Adult Patient Risk Stratification Using a Risk Score for Periodontitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation and Sampling
2.2. Variables Definition
2.3. Statistical Analysis
2.3.1. Identification of Risk Factors
2.3.2. Development of a Risk Score
3. Results
3.1. Participants
3.2. Risk Model
3.3. Risk Score
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Petersen, P.K.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar] [PubMed]
- Kassebaum, N.J.; Bernabém, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.J.; Lamster, I.B.; Greenspan, J.S.; Pitts, N.B.; Scully, C.; Warnakulasuriva, S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral. Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.L.; Asma, S. Smoking-attributable periodontitis in the United States: Findings from NHANES III. National Health and Nutrition Examination Survey. J. Periodontol. 2000, 71, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C.; Martin, J.; Krall, E.A.; Mancl, L.; Garcia, R. Longitudinal validation of a risk calculator for periodontal disease. J. Clin. Periodontol. 2003, 30, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Tonetti, M.S. Periodontal risk assessment (PRA) for patients in supportive periodontal therapy (SPT). Oral. Health Prev. Dent. 2003, 1, 7–16. [Google Scholar] [PubMed]
- Van Dyke, T.E.; Sheilesh, D. Risk factors for Periodontitis. J. Int. Acad. Periodontol. 2005, 7, 3–7. [Google Scholar] [PubMed]
- Chaffee, B.W.; Weston, S.J. Association between chronic periodontal disease and obesity: A systematic review and meta-analysis. J. Periodontol. 2010, 81, 1708–1724. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Chambrone, D.; Lima, L.A.; Chambrone, L.A. Predictors of tooth loss during long-term periodontal maintenance: A systematic review of observational studies. J. Clin. Periodontol. 2010, 37, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Leininger, M.; Tenenbaum, H.; Davideau, J.L. Modified periodontal risk assessment score: Long-term predictive value of treatment outcomes. A retrospective study. J. Clin. Periodontol. 2010, 37, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Boillot, A.; El Halabi, B.; Batty, G.D.; Rangé, H.; Czernichow, S.; Bouchard, P. Education as a predictor of chronic periodontitis: A systematic review with meta-analysis population-based studies. PLoS ONE 2011, 6, e21508. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Genco, F.D. Common risk factors in the management of periodontal and associated systemic diseases: The dental setting and interprofissional collaboration. J. Evid. Based. Dent. Pract. 2014, 14, S4–S16. [Google Scholar] [CrossRef] [PubMed]
