Targeting Tyrosine Phosphatases by 3-Bromopyruvate Overcomes Hyperactivation of Platelets from Gastrointestinal Cancer Patients
Abstract
:1. Introduction
2. Material and Methods
2.1. Antibodies and Reagents
2.2. Cell Culture
2.3. Platelet Preparation
2.4. Patient Information
2.5. Platelet Aggregation Assay by Light Transmission
2.6. Platelet Activation Assay
2.7. Platelet-Cancer Cells Interaction Assays
2.8. Western Blot
2.9. Immunoprecipitation Phosphatases
2.10. Phosphatase Activity Assay
2.11. MTT Assay
2.12. Statistical Analysis
3. Results
3.1. Protein Tyrosine Phosphatases Are Selectively Activated by Classic Platelet Agonists in Healthy Blood Donors
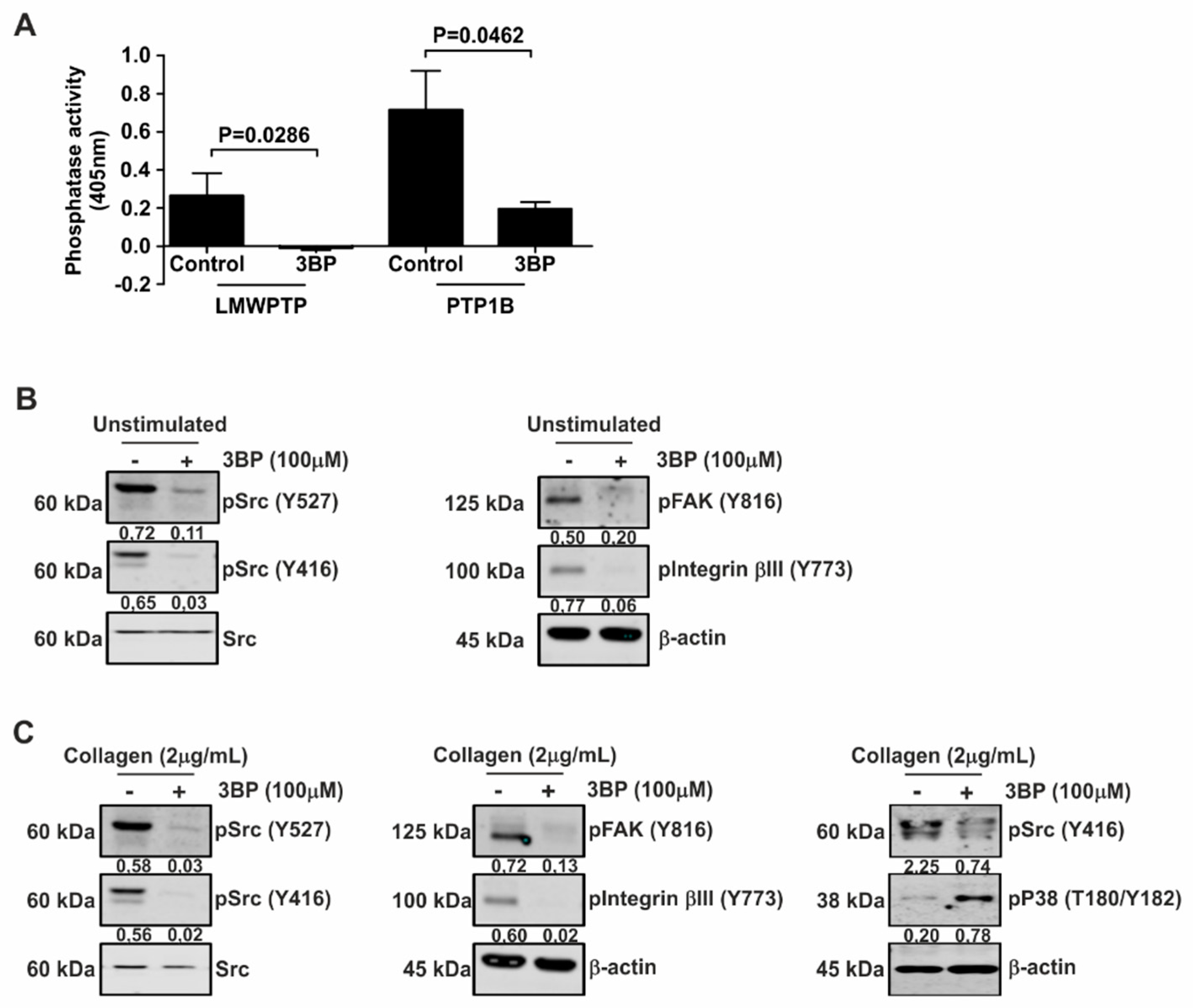
3.2. Collagen-Induced Intracellular Signaling in Platelets from Healthy Donors is Inhibited by 3-BP
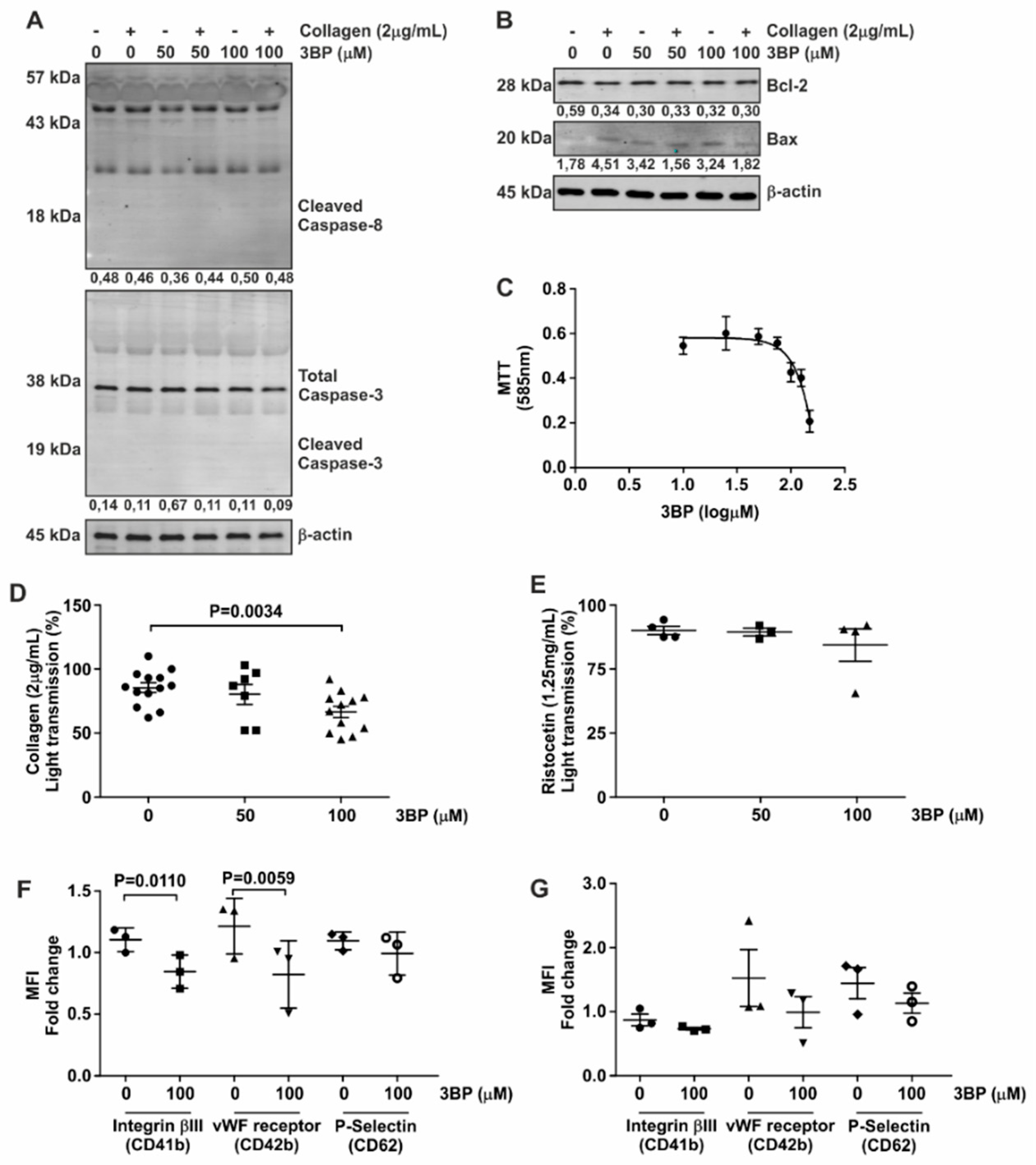
3.3. 3-BP Abrogates Collagen-Induced Platelet Aggregation
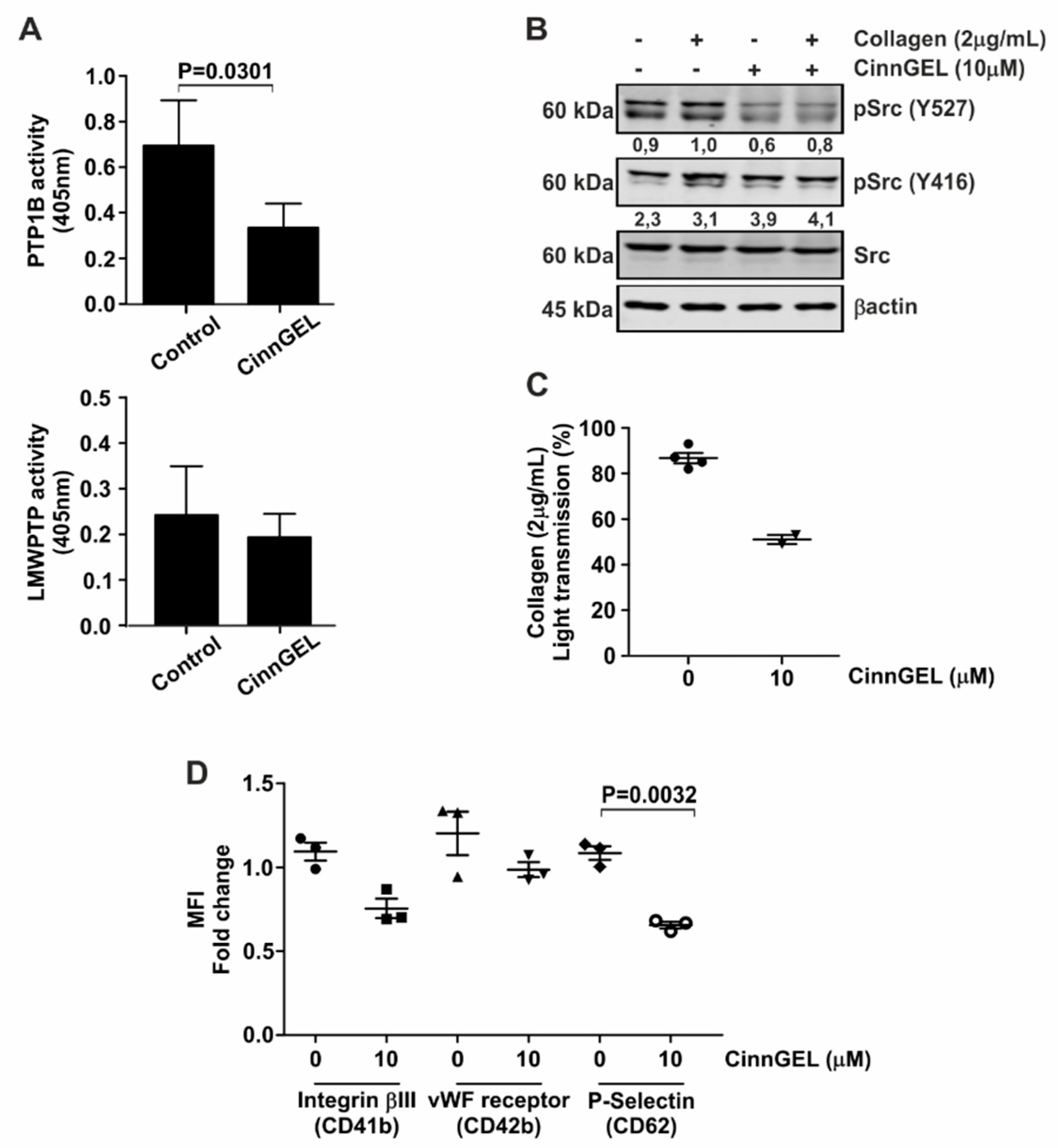
3.4. Platelet Function Is Dependent on Specific Phosphatases
3.5. 3-BP Decreases the Capacity of Colorectal Cancer Cell Lines to Activate Platelets
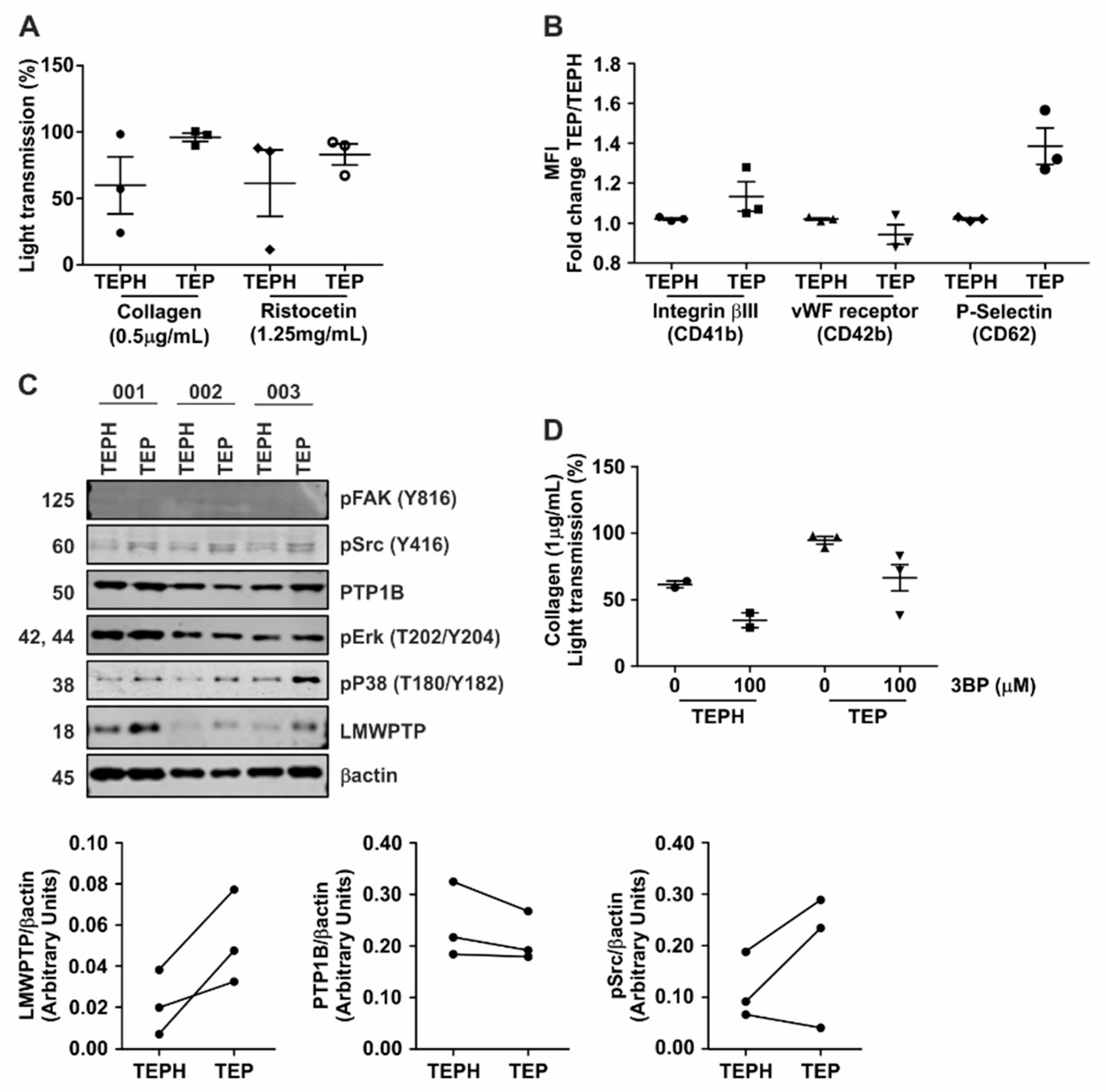
3.6. Hyperactivity of Platelets from Gastrointestinal Cancer Patients Is Reduced upon Treatment with 3-BP
4. Discussion and Conclusion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandén, P.; Svensson, P.J.; Själander, A. Venous thromboembolism and cancer risk. J. Thromb. Thrombolysis 2017, 43, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Culakova, E.; Poniewierski, M.S.; Kuderer, N.M. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb. Res. 2018, 164 (Suppl. 1), S112–S118. [Google Scholar] [CrossRef] [PubMed]
- Trousseau, A. Phlegmasia alba dolens. Clin. Med. Hotel-Dieu Paris 1865, 3, 654–712. [Google Scholar]
