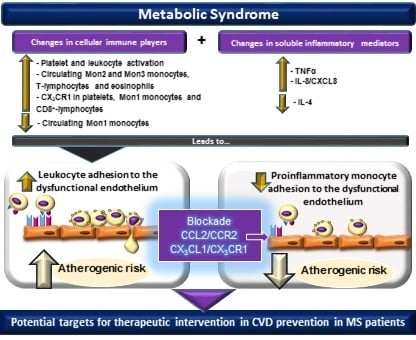
Systemic Inflammation in Metabolic Syndrome: Increased Platelet and Leukocyte Activation, and Key Role of CX3CL1/CX3CR1 and CCL2/CCR2 Axes in Arterial Platelet-Proinflammatory Monocyte Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Human Study Populations
2.3. Flow Cytometry
2.4. Measurement of Soluble Metabolic and Inflammatory Markers
2.5. Leukocyte-Endothelial Cell Interactions under Flow Conditions
2.6. Statistical Analysis
3. Results
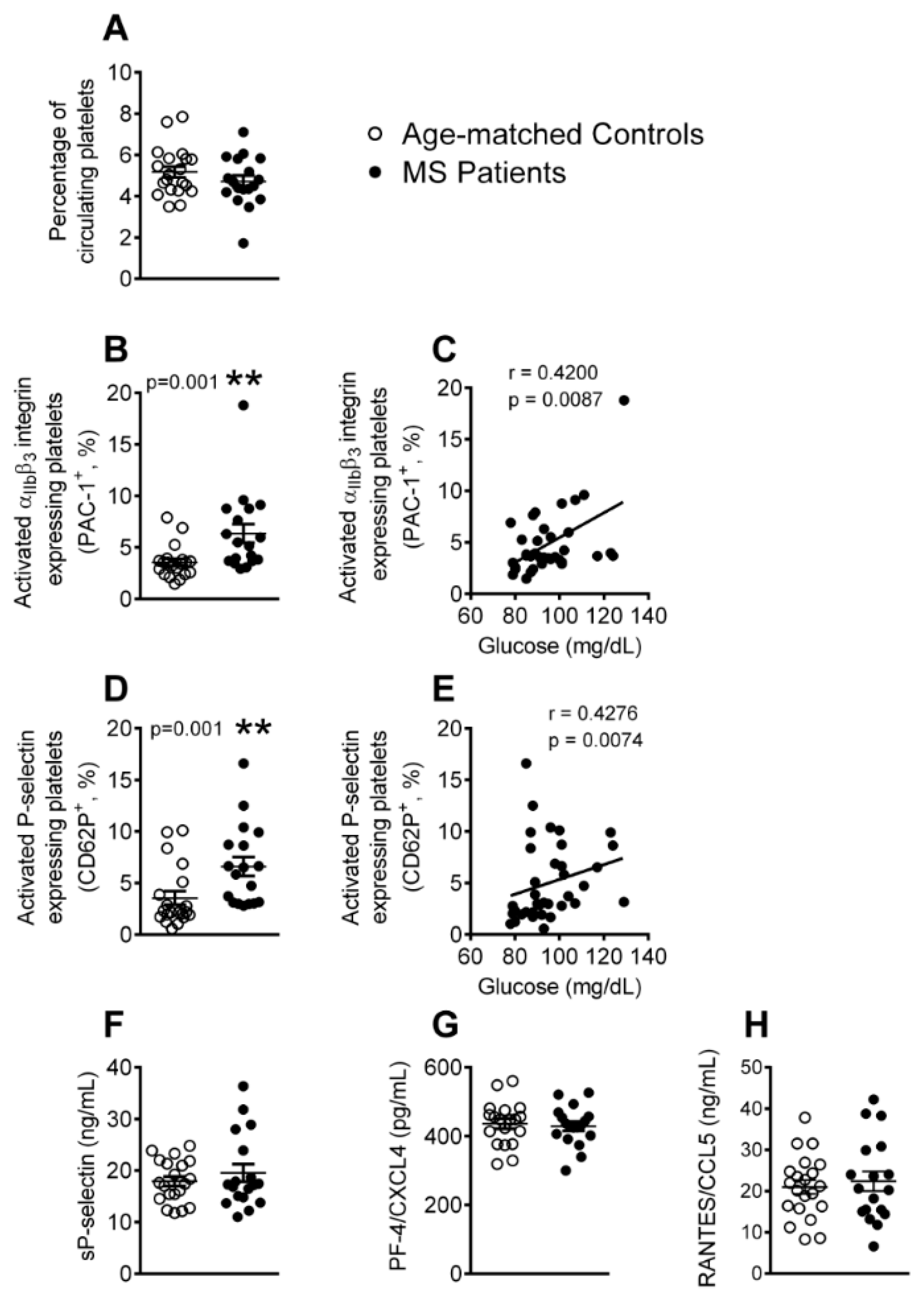
3.1. Platelet Activation is Enhanced in Patients with Metabolic Syndrome
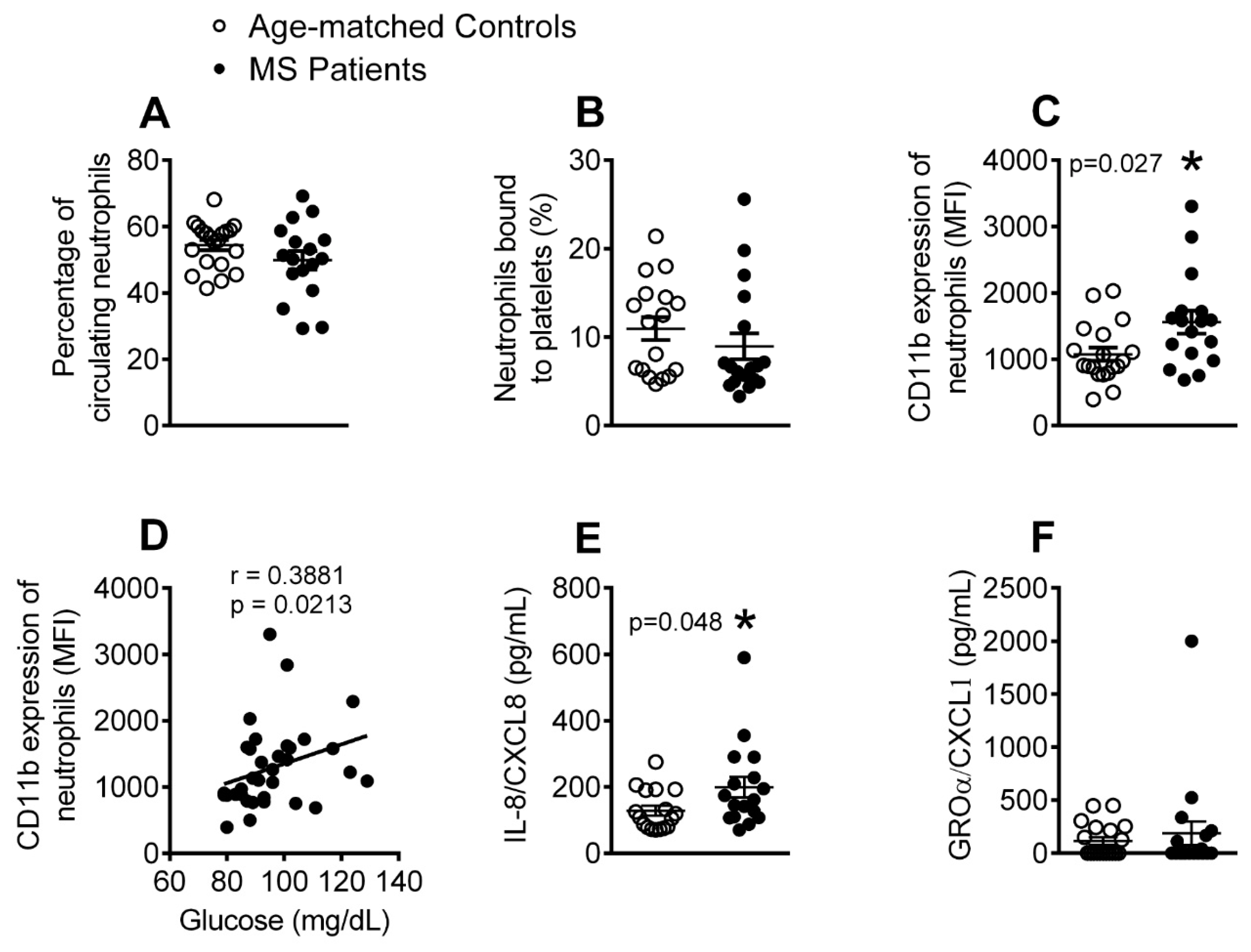
3.2. The Percentage of Activated Neutrophils and Circulating Levels of IL-8 Are Elevated in Patients with Metabolic Syndrome
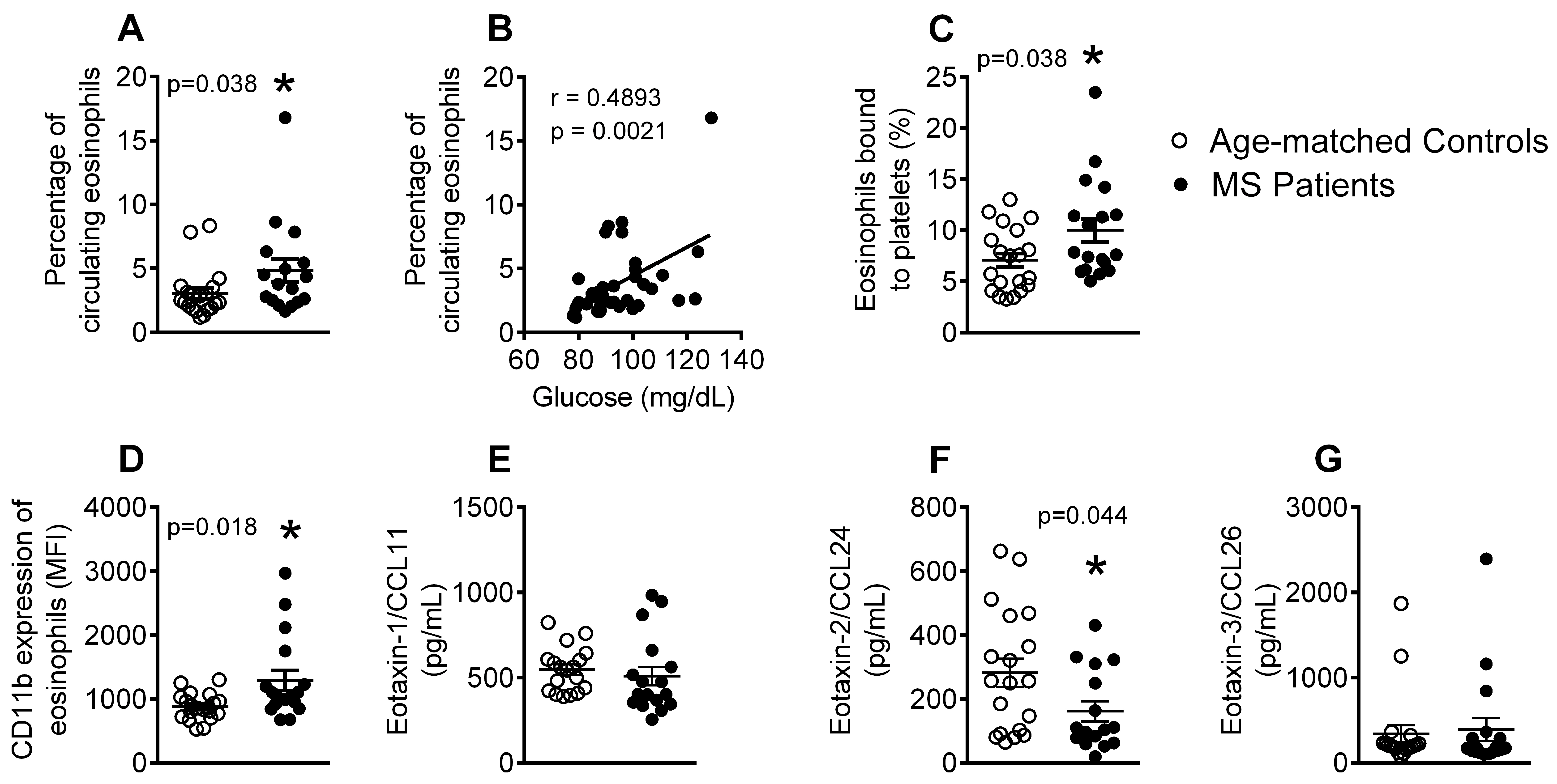
3.3. Patients with Metabolic Syndrome Present a Higher Percentage of Circulating Eosinophils, Increased Number of Eosinophil-Platelet Aggregates, Enhanced Eosinophil Activation and Decreased Eotaxin-2/CCL24 Plasma Levels
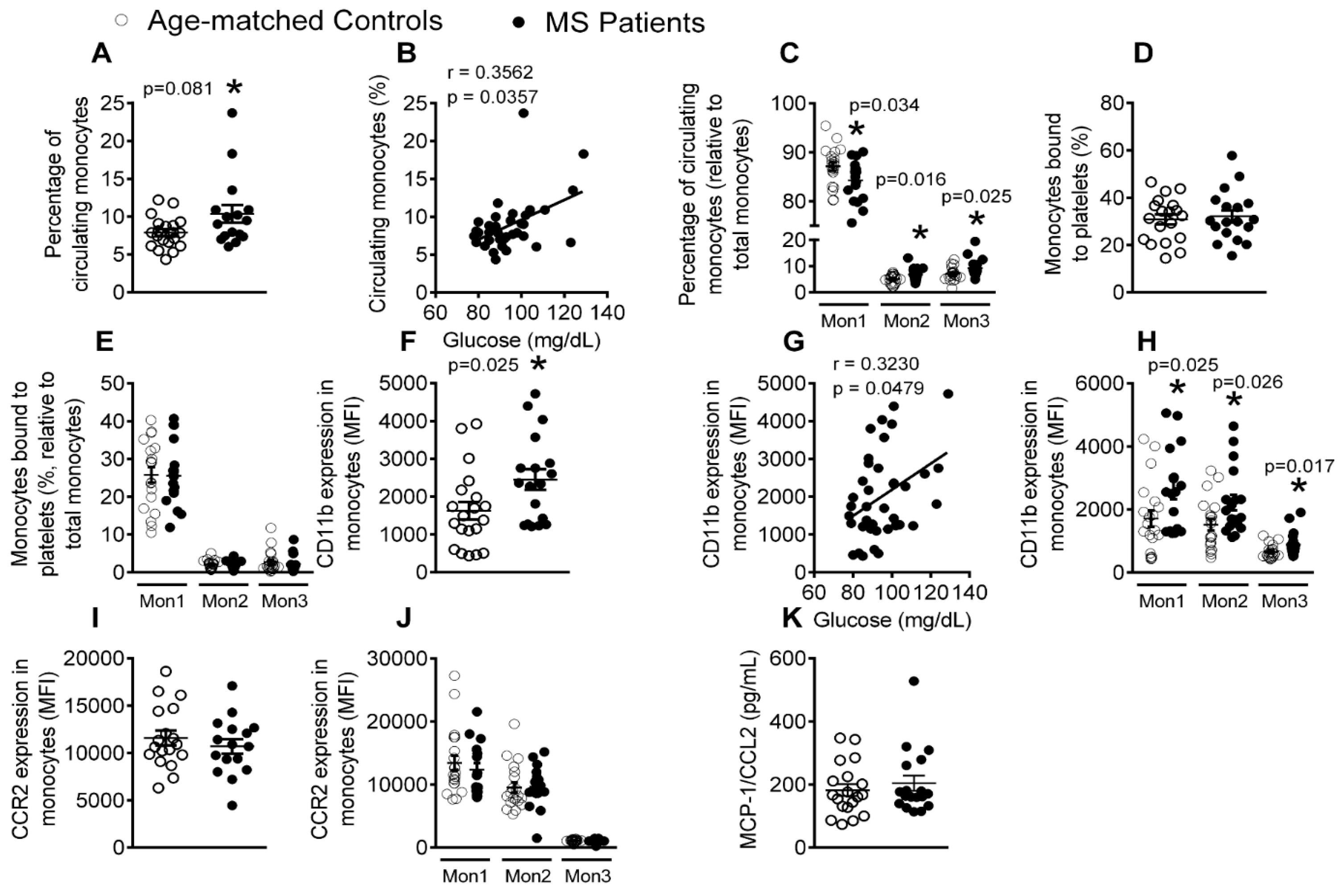
3.4. Patients with Metabolic Syndrome Present a Higher Percentage of Circulating Monocytes and Enhanced Monocyte Activation
3.5. The Percentages of Circulating Lymphocytes, Lymphocyte-Platelet Aggregates and Activated CD8+ Lymphocytes Are Elevated in Patients with Metabolic Syndrome
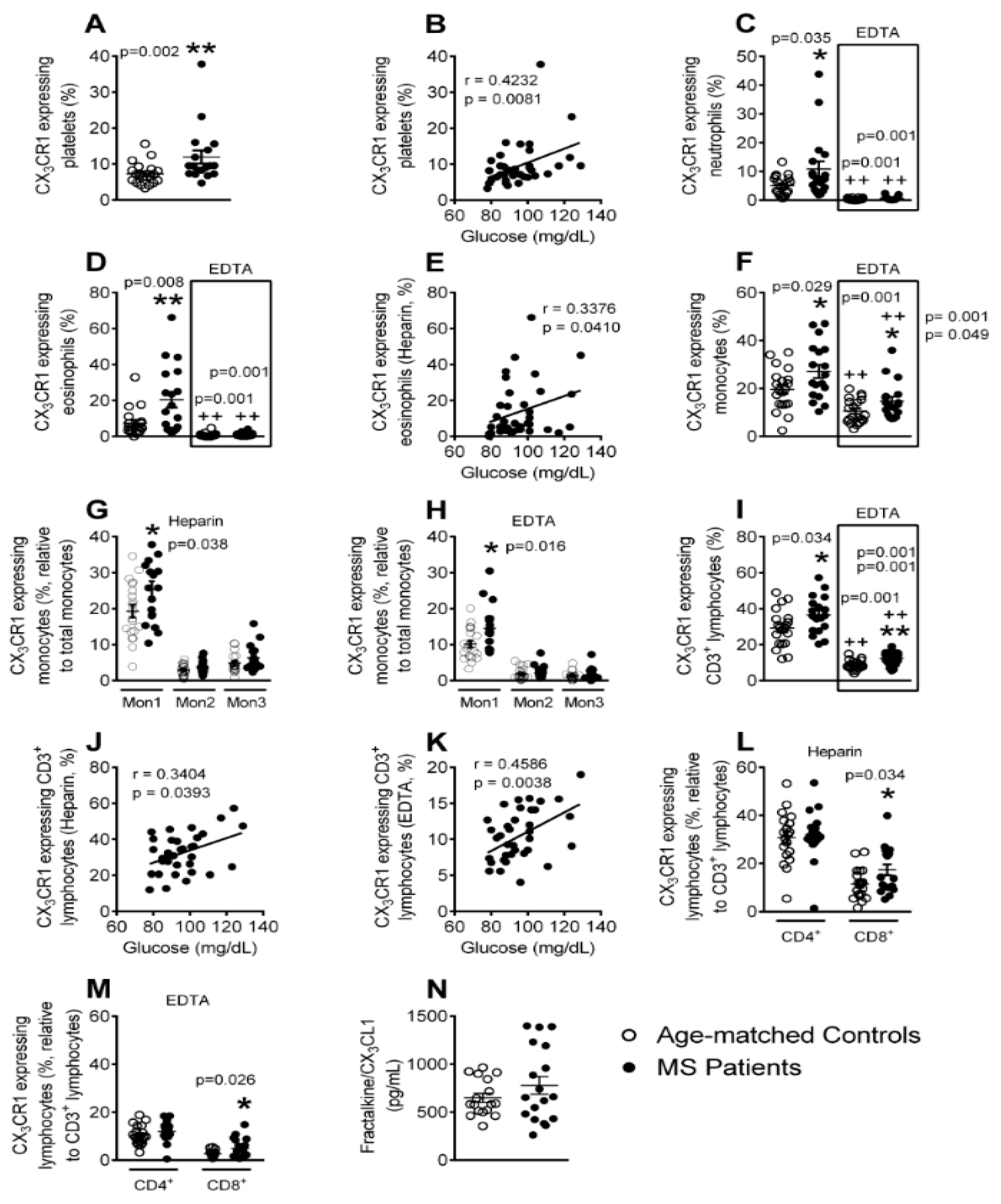
3.6. Enhanced CX3CR1 Expression in Platelets, Different Leukocyte Subsets and Leukocyte-Platelet Aggregates in Patients with Metabolic Syndrome
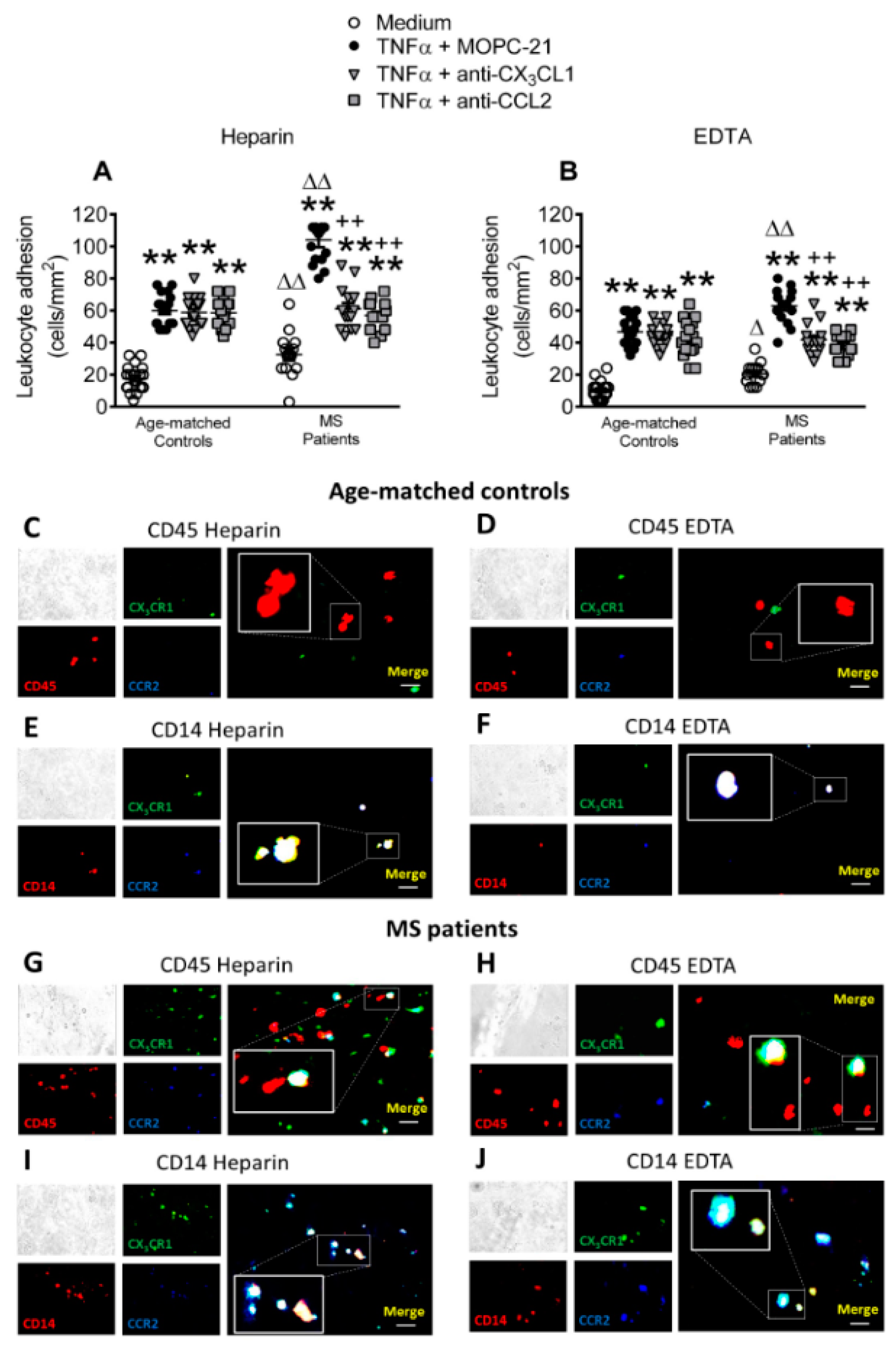
3.7. Circulating Leukocytes from Patients with Metabolic Syndrome Show Superior Adhesiveness to TNFα-Stimulated Arterial Endothelium, Which is Partly Dependent on CX3CL1 and CCL2 Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Novo, S.; Peritore, A.; Guarneri, F.P.; Corrado, E.; Macaione, F.; Evola, S.; Novo, G. Metabolic