Risk of Acute Myocardial Infarction Among New Users of Allopurinol According to Serum Urate Level: A Nested Case-Control Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Data source and Study Design
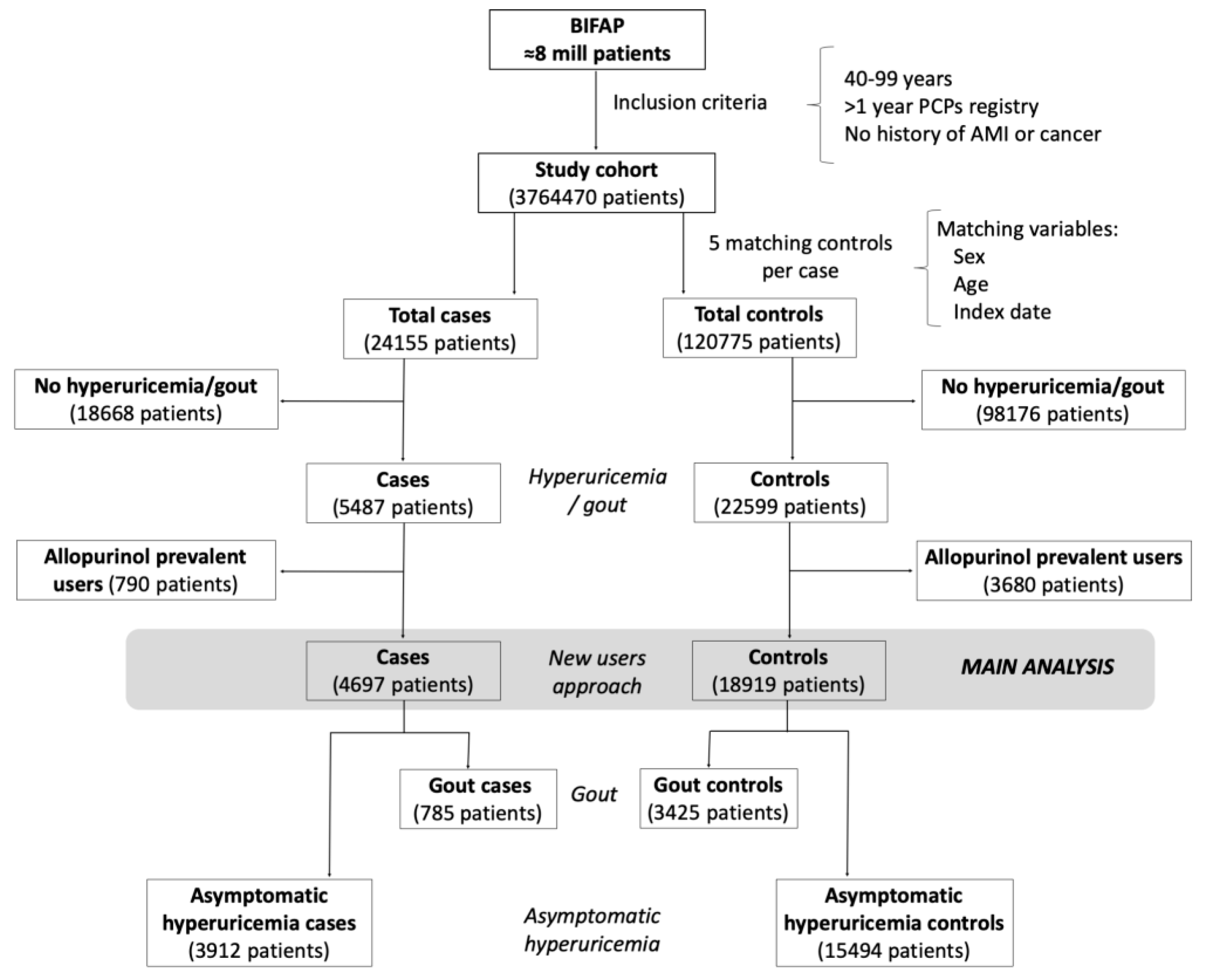
2.2. Selection of Cases and Controls
2.3. Definitions of Gout and Asymptomatic Hyperuricemia
2.4. New Users Design
2.5. Exposure Definition
2.6. Serum Uric Acid (SUA) Levels Attained
2.7. Potential Confounding Factors
2.8. Statistical Analysis
2.9. Sensitivity Analyses
2.10. Ethics Review
3. Results
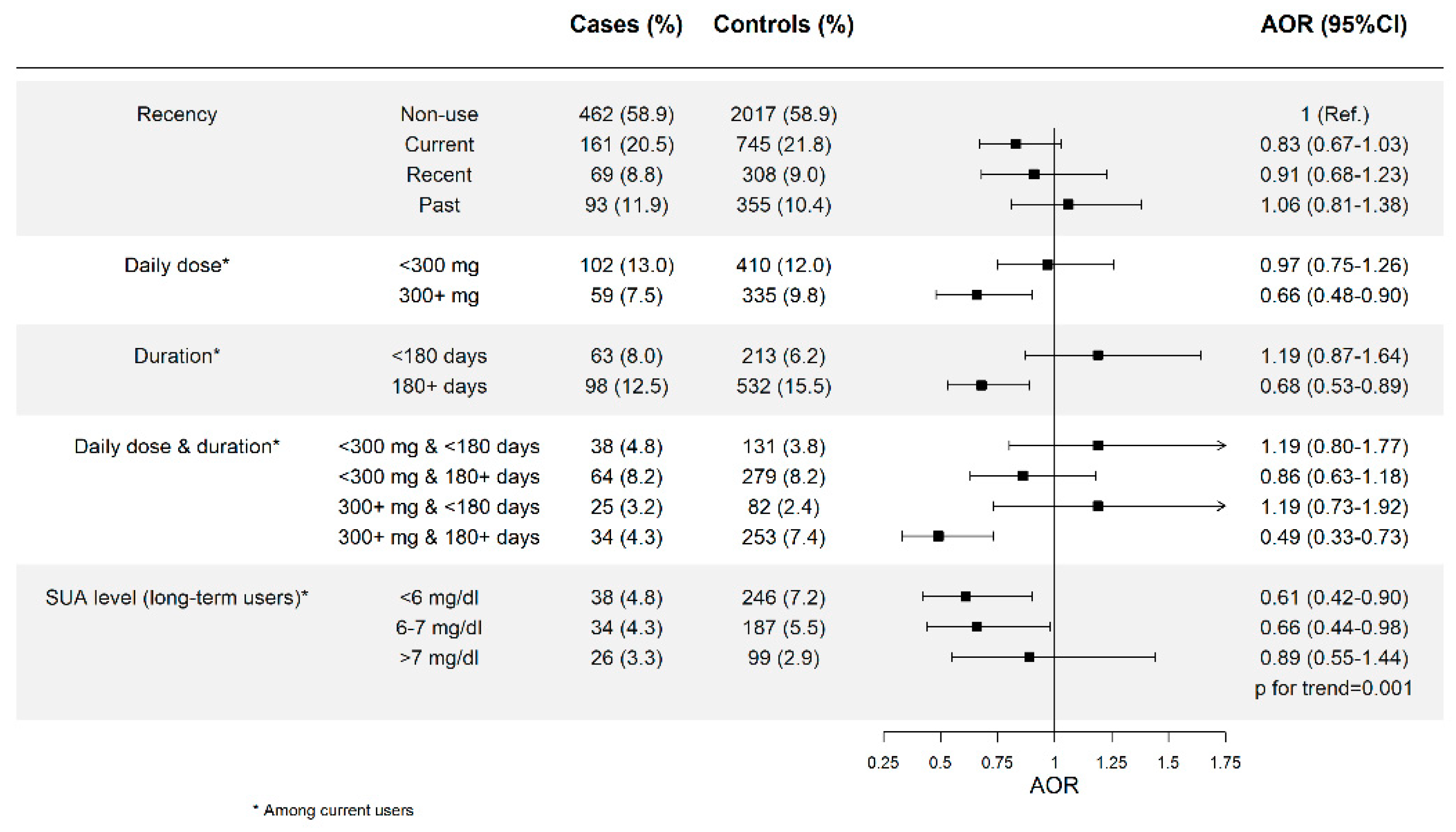
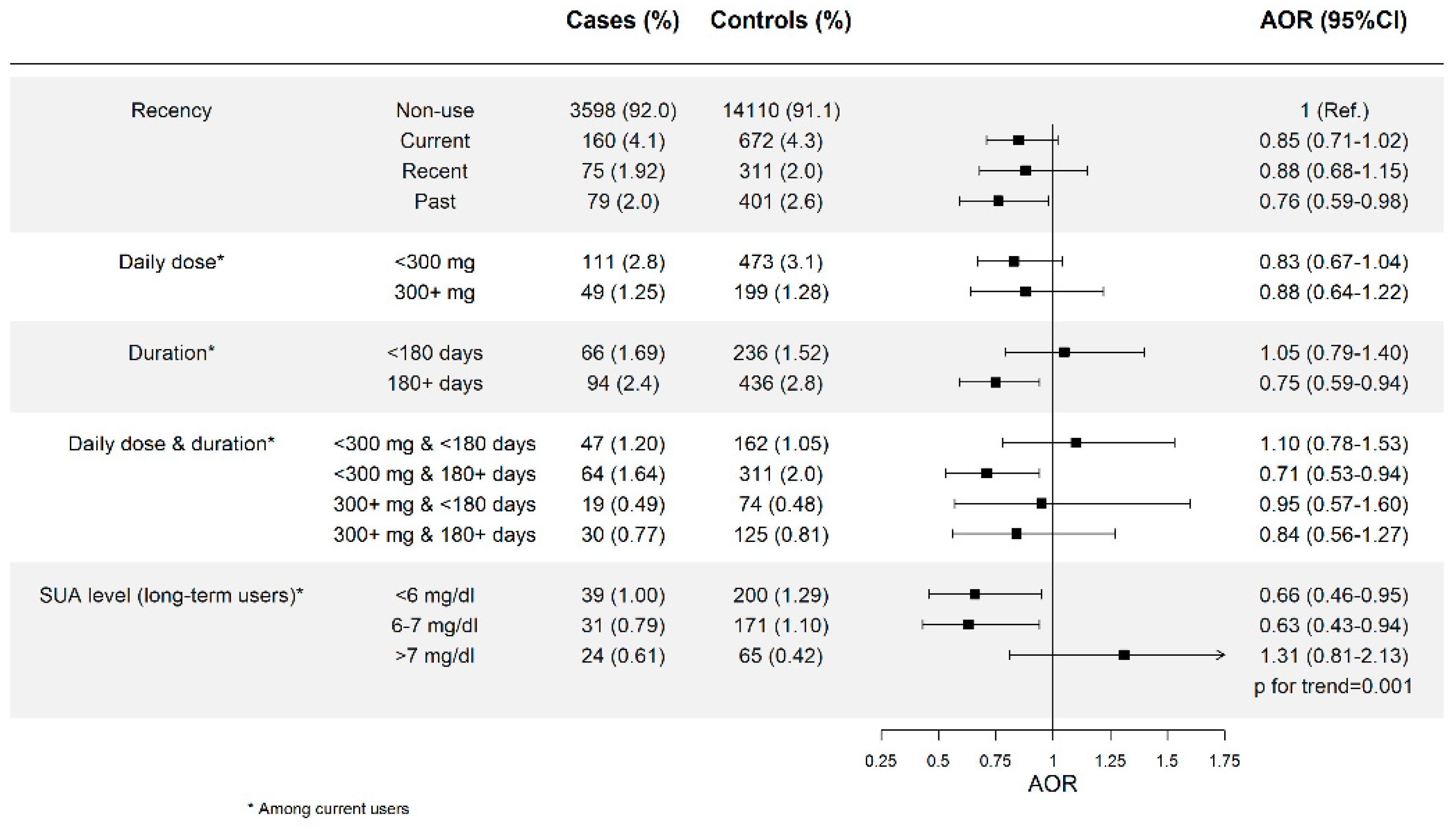
3.1. Allopurinol Use and Risk of AMI and Effect of Dose and Duration
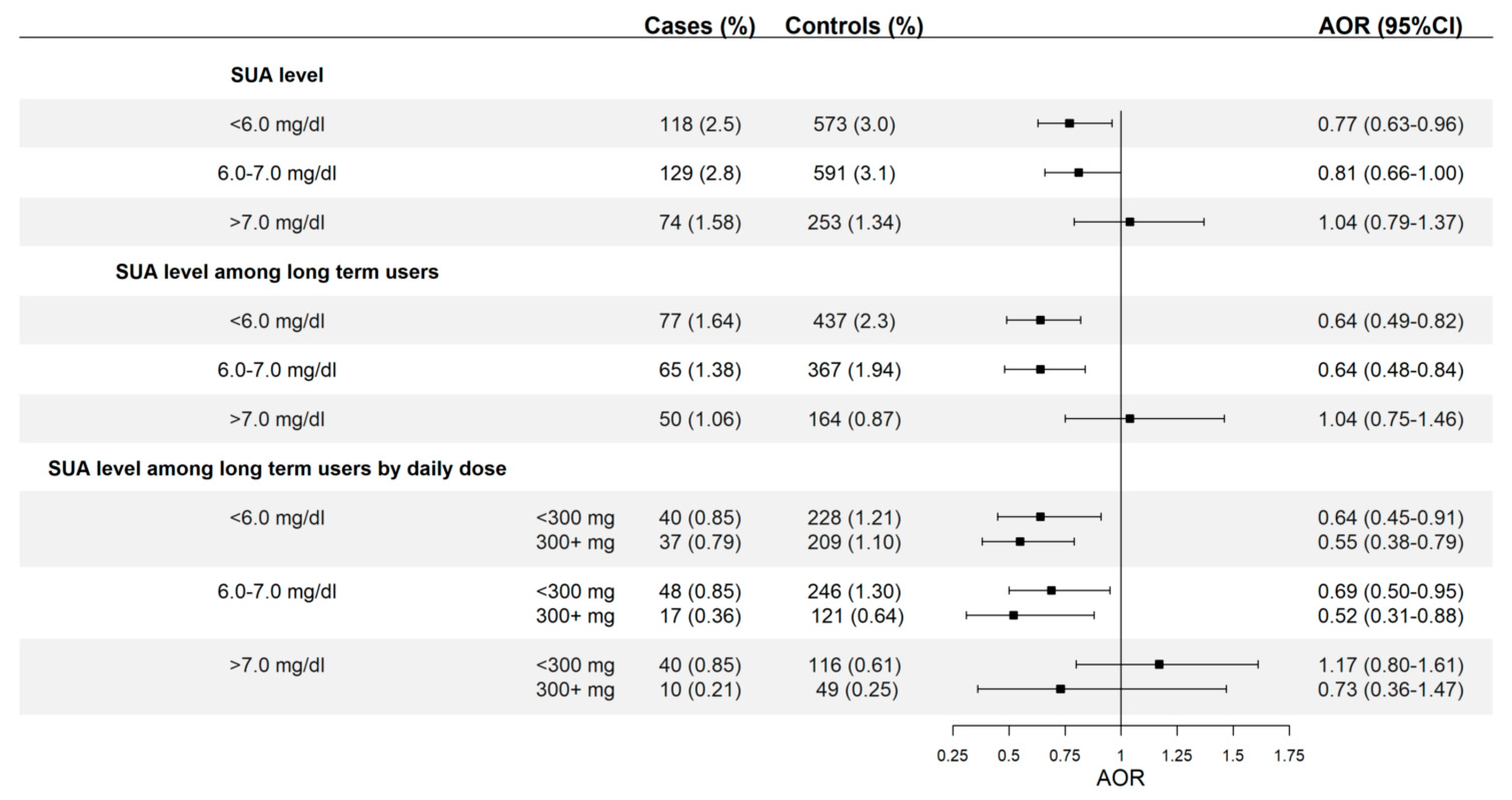
3.2. Allopurinol Use and Risk of AMI According to SUA Levels Attained
3.3. Allopurinol Use and AMI Stratified by Gout and Asymptomatic Hyperuricemia