- Donnellan, E.; Khorana, A.A. Cancer and Venous Thromboembolic Disease: A Review. Oncologist 2017, 22, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palumbo, A.; Cavo, M.; Bringhen, S.; Zamagni, E.; Romano, A.; Patriarca, F.; Rossi, D.; Gentilini, F.; Crippa, C.; Galli, M.; et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: A phase III, open-label, randomized trial. J. Clin. Oncol. 2011, 29, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Otto, F.; A Baron, J.; Brown, P.H.; Burn, J.; Greenwald, P.; Jankowski, J.; La Vecchia, C.; Meyskens, F.; Senn, H.J.; et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: An international consensus statement. Lancet Oncol. 2009, 10, 501–507. [Google Scholar] [CrossRef]
- Blom, J.W.; Doggen, C.J.M.; Osanto, S.; Rosendaal, F.R. Malignancies, Prothrombotic Mutations, and the Risk of Venous Thrombosis. JAMA 2005, 293, 715–722. [Google Scholar] [CrossRef]
- Menter, D.G.; Tucker, S.C.; Kopetz, S.; Sood, A.K.; Crissman, J.D.; Honn, K.V. Platelets and cancer: A casual or causal relationship: Revisited. Cancer Metastasis Rev. 2014, 33, 231–269. [Google Scholar] [CrossRef]
- Falanga, A.; Russo, L.; Milesi, V.; Vignoli, A. Mechanisms and risk factors of thrombosis in cancer. Crit. Rev. Oncol./Hematol. 2017, 118, 79–83. [Google Scholar] [CrossRef]
- Rezania, S.; Puskarich, M.A.; Petrusca, D.N.; Neto-Neves, E.M.; Rondina, M.T.; Kline, J.A. Platelet hyperactivation, apoptosis and hypercoagulability in patients with acute pulmonary embolism. Thromb. Res. 2017, 155, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Zhang, D.; Oswald, B.E.; Carrim, N.; Wang, X.; Hou, Y.; Zhang, Q.; LaValle, C.; McKeown, T.; Marshall, A.H.; et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit. Rev. Clin. Lab. Sci. 2016, 53, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Winter, W.E.; Flax, S.D.; Harris, N.S. Coagulation Testing in the Core Laboratory. Lab. Med. 2017, 48, 295–313. [Google Scholar] [CrossRef] [PubMed]