syndrome (MetS) predicts cardio and cerebrovascular events in a twenty years follow-up. A prospective study. Atherosclerosis 2012, 223, 468–472. [Google Scholar] [CrossRef]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Avogaro, A.; de Kreutzenberg, S.V. Mechanisms of endothelial dysfunction in obesity. Clin. Chim. Acta Int. J. Clin. Chem. 2005, 360, 9–26. [Google Scholar] [CrossRef]
- Esser, N.; Paquot, N.; Scheen, A.J. Inflammatory markers and cardiometabolic diseases. Acta Clin. Belg. 2015, 70, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [PubMed]
- van Rooy, M.J.; Pretorius, E. Metabolic syndrome, platelet activation and the development of transient ischemic attack or thromboembolic stroke. Thromb. Res. 2015, 135, 434–442. [Google Scholar] [CrossRef]
- Dinh, Q.N.; Chrissobolis, S.; Diep, H.; Chan, C.T.; Ferens, D.; Drummond, G.R.; Sobey, C.G. Advanced atherosclerosis is associated with inflammation, vascular dysfunction and oxidative stress, but not hypertension. Pharm. Res. 2017, 116, 70–76. [Google Scholar] [CrossRef]
- Van Huisstede, A.; Cabezas, M.C.; Birnie, E.; van de Geijn, G.J.; Rudolphus, A.; Mannaerts, G.; Njo, T.L.; Hiemstra, P.S.; Braunstahl, G.J. Systemic inflammation and lung function impairment in morbidly obese subjects with the metabolic syndrome. J. Obes. 2013, 2013, 131349. [Google Scholar] [CrossRef]
- Landmesser, U.; Hornig, B.; Drexler, H. Endothelial function: A critical determinant in atherosclerosis? Circulation 2004, 109, II27–II33. [Google Scholar] [CrossRef]
- Collado, A.; Marques, P.; Escudero, P.; Rius, C.; Domingo, E.; Martinez-Hervas, S.; Real, J.T.; Ascaso, J.F.; Piqueras, L.; Sanz, M.J. Functional role of endothelial CXCL16/CXCR6-platelet-leucocyte axis in angiotensin II-associated metabolic disorders. Cardiovasc. Res. 2018, 114, 1764–1775. [Google Scholar] [CrossRef]