- Koshi, E.; Rajesh, S.; Koshi, P.; Arunima, P.R. Risk assessment of periodontal disease. J. Indian. Soc. Periodontol. 2012, 16, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Zweig, M.H.; Campbell, G. Receiver-Operating Characteristic (ROC) Plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Nobre, M.; Maló, P. Prevalence of periodontitis, dental caries, peri-implant pathology and their relation with systemic status and smoking habits: Results of an open-cohort study with 22009 patients in a private rehabilitation center. J. Dent. 2017, 67, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.C.; Murphy, N.; Johansson, M.; Ferrari, P.; Tsilidis, K.K.; Boutron-Ruault, M.C.; Clavel, F.; Dartois, L.; Li, K.; Kaaks, R. Modifiable causes of premature death in middle-age in Western Europe: Results from the EPIC cohort study. BMC Med. 2016, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Le Polain de Waroux, O.; Maguire, H.; More, A. The case-cohort design in outbreak investigations. Euro. Surveill. 2012, 17, 20202. [Google Scholar] [PubMed]
- American Academy of Periodontology. Parameter on Chronic Periodontitis with Slight to Moderate Loss of Periodontal Support. J. Periodontol. 2000, 71, 853–855. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Periodontology. Parameter on plaque-induced gingivitis. J. Periodontol. 2000, 71, 851–852. [Google Scholar] [CrossRef] [PubMed]
- Goldthorpe, J.H. On Sociology, 2nd ed.; Goldthorpe, J.H., Ed.; Stanford University Press: Stanford, CA, USA, 2000. [Google Scholar]
- Schouten, E.G.; Dekker, J.M.; Kok, F.J.; Le Cessie, S.; van Houwelingen, H.C.; Pool, J.; Vandenbroucke, J.B. Risk ratio and rate ratio estimation in case-cohort designs: Hypertension and cardiovascular mortality. Stat. Med. 1993, 12, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.M.; Massaro, J.M.; D’Agostino, R.B., Sr. Presentation of the multivariate data for clinical use: The Framingham Study risk score functions. Stat. Med. 2004, 30, 1631–1660. [Google Scholar] [CrossRef] [PubMed]
- Mrdovic, I.; Savic, L.; Krljanac, G.; Asanin, M.; Lasica, R.; Djuricic, N.; Brdar, N.; Marinkovic, J.; Kocev, N.; Perunicic, J. Simple risk algorithm to predict serious bleeding in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: RISK-PCI bleeding score. Circ. J. 2013, 77, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Nobre, M.; Mano Azul, A.; Rocha, E.; Maló, P.; Salvado, F. Attributable fractions, modifiable risk factors and risk stratification using a risk score for peri-implant pathology. J. Prosthodont. Res. 2017, 61, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986, 73, 1–11. [Google Scholar] [CrossRef]