- van Geffen, J.P.; Swieringa, F.; Heemskerk, J.W. Platelets and coagulation in thrombus formation: Aberrations in the Scott syndrome. Thromb. Res. 2016, 141 (Suppl. 2), S12–S16. [Google Scholar] [CrossRef]
- Ezumi, Y.; Shindoh, K.; Tsuji, M.; Takayama, H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor gamma chain complex on human platelets. J. Exp. Med. 1998, 188, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meijden, P.E.J.; Feijge, M.A.H.; Swieringa, F.; Gilio, K.; Nergiz-Unal, R.; Hamulyak, K.; Heemskerk, J.W.M.; Meijden, P.E.J. Key role of integrin α(IIb)β(3) signaling to Syk kinase in tissue factor-induced thrombin generation. Cell. Mol. Life Sci. 2012, 69, 3481–3492. [Google Scholar] [CrossRef]
- Ferreira, P.A.; Ruela de Sousa, R.R.; Queiroz, K.C.S.; Souza, A.C.S.; Milani, R.; Pilli, R.A.; Peppelenbosch, M.P.; Hertog, J.; Ferreira, C.V. Knocking down Low Molecular Weight Protein Tyrosine Phosphatase (LMW-PTP) Reverts Chemoresistance through Inactivation of Src and Bcr-Abl Proteins. PLoS ONE 2012, 7, e44312. [Google Scholar] [CrossRef]
- Ko, Y.H.; Verhoeven, H.A.; Lee, M.J.; Corbin, D.J.; Vogl, T.J.; Pedersen, P.L. A translational study “case report” on the small molecule “energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: From bench side to bedside. J. Bioenerg. Biomembr. 2012, 44, 163–170. [Google Scholar] [CrossRef]
- El Sayed, S.M. Enhancing anticancer effects, decreasing risks and solving practical problems facing 3-bromopyruvate in clinical oncology: 10 years of research experience. Int. J. Nanomed. 2018, 13, 4699–4709. [Google Scholar] [CrossRef]
