, 3.33) | 0.97 |
| ΔRV (mL/beat) | −3.73 (−9.22, 12.04) | −7.95 (−21.67, 1.32) | 0.12 |
| β-Coefficient | Standard Error | p-Value | |
|---|---|---|---|
| ΔHR (beat/min) | 0.00681 | 0.143 | 0.96 |
| ΔSBP (mmHg) | 0.023 | 0.118 | 0.85 |
| Group T | Reference | ||
| Group I | −9.181 | 4.467 | 0.0451 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.H.; Ahn, H.J.; Yi, J.-W. Total Intravenous Anesthesia Maintained the Degree of Pre-Existing Mitral Regurgitation Better than Isoflurane Anesthesia in Cardiac Surgery: A Randomized Controlled Trial. J. Clin. Med. 2019, 8, 1104. https://doi.org/10.3390/jcm8081104
Ahn JH, Ahn HJ, Yi J-W. Total Intravenous Anesthesia Maintained the Degree of Pre-Existing Mitral Regurgitation Better than Isoflurane Anesthesia in Cardiac Surgery: A Randomized Controlled Trial. Journal of Clinical Medicine. 2019; 8(8):1104. https://doi.org/10.3390/jcm8081104
Chicago/Turabian StyleAhn, Jin Hee, Hyun Joo Ahn, and Jae-Woo Yi. 2019. "Total Intravenous Anesthesia Maintained the Degree of Pre-Existing Mitral Regurgitation Better than Isoflurane Anesthesia in Cardiac Surgery: A Randomized Controlled Trial" Journal of Clinical Medicine 8, no. 8: 1104. https://doi.org/10.3390/jcm8081104
APA StyleAhn, J. H., Ahn, H. J., & Yi, J.-W. (2019). Total Intravenous Anesthesia Maintained the Degree of Pre-Existing Mitral Regurgitation Better than Isoflurane Anesthesia in Cardiac Surgery: A Randomized Controlled Trial. Journal of Clinical Medicine, 8(8), 1104. https://doi.org/10.3390/jcm8081104