- Ishii, M.; Araki, S.; Goto, M.; Yamamoto, Y.; Kusuhara, K. CCL2 level is elevated with metabolic syndrome and CXCL10 level is correlated with visceral fat area in obese children. Endocr. J. 2016, 63, 795–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazidi, M.; Toth, P.P.; Banach, M. C-reactive Protein Is Associated With Prevalence of the Metabolic Syndrome, Hypertension, and Diabetes Mellitus in US Adults. Angiology 2018, 69, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.Q.; Qin, R.R.; Li, Y.H.; Song, D.J.; Chen, T.S.; Zhang, W.; Zhong, M.; Zhang, Y.; Xing, Y.Q.; Wang, Z.H. Endothelial cells microparticle-associated protein disulfide isomerase promotes platelet activation in metabolic syndrome. Oncotarget 2016, 7, 83231–83240. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.M.; Pokharel, Y.; Dadu, R.T.; Lewis, D.E.; Hoogeveen, R.C.; Wu, H.; Ballantyne, C.M. Postprandial Monocyte Activation in Individuals With Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 4195–4204. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.S.; Kim, H.J.; Kang, E.S.; Ahn, C.W.; Lim, S.K.; Lee, H.C.; Cha, B.S. The association of total and differential white blood cell count with metabolic syndrome in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2006, 73, 284–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genc, H.; Dogru, T.; Tapan, S.; Tasci, I.; Bozoglu, E.; Gok, M.; Aslan, F.; Celebi, G.; Erdem, G.; Avcu, F.; et al. Soluble CD40 ligand, soluble P-selectin and von Willebrand factor levels in subjects with prediabetes: The impact of metabolic syndrome. Clin. Biochem. 2012, 45, 92–95. [Google Scholar] [CrossRef]
- Grun, J.L.; Manjarrez-Reyna, A.N.; Gomez-Arauz, A.Y.; Leon-Cabrera, S.; Ruckert, F.; Fragoso, J.M.; Bueno-Hernandez, N.; Islas-Andrade, S.; Melendez-Mier, G.; Escobedo, G. High-Density Lipoprotein Reduction Differentially Modulates to Classical and Nonclassical Monocyte Subpopulations in Metabolic Syndrome Patients and in LPS-Stimulated Primary Human Monocytes In Vitro. J. Immunol. Res. 2018, 2018, 2737040. [Google Scholar] [CrossRef]