- Andrade, S.S.; Gouvea, I.E.; Silva, M.C.C.; Castro, E.D.; De Paula, C.A.A.; Okamoto, D.; Oliveira, L.; Peres, G.B.; Ottaiano, T.; Facina, G.; et al. Cathepsin K induces platelet dysfunction and affects cell signaling in breast cancer-molecularly distinct behavior of cathepsin K in breast cancer. BMC Cancer 2016, 16, 173. [Google Scholar] [CrossRef]
- Born, G.V.R.; Cross, M.J. The aggregation of blood platelets. J. Physiol. 1963, 168, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.M.; Fuhler, G.M.; Queiroz, K.C.; Scholma, J.; Goorden, S.; Anink, J.; Spek, C.A.; Hoogeveen-Westerveld, M.; Bruno, M.J.; Nellist, M.; et al. PAK2 is an effector of TSC1/2 signaling independent of mTOR and a potential therapeutic target for Tuberous Sclerosis Complex. Sci. Rep. 2015, 28, 14534. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Jurasz, P.; Santos-Martinez, M.J.; Jeong, S.S.; Mitsky, T.; Chen, R.; Radomski, M.W. Platelet aggregation-induced by caco-2 cells: Regulation by matrix metalloproteinase-2 and adenosine diphosphate. J. Pharmacol. Exp. Ther. 2006, 317, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, E.; Das, A.M.; Swets, M.; Cao, W.; Van Der Woude, C.J.; Bruno, M.J.; Peppelenbosch, M.P.; Kuppen, P.J.K.; Hagen, T.L.M.T.; Fuhler, G.M. Increased PTP1B expression and phosphatase activity in colorectal cancer results in a more invasive phenotype and worse patient outcome. Oncotarget 2016, 7, 21922–21938. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, E.; Kodach, L.L.; Das, A.M.; Ruela-De-Sousa, R.R.; Ferreira, C.V.; Hardwick, J.C.; Van Der Woude, C.J.; Peppelenbosch, M.P.; Hagen, T.L.T.; Fuhler, G.M. Low molecular weight protein tyrosine phosphatase (LMWPTP) upregulation mediates malignant potential in colorectal cancer. Oncotarget 2015, 6, 8300–8312. [Google Scholar] [CrossRef] [Green Version]