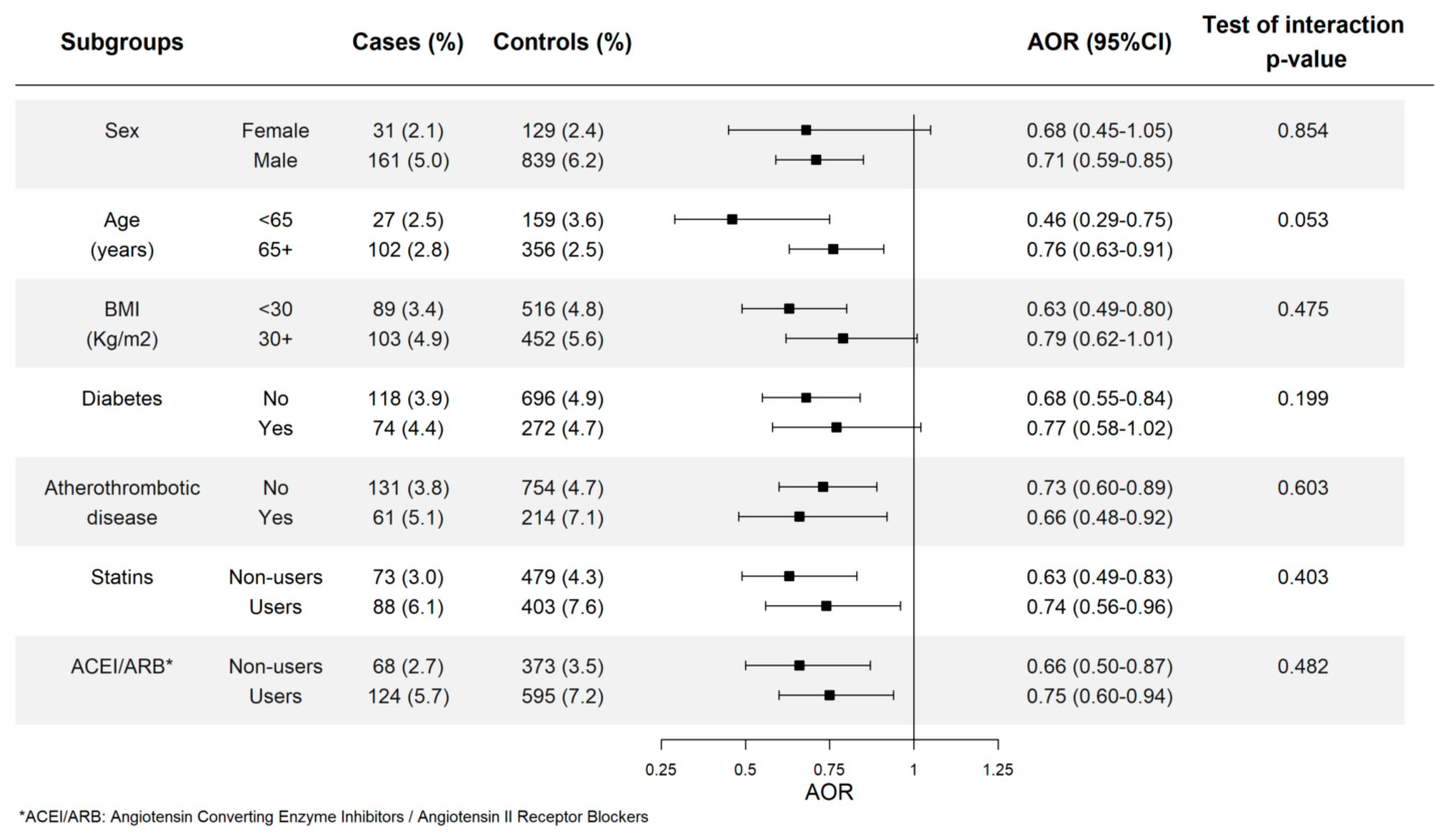
3.4. Allopurinol Use and Risk of AMI in Different Subgroups
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Reference
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Data Source
Appendix B. Selection of Cases and Controls
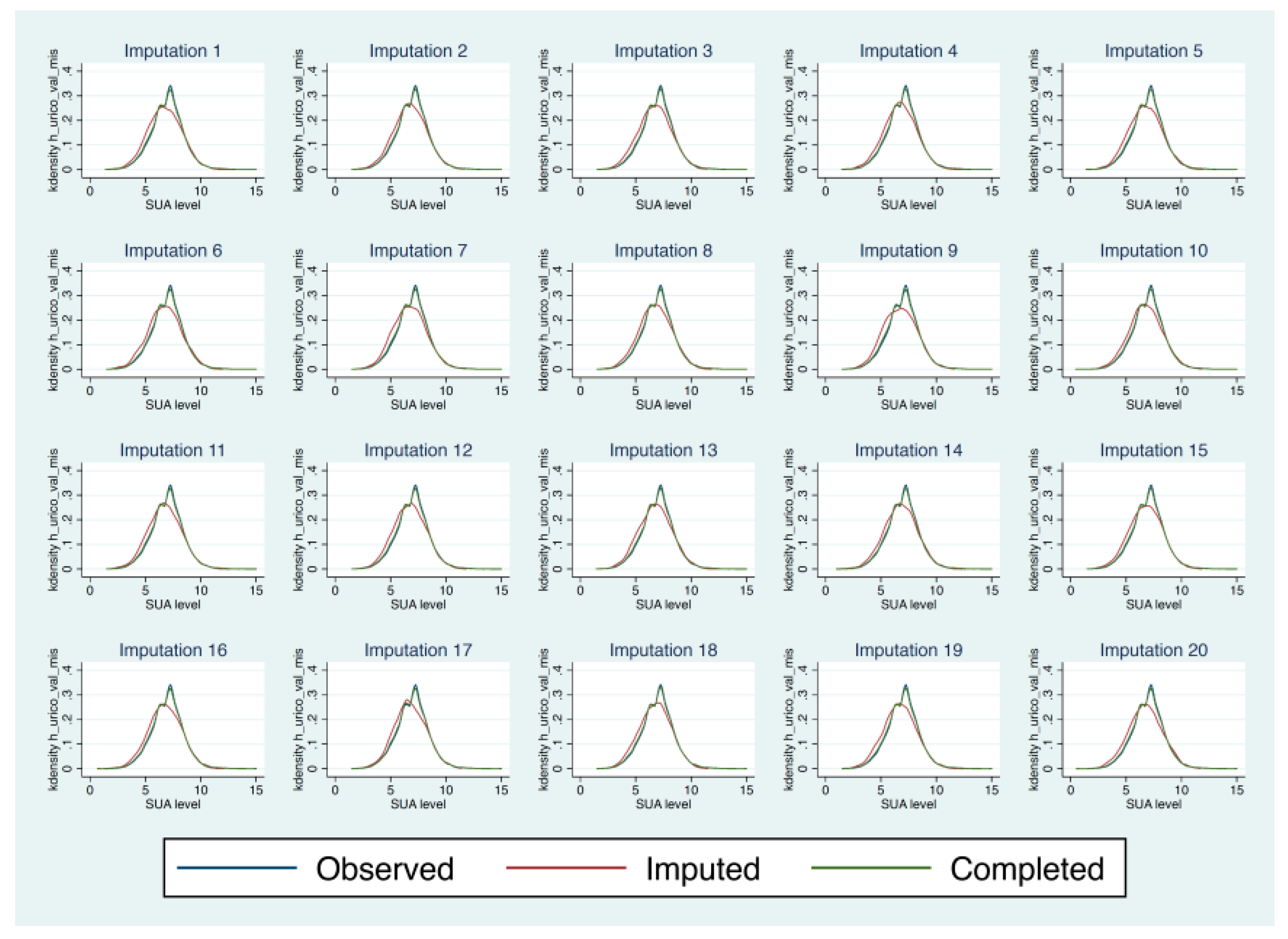
Appendix C. Multiple Imputation by Chained Equations (MICE) Models
References
- Abeles, A.M.; Pillinger, M.H. Gout and cardiovascular disease: Crystallized confusion. Curr. Opin. Rheumatol. 2019, 31, 118–124. [Google Scholar] [CrossRef]
- Landolfo, M.; Borghi, C. Hyperuricaemia and vascular risk: The debate continues. Curr. Opin. Cardiol. 2019, 34, 399–405. [Google Scholar] [CrossRef]
- Roddy, E.; Doherty, M. Epidemiology of gout. Arthritis Res. Ther. 2010, 12, 223. [Google Scholar] [CrossRef]