- Apostolakis, S.; Amanatidou, V.; Papadakis, E.G.; Spandidos, D.A. Genetic diversity of CX3CR1 gene and coronary artery disease: New insights through a meta-analysis. Atherosclerosis 2009, 207, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Damas, J.K.; Boullier, A.; Waehre, T.; Smith, C.; Sandberg, W.J.; Green, S.; Aukrust, P.; Quehenberger, O. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, is elevated in coronary artery disease and is reduced during statin therapy. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Franca, C.N.; Izar, M.C.O.; Hortencio, M.N.S.; do Amaral, J.B.; Ferreira, C.E.S.; Tuleta, I.D.; Fonseca, F.A.H. Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease. Clin. Sci. 2017, 131, 1215–1224. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, X.; Cai, W.; Liu, T.; Liang, Z.; Sun, Y.; Yan, C.; Han, Y. Chemokine CX3CL1 and its receptor CX3CR1 are associated with human atherosclerotic lesion volnerability. Thrombolysis Res. 2015, 135, 1147–1153. [Google Scholar] [CrossRef]
- Ludwig, A.; Weber, C. Transmembrane chemokines: Versatile ‘special agents’ in vascular inflammation. Thromb. Haemost. 2007, 97, 694–703. [Google Scholar] [PubMed]
- Rius, C.; Piqueras, L.; Gonzalez-Navarro, H.; Albertos, F.; Company, C.; Lopez-Gines, C.; Ludwig, A.; Blanes, J.I.; Morcillo, E.J.; Sanz, M.J. Arterial and venous endothelia display differential functional fractalkine (CX3CL1) expression by angiotensin-II. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Kusano, K.F.; Nakamura, K.; Kusano, H.; Nishii, N.; Banba, K.; Ikeda, T.; Hashimoto, K.; Yamamoto, M.; Fujio, H.; Miura, A.; et al. Significance of the level of monocyte chemoattractant protein-1 in human atherosclerosis. Circ. J. Off. J. Jpn. Circ. Soc. 2004, 68, 671–676. [Google Scholar] [CrossRef]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, A.W.; Wong, C.K.; Lam, C.W. Tumor necrosis factor-alpha up-regulates the expression of CCL2 and adhesion molecules of human proximal tubular epithelial cells through MAPK signaling pathways. Immunobiology 2008, 213, 533–544. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Mummidi, S.; Perla, R.P.; Bysani, S.; Dulin, N.O.; Liu, F.; Melby, P.C. Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem. J. 2003, 373, 547–558. [Google Scholar] [CrossRef] [Green Version]
- Jose, P.J.; Griffiths-Johnson, D.A.; Collins, P.D.; Walsh, D.T.; Moqbel, R.; Totty, N.F.; Truong, O.; Hsuan, J.J.; Williams, T.J. Eotaxin: A potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J. Exp. Med. 1994, 179, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, S.; Krambovitis, E.; Vlata, Z.; Kochiadakis, G.E.; Baritaki, S.; Spandidos, D.A. CX3CR1 receptor is up-regulated in monocytes of coronary artery diseased patients: Impact of pre-inflammatory stimuli and renin-angiotensin system modulators. Thromb. Res. 2007, 121, 387–395. [Google Scholar] [CrossRef]
- Olson, T.S.; Ley, K. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R7–R28. [Google Scholar] [CrossRef] [PubMed]
- von Hundelshausen, P.; Schmitt, M.M. Platelets and their chemokines in atherosclerosis-clinical applications. Front. Physiol. 2014, 5, 294. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Stroes, E.S.; Meijers, J.C.; Buller, H. Hypercoagulability in the metabolic syndrome. Curr. Opin. Pharm. 2005, 5, 155–159. [Google Scholar] [CrossRef]