- Shiri, R.; Yari, F.; Ahmadinejad, M.; Vaeli, S.; Tabatabaei, M.R. The caspase-3 inhibitor (peptide Z-DEVD-FMK) affects the survival and function of platelets in platelet concentrate during storage. Blood Res. 2014, 49, 49–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tison, P.; Kubisz, P.; Cernácek, P.; Dzúrik, R. Influence of inhibitor of glucose utilization on the blood platelet function. Nephron 1983, 33, 253–256. [Google Scholar] [CrossRef]
- Davison, K.; Mann, K.K.; Waxman, S.; Miller, W.H., Jr. JNK activation is a mediator of arsenic trioxide-induced apoptosis in acute promyelocytic leukemia cells. Blood 2004, 103, 3496–3502. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Chen, M.; Zhao, L.; Zhao, Q.; Hu, R.; Zhu, J.; Yan, R.; Dai, K. Ethanol Induces Platelet Apoptosis. Alcohol. Clin. Exp. Res. 2017, 41, 291–298. [Google Scholar] [CrossRef]
- Ravi, S.; Chacko, B.; Sawada, H.; Kramer, P.A.; Johnson, M.S.; Benavides, G.A.; O’Donnell, V.; Marques, M.B.; Darley-Usmar, V.M. Metabolic plasticity in resting and thrombin activated platelets. PLoS ONE 2015, 10, e0123597. [Google Scholar] [CrossRef]
- Nayak, M.K.; Dhanesha, N.; Doddapattar, P.; Rodriguez, O.; Sonkar, V.K.; Dayal, S.; Chauhan, A.K. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Adv. 2018, 2, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Silva, J.; Queirós, O.; Ribeiro, A.; Baltazar, F.; Young, K.H.; Pedersen, P.L.; Preto, A.; Casal, M. The cytotoxicity of 3-bromopyruvate in breast cancer cells depends on extracellular pH. Biochem. J. 2015, 467, 247–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vistica, D.T.; Skehan, P.; Scudiero, D.; Monks, A.; Pittman, A.; Boyd, M.R. Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res. 1991, 51, 2515–2520. [Google Scholar] [PubMed]