- Johannsen, A.; Susin, C.; Gustafsson, A. Smoking and inflammation: Evidence for a synergistic role in chronic disease. Periodontol. 2000 2014, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Zimmermann, N.; Hagenfeld, D.; Veile, A.; Kim, T.S.; Becher, H. Is frequency of tooth brushing a risk factor for periodontitis? A systematic review and meta-analysis. Community Dent. Oral. Epidemiol. 2015, 43, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Winterfeld, K.; Quera, V.; Winterfeld, T.; Ganss, C. Toothbrushing Systematics Index (TSI)—A new tool for quantifying systematics in toothbrushing behaviour. PLoS ONE 2018, 13, e0196497. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Suvan, J.E.; Tonetti, M.S. Risk factor assessment tools for the prevention of periodontitis progression: A systematic review. J. Clin. Periodontol. 2015, 42, 59S–70S. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Schmietendorf, E.; Plaumann, A.; Sälzer, S.; Dörfer, C.E.; Graetz, C. Validation of multivariable models for predicting tooth loss in periodontitis patients. J. Clin. Periodontol. 2018, 45, 701–710. [Google Scholar] [CrossRef] [PubMed]


| Variables | Total | Cases | Controls |
|---|---|---|---|
| Number (%) | 330 (100%) | 155 (47%) | 175 (53%) |
| Age mean (standard deviation) | 53.1 (13.9) | 54.5 (12.9) | 51.8 (14.7) |
| Gender distribution | |||
| Female | 164 (49.7%) | 76 (46.3%) | 88 (53.7%) |
| Male | 166 (51.3%) | 79 (47.6%) | 87 (52.4%) |
| Systemic condition number (%) | 119(100%) | 58 (48.7%) | 61 (51.3%) |
| Hepatitis number (%) | 5 (100%) | 3 (60%) | 2 (40%) |
| Cardiovascular condition number (%) | 62 (100%) | 30 (48.4%) | 32 (51.6%) |
| Thyroid number (%) | 9 (100%) | 6 (66.7%) | 3 (33.3%) |
| Diabetes number (%) | 14 (100%) | 7 (50%) | 7 (50%) |
| Rheumatologic condition number (%) | 17 (100%) | 7 (41.2%) | 10 (58.8%) |
| Oncological number (%) | 6 (100%) | 4 (66.7%) | 2 (33.3%) |
| Inflammatory condition number (%) | 2 (100%) | 0 (0%) | 2 (100%) |
| Neurologic condition number (%) | 1 (100%) | 1 (100%) | 0 (0%) |
| Autoimmune condition number (%) | 3 (100%) | 0 (0%) | 3 (100%) |
| More than one condition number (%) | 17 (100%) | 9 (52.9%) | 8 (47.1%) |
| Smoking habits number (%) | 63 (100%) | 40 (63.5%) | 23 (36.5%) |
| 1st year number of observations mean (standard deviation) | 2.4 (1.0) | 2.3 (1.1) | 2.6 (0.9) |
| Gingivitis at baseline (%) | 110 (100%) | 69 (62.7%) | 41 (37.3%) |
| Socioeconomic Status Code (%) | 330 (100%) | 155 (47%) | 175 (53%) |
| Level 1 (%) | 111 (100%) | 56 (50.5%) | 55 (49.5%) |
| Level 2 (%) | 77 (100%) | 34 (44.2%) | 43 (55.8%) |