- Santilli, F.; Vazzana, N.; Liani, R.; Guagnano, M.T.; Davi, G. Platelet activation in obesity and metabolic syndrome. Obes. Rev. 2012, 13, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Khoury, P.; Grayson, P.C.; Klion, A.D. Eosinophils in vasculitis: Characteristics and roles in pathogenesis. Nat. Rev. Rheumatol. 2014, 10, 474–483. [Google Scholar] [CrossRef]
- Weber, C.; Shantsila, E.; Hristov, M.; Caligiuri, G.; Guzik, T.; Heine, G.H.; Hoefer, I.E.; Monaco, C.; Peter, K.; Rainger, E.; et al. Role and analysis of monocyte subsets in cardiovascular disease. Joint consensus document of the European Society of Cardiology (ESC) Working Groups “Atherosclerosis & Vascular Biology” and “Thrombosis”. Thromb. Haemost. 2016, 116, 626–637. [Google Scholar]
- Wu, H.; Ballantyne, C.M. Dyslipidaemia: PCSK9 inhibitors and foamy monocytes in familial hypercholesterolaemia. Nat. Rev. Cardiol. 2017, 14, 385–386. [Google Scholar] [CrossRef]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocyte Conversion During Inflammation and Injury. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef]
- Urra, X.; Villamor, N.; Amaro, S.; Gomez-Choco, M.; Obach, V.; Oleaga, L.; Planas, A.M.; Chamorro, A. Monocyte subtypes predict clinical course and prognosis in human stroke. J. Cereb. Blood Flow Metab. 2009, 29, 994–1002. [Google Scholar] [CrossRef]
- Kyaw, T.; Peter, K.; Li, Y.; Tipping, P.; Toh, B.H.; Bobik, A. Cytotoxic lymphocytes and atherosclerosis: Significance, mechanisms and therapeutic challenges. Br. J. Pharm. 2017, 174, 3956–3972. [Google Scholar] [CrossRef] [PubMed]
- Kolbus, D.; Ljungcrantz, I.; Andersson, L.; Hedblad, B.; Fredrikson, G.N.; Bjorkbacka, H.; Nilsson, J. Association between CD8+ T-cell subsets and cardiovascular disease. J. Intern. Med. 2013, 274, 41–51. [Google Scholar] [CrossRef]