- Roberts, D.E.; McNicol, A.; Bose, R. Mechanism of Collagen Activation in Human Platelets. J. Biol. Chem. 2004, 279, 19421–19430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farndale, R.W. Collagen-induced platelet activation. Blood Cells Mol. Dis. 2006, 36, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Kornblith, L.Z.; Robles, A.J.; Conroy, A.S.; Hendrickson, C.M.; Calfee, C.S.; Fields, A.T.; Callcut, R.A.; Cohen, M.J. Perhaps it’s not the platelet: Ristocetin uncovers the potential role of von Willebrand factor in impaired platelet aggregation following traumatic brain injury. J. Trauma Acute Care Surg. 2018, 85, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Jurasz, P.; Alonso-Escolano, D.; Radomski, M.W. Platelet—Cancer interactions: Mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br. J. Pharmacol. 2004, 143, 819–826. [Google Scholar] [CrossRef]
- Van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Brunelle, J.K.; Ryan, J.; Yecies, D.; Opferman, J.T.; Letai, A. MCL-1-dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts. J. Cell Biol. 2009, 187, 429–442. [Google Scholar] [CrossRef]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [CrossRef] [Green Version]
- Estevez, B.; Du, X. New Concepts and Mechanisms of Platelet Activation Signaling. Physiology 2017, 32, 162–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavergne, M.; Janus-Bell, E.; Schaff, M.; Gachet, C.; Mangin, P.H. Platelet Integrins in Tumor Metastasis: Do They Represent a Therapeutic Target? Cancers 2017, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, L.; Kuijpers, M.J.; Gilio, K.; Hego, A.; Théâtre, E.; Maurissen, L.; Vandereyken, M.; Diogo, C.V.; Lecut, C.; Guilmain, W.; et al. Dual-specificity phosphatase 3 deficiency or inhibition limits platelet activation and arterial