| Level 3 (%) | 101 (100%) | 45 (42.6%) | 56 (57.4%) |
| Nonconsidered level (Pensionaries) (%) | 41 (100%) | 21 (51.2%) | 20 (48.8%) |
| Presence of periodontitis number (%) | 169 (100%) | 155 (91.7%) | 14 (8.3%) |
| Type of dentition | |||
| Single-rooted (%) | 46 (100%) | 44 (95.7%) | 2 (4.3%) |
| Multiradicular (%) | 102 (100%) | 92 (90.2%) | 10 (9.8%) |
| Both type of teeth (%) | 182 (100%) | 19 (10.4%) | 163 (89.6%) |
| Presence of subgingival calculus (%) | 195 (100%) | 105 (53.8%) | 90 (46.2%) |
| Mean number teeth present (standard deviation) | 22.5 (7.0) | 23.4 (6.4) | 21.7 (7.4) |
| Dental crowding (%) | 184 (100%) | 89 (48.4%) | 95 (51.6%) |
| Fixed prosthesis supported (%) | 44 (100%) | 23 (52.3%) | 21 (47.7%) |
| Number patients with bruxism (%) | 10 (100%) | 4 (40%) | 6 (60%) |
| History of periodontitis (%) | 177 (100%) | 103 (58.2%) | 74 (41.8%) |
| Variables | Univariable Risk Ratio (95% Confidence Intervals) | Univariable p-Value | Multivariable Risk Ratio (95% Confidence Intervals) | Multivariable p-Value | Multivariable β Coefficient after Bootstrap Validation (Standard Error) | Risk Score Points |
|---|---|---|---|---|---|---|
| Age | ||||||
| >53 | 1.0 (reference) | 1.0 (reference) | ||||
| ≤53 | 0.67 (0.43; 1.02) | 0.062 | 0.53 (0.31; 0.88) | 0.015 | −0.64 (0.111) | −1 |
| Gender | ||||||
| Female | 1.0 (reference) | |||||
| Male | 1.15 (0.76; 1.76) | 0.511 | ||||
| Systemic Problems | ||||||
| Absent | 1.0 (reference) | |||||
| Present | 0.97 (0.61; 1.54) | 0.887 | ||||
| Diabetes | ||||||
| Absent | 1.0 (reference) | |||||
| Present | 1.19 (0.42; 3.36) | 0.739 | ||||
| Cardiovascular | ||||||
| Absent | 1.0 (reference) | |||||
| Present | 0.86 (0.49; 1.48) | 0.578 | ||||
| Rheumatologic | ||||||
| Absent | 1.0 (reference) | |||||
| Present | 0.47 (0.16; 1.45) | 0.190 | ||||
| More than One Condition | ||||||
| Absent | 1.0 (reference) | |||||
| Present | 0.65 (0.22; 1.91) | 0.432 | ||||
| Smoking | ||||||
| Nonsmoker | 1.0 (reference) | 1.0 (reference) | ||||
| Smoker | 2.40 (1.38; 4.18) | 0.002 | 2.87 (1.51; 5.42) | 0.001 | 1.05 (0.115) | 2 |
| N of observations in the first year | ||||||
| 2 or more | 1.0 (reference) | 1.0 (reference) | ||||
| Less than 2 | 2.79 (1.51; 5.16) | 0.001 | 3.72 (1.88; 7.34) | <0.001 | 1.31 (0.115) | 2 |
| Gingivitis at baseline | ||||||
| Absent | 1.0 (reference) | 1.0 (reference) | ||||
| Present | 2.43 (1.53; 3.86) | <0.001 | 3.05 (1.81; 5.14) | <0.001 | 1.126 (0.104) | 2 |
| Dental crowding | ||||||
| Absent | 1.0 (reference) | |||||
| Present | 1.14 (0.74; 1.74) | 0.561 | ||||
| Subgingival Calculus at Baseline | ||||||
| Absent | 1.0 (reference) | 1.0 (reference) | ||||