- Pilatz, A.; Hudemann, C.; Wolf, J.; Halefeld, I.; Paradowska-Dogan, A.; Schuppe, H.C.; Hossain, H.; Jiang, Q.; Schultheiss, D.; Renz, H.; et al. Metabolic syndrome and the seminal cytokine network in morbidly obese males. Andrology 2017, 5, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Zanuso, S.; Nicolucci, A.; Fernando, F.; Cavallo, S.; Cardelli, P.; Fallucca, S.; Alessi, E.; Letizia, C.; Jimenez, A.; et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Quandt, D.; Rothe, K.; Scholz, R.; Baerwald, C.W.; Wagner, U. Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis. PLoS ONE 2014, 9, e93293. [Google Scholar] [CrossRef]
- Yoshimoto, T. The Hunt for the Source of Primary Interleukin-4: How We Discovered That Natural Killer T Cells and Basophils Determine T Helper Type 2 Cell Differentiation In Vivo. Front. Immunol. 2018, 9, 716. [Google Scholar] [CrossRef]
- Flierl, U.; Bauersachs, J.; Schafer, A. Modulation of platelet and monocyte function by the chemokine fractalkine (CX3 CL1) in cardiovascular disease. Eur. J. Clin. Investig. 2015, 45, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Njerve, I.U.; Pettersen, A.A.; Opstad, T.B.; Arnesen, H.; Seljeflot, I. Fractalkine and its receptor (CX3CR1) in patients with stable coronary artery disease and diabetes mellitus. Metab. Syndr. Relat. Disord. 2012, 10, 400–406. [Google Scholar] [CrossRef]

| Control Volunteers (n = 21) | Metabolic Syndrome Patients (n = 18) | p Value | |
|---|---|---|---|
| Age (years) | 48.8 ± 2.7 | 52.2 ± 3.2 | 0.42 |
| Gender M/F (%) | 5/16 (23.8/76.2) | 3/15 (16.7/83.3) | 0.59 |
| BMI (kg/m2) | 25.5 ± 0.7 | 31.0 ± 0.9 ** | <0.01 |
| Weight (kg) | 69.4 ± 2.6 | 83.3 ± 2.9 ** | <0.01 |
| Height (cm) | 164.9 ± 2.4 | 163.8 ± 1.7 | 0.71 |
| Waist circumference (cm) total | 85.4 ± 2.0 | 100.0 ± 1.7 ** | <0.01 |
| Waist circumference (cm) male | 92.4 ± 2.1 | 105.0 ± 1.2 ** | <0.01 |
| Waist circumference (cm) female | 83.0 ± 2.3 | 99.0 ± 1.9 ** | <0.01 |
| Systolic blood pressure (mmHg) | 116.6 ± 2.0 | 130.3 ± 2.1 ** | <0.01 |
| Diastolic blood pressure (mmHg) | 71.7 ± 1.9 | 79.9 ± 1.2 ** | <0.01 |