thrombosis. Circulation 2015, 131, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Tautz, L.; Senis, Y.A.; Oury, C.; Rahmouni, S. Perspective: Tyrosine phosphatases as novel targets for antiplatelet therapy. Bioorg. Med. Chem. 2015, 23, 2786–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchay, S.M.; Kim, N.; Grunz, E.A.; Fay, W.P.; Chishti, A.H. Double knockouts reveal that protein tyrosine phosphatase 1B is a physiological target of calpain-1 in platelets. Mol. Cell Biol. 2007, 27, 6038–6052. [Google Scholar] [CrossRef] [PubMed]
- Senis, Y.A. Protein-tyrosine phosphatases: A new frontier in platelet signal transduction. J. Thromb. Haemost. 2013, 11, 1800–1813. [Google Scholar] [CrossRef]
- Zacharski, L.R. The biologic basis for anticoagulant treatment of cancer. Prog. Clin. Biol. Res. 1982, 89, 113–129. [Google Scholar]
- Takahashi, K.; Ito, H.; Hashimoto, M.; Mita, K.; Asakawa, H.; Hayashi, T.; Fujino, K. Does antithrombotic therapy improve survival with colorectal cancer? World J. Surg. Oncol. 2017, 15, 161. [Google Scholar] [CrossRef]
- Cooke, N.M.; Egan, K.; McFadden, S.; Grogan, L.; Breathnach, O.S.; O’Leary, J.; Hennessy, B.T.; Kenny, D. Increased platelet reactivity in patients with late-stage metastatic cancer. Cancer Med. 2013, 2, 564–570. [Google Scholar] [CrossRef]
- Basu, A.; Gosain, R.; Tantry, U.; Miller, K.; Gurbel, P.A. Platelet Activation and Aggregation in Patients with Advanced Adenocarcinoma Undergoing Chemotherapy: Correlation with a Validated Venous Thromboembolism Risk Score. Blood 2015, 126, 3445. [Google Scholar]