| Present | 1.96 (1.26; 3.03) | 0.003 | 1.90 (1.17; 3.10) | 0.01 | 0.64 (0.119) | 1 |
| Dental Crowns | ||||||
| Absent | 1.0 (reference) | |||||
| Present | 1.21 (0.65; 2.28) | 0.545 | ||||
| History of Periodontitis | ||||||
| Absent | 1.0 (reference) | 1.0 (reference) | ||||
| Present | 2.61 (1.69; 4.04) | <0.001 | 2.26 (1.38; 3.69) | 0.001 | 0.81 (0.124) | 1 |
| Type of Dentition | 0.207 | |||||
| Both | 1.0 (reference) | |||||
| Single-root | 4.33 (0.21; 90.85) | 0.345 | ||||
| Multiradicular | 4.44 (0.97; 15.88) | 0.107 | ||||
| Number of Teeth Lost | ||||||
| ≤8 teeth | 1.0 (reference) | |||||
| > 8 teeth | 0.75 (0.48; 1.15) | 0.184 | ||||
| Bruxism | ||||||
| Absent | 1.0 (reference) | |||||
| Present | 0.86 (0.26; 2.87) | 0.805 | ||||
| Socioeconomic Status | 0.371 | |||||
| 1st category | 1.0 (reference) | |||||
| 2nd category | 0.73 (0.41; 1.28) | 0.273 | ||||
| 3rd category | 0.71 (0.42; 1.21) | 0.204 | ||||
| R2 = 0.242; Sensitivity = 52%; Specificity = 73%; Accuracy = 67% | ||||||
| Risk Score (Sum of Points) | Risk Group and Predicted Probability Estimated from the Risk Score | Within Group Incidence of Periodontitis | Observed Incidence of Periodontitis |
|---|---|---|---|
| −1–0 points | <26.6%—Low risk (<0.5 preanalysis risk) | 14/61 = 23% | 14/344 = 4.1% |
| 1–2 points | 26.6–53.2%—Moderate risk (0.5 to <1 times preanalysis risk) | 74/159 = 46.5% | 74/344 = 21.5% |
| ≥3 points | > 53.2%—High risk (>1 times preanalysis risk) | 95/124 = 76.6% | 95/344 = 27.6% |
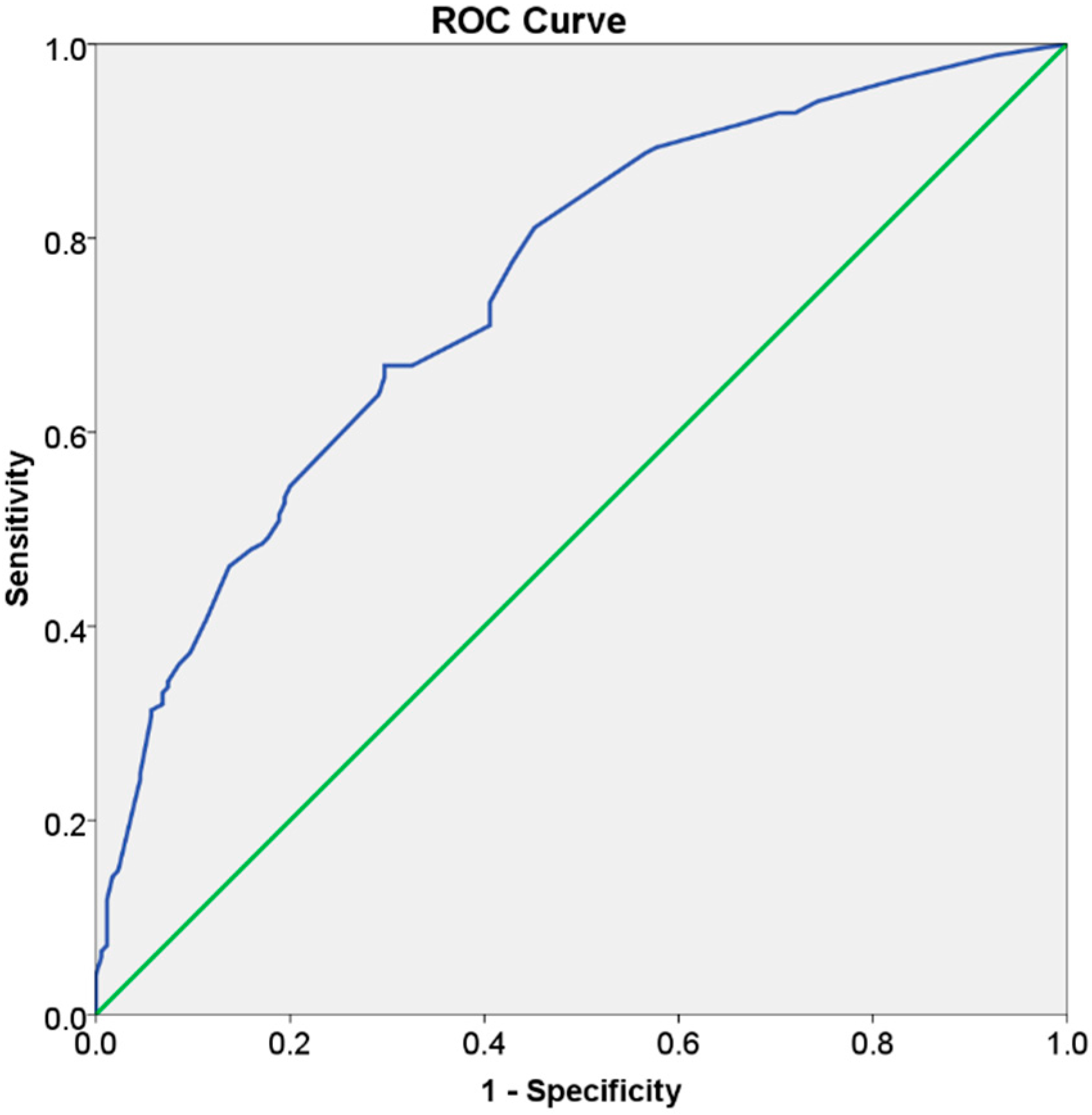
| C-statistic (95% confidence interval) = 0.75 (0.70; 0.80); p < 0.001 | |||
| Variables | Status | Score |
|---|---|---|
| Age less than 53 years | No | 0 |
| Smoker | No | 0 |
| Less than two observations in the first year of follow-up | No | 0 |
| Presence of gingivitis | Yes | 2 |
| Presence of sub-gingival calculus | Yes | 1 |
| History of periodontitis | Yes | 1 |
| Total points | 4 | |
| Risk profile | High risk | |
| Probability of periodontitis | >53.2% | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo Nobre, M.; Ferro, A.; Maló, P. Adult Patient Risk Stratification Using a Risk Score for Periodontitis. J. Clin. Med. 2019, 8, 307. https://doi.org/10.3390/jcm8030307
de Araújo Nobre M, Ferro A, Maló P. Adult Patient Risk Stratification Using a Risk Score for Periodontitis. Journal of Clinical Medicine. 2019; 8(3):307. https://doi.org/10.3390/jcm8030307
Chicago/Turabian Stylede Araújo Nobre, Miguel, Ana Ferro, and Paulo Maló. 2019. "Adult Patient Risk Stratification Using a Risk Score for Periodontitis" Journal of Clinical Medicine 8, no. 3: 307. https://doi.org/10.3390/jcm8030307