| Glucose (mg/dL) | 86.7 ± 1.5 | 103.8 ± 3.0 ** | <0.01 |
| Insulin (mIU/L) | 7.6 ± 0.9 | 18.4 ± 2.0 ** | <0.01 |
| HOMA Index | 1.7 ±0.2 | 4.7 ± 0.5 ** | <0.01 |
| Total Cholesterol levels (mg/dL) | 206.1 ± 6.8 | 239.5 ± 12.8 * | <0.05 |
| LDL levels (mg/dL) | 130.6 ± 5.4 | 156.2 ± 11.2 * | <0.05 |
| HDL levels (mg/dL) | 66.0 ± 2.5 | 50.5 ± 2.1 ** | <0.01 |
| Triglycerides (mg/dL) | 81.0 ± 7.3 | 181.6 ± 30.0 ** | <0.01 |
| IgG (mg/dL) | 966.7 ± 41.1 | 985.8 ± 48.9 | 0.76 |
| IgM (mg/dL) | 100.4 ± 7.6 | 104.5 ± 13.0 | 0.78 |
| IgE (mg/dL) | 42.6 ± 12.0 | 57.2 ± 17.3 | 0.48 |
| CRP (mg/L) | 1.4 ± 0.2 | 2.1 ± 0.4 | 0.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, P.; Collado, A.; Martinez-Hervás, S.; Domingo, E.; Benito, E.; Piqueras, L.; Real, J.T.; Ascaso, J.F.; Sanz, M.-J. Systemic Inflammation in Metabolic Syndrome: Increased Platelet and Leukocyte Activation, and Key Role of CX3CL1/CX3CR1 and CCL2/CCR2 Axes in Arterial Platelet-Proinflammatory Monocyte Adhesion. J. Clin. Med. 2019, 8, 708. https://doi.org/10.3390/jcm8050708
Marques P, Collado A, Martinez-Hervás S, Domingo E, Benito E, Piqueras L, Real JT, Ascaso JF, Sanz M-J. Systemic Inflammation in Metabolic Syndrome: Increased Platelet and Leukocyte Activation, and Key Role of CX3CL1/CX3CR1 and CCL2/CCR2 Axes in Arterial Platelet-Proinflammatory Monocyte Adhesion. Journal of Clinical Medicine. 2019; 8(5):708. https://doi.org/10.3390/jcm8050708
Chicago/Turabian StyleMarques, Patrice, Aida Collado, Sergio Martinez-Hervás, Elena Domingo, Esther Benito, Laura Piqueras, José T. Real, Juan F. Ascaso, and Maria-Jesus Sanz. 2019. "Systemic Inflammation in Metabolic Syndrome: Increased Platelet and Leukocyte Activation, and Key Role of CX3CL1/CX3CR1 and CCL2/CCR2 Axes in Arterial Platelet-Proinflammatory Monocyte Adhesion" Journal of Clinical Medicine 8, no. 5: 708. https://doi.org/10.3390/jcm8050708