- Geddings, J.E.; Hisada, Y.; Boulaftali, Y.; Getz, T.M.; Whelihan, M.; Fuentes, R.; Dee, R.; Cooley, B.C.; Key, N.S.; Wolberg, A.S.; et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J. Thromb. Haemost. 2016, 14, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Cazenave, J.P.; Ohlmann, P.; Cassel, D.; Eckly, A.; Hechler, B.; Gachet, C. Preparation of washed platelet suspensions from human and rodent blood. Methods Mol. Biol. 2004, 272, 13–28. [Google Scholar] [PubMed]
- Jankowski, J.; Henning, L.; Schlüter, H. Analysis of the releasable nucleotides of platelets. Methods Mol. Biol. 2004, 272, 97–108. [Google Scholar] [PubMed]
- Canault, M.; Duerschmied, D.; Brill, A.; Stefanini, L.; Schatzberg, D.; Cifuni, S.M.; Bergmeier, W.; Wagner, D.D. p38 mitogen-activated protein kinase activation during platelet storage: Consequences for platelet recovery and hemostatic function In Vivo. Blood 2010, 115, 1835–1842. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, A.V.S.; Andrade, S.S.; Reijm, A.N.; Spaander, M.C.W.; de Maat, M.P.M.; Peppelenbosch, M.P.; Ferreira-Halder, C.V.; Fuhler, G.M. Targeting Tyrosine Phosphatases by 3-Bromopyruvate Overcomes Hyperactivation of Platelets from Gastrointestinal Cancer Patients. J. Clin. Med. 2019, 8, 936. https://doi.org/10.3390/jcm8070936
Faria AVS, Andrade SS, Reijm AN, Spaander MCW, de Maat MPM, Peppelenbosch MP, Ferreira-Halder CV, Fuhler GM. Targeting Tyrosine Phosphatases by 3-Bromopyruvate Overcomes Hyperactivation of Platelets from Gastrointestinal Cancer Patients. Journal of Clinical Medicine. 2019; 8(7):936. https://doi.org/10.3390/jcm8070936
Chicago/Turabian StyleFaria, Alessandra V. S., Sheila S. Andrade, Agnes N. Reijm, Manon C. W. Spaander, Moniek P. M. de Maat, Maikel P. Peppelenbosch, Carmen V. Ferreira-Halder, and Gwenny M. Fuhler. 2019. "Targeting Tyrosine Phosphatases by 3-Bromopyruvate Overcomes Hyperactivation of Platelets from Gastrointestinal Cancer Patients" Journal of Clinical Medicine 8, no. 7: 936. https://doi.org/10.3390/jcm8070936