- Choi, H.K.; Curhan, G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007, 116, 894–900. [Google Scholar] [CrossRef]
- Richette, P.; Latourte, A.; Bardin, T. Cardiac and renal protective effects of urate-lowering therapy. Rheumatology (Oxford) 2018, 57, i47–i50. [Google Scholar] [CrossRef]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castañeda-Sanabria, J.; Coyfish, M.; Guillo, S.; Jansen, T.L.; Janssens, H. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann. Rheum. Dis. 2017, 76, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Fitzgerald, J.D.; Khanna, P.P.; Bae, S.; Singh, M.K.; Neogi, T.; Pillinger, M.H.; Merill, J.; Lee, S.; Prakash, S. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. (Hoboken) 2012, 64, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.; Dalbeth, N. Urate-lowering therapy for asymptomatic hyperuricaemia: A need for caution. Semin. Arthritis Rheum. 2017, 46, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.J.; Anoopkumar, K.; Krishnan, V. Asymptomatic hyperuricemia: Is it time to intervene? Clin. Rheumatol. 2017, 36, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Struthers, A.; Shearer, F. Allopurinol: Novel indications in cardiovascular disease. Heart 2012, 98, 1543–1545. [Google Scholar] [CrossRef]
- De Abajo, F.J.; Gil, M.J.; Rodríguez, A.; García-Poza, P.; Álvarez, A.; Bryant, V.; García-Rodríguez, L.A. Allopurinol use and risk of non-fatal acute myocardial infarction. Heart 2015, 101, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi-Bensouda, L.; Alpérovitch, A.; Aubrun, E.; Danchin, N.; Rossignol, M.; Abenhaim, L.; Richette, P.; PGRx MI Group. Impact of allopurinol on risk of myocardial infarction. Ann. Rheum. Dis. 2015, 74, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.S.; Pottegard, A.; Lindegaard, H.M.; Hallas, J. Effect of allopurinol on cardiovascular outcomes in hyperuricemic patients: A cohort study. Am. J. Med. 2016, 129, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Ramachandaran, R.; Yu, S.; Curtis, J.R. Allopurinol use and the risk of acute cardiovascular events in patients with gout and diabetes. BMC Cardiovasc. Disord. 2017, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Lan, J.L.; Cheng, C.F.; Liang, W.M.; Lin, H.Y.; Tsay, G.J.; Yeh, W.T.; Pan, W.H. Effect of Urate-lowering Therapy on the Risk of Cardiovascular Disease and All-cause Mortality in Patients with Gout: A Case-matched Cohort Study. J. Rheumatol. 2015, 42, 1694–16701. [Google Scholar] [CrossRef]
- Søltoft Larsen, K.; Pottegard, A.; Lindegaard, H.M.; Hallas, J. Impact of Urate Level on Cardiovascular Risk in Allopurinol Treated Patients. A Nested Case-Control Study. PLoS ONE 2016, 11, e0146172. [Google Scholar]
- Kim, S.C.; Schneeweiss, S.; Choudhry, N.; Liu, J.; Glynn, R.J.; Solomon, D.H. Effects of xanthine oxidase inhibitors on cardiovascular disease in patients with gout: A cohort study. Am. J. Med. 2015, 128, 653-e7. [Google Scholar] [CrossRef]
- Zhang, T.; Pope, J.E. Cardiovascular effects of urate-lowering therapies in patients with chronic gout: A systematic review and meta-analysis. Rheumatology (Oxford) 2017, 56, 1144–1153. [Google Scholar] [CrossRef]
- Kok, V.C.; Horng, J.T.; Chang, W.S.; Hong, Y.F.; Chang, T.H. Allopurinol therapy in gout patients does not associate with beneficial cardiovascular outcomes: A population-based matched-cohort study. PLoS ONE 2014, 9, e99102. [Google Scholar] [CrossRef]
- Richette, P.; Perez-Ruiz, F.; Doherty, M.; Jansen, T.L.; Nuki, G.; Pascual, E.; Punzi, L.; So, A.K.; Bardin, T. Improving cardiovascular and renal outcomes in gout: What should we target? Nat. Rev. Rheumatol. 2014, 10, 654–661. [Google Scholar] [CrossRef]
- BIFAP: Base de Datos para la Investigación Farmacoepidemiológica en Atención Primaria. Available online: http://www.bifap.org (accessed on 15 April 2019).
- Ray, W.A. Evaluating medication effects outside of clinical trials: New-user designs. Am. J. Epidemiol. 2003, 158, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Interaction revisited: The difference between two estimates. BMJ 2003, 326, 219. [Google Scholar] [CrossRef] [PubMed]
- Azur, M.J.; Stuart, E.A.; Frangakis, C.; Leaf, P.J. Multiple imputation by chained equations: What is it and how does it work? Int. J. Methods Psychiatr. Res. 2011, 20, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P.C.; Kim, S.Y.; LaValley, M.; Choi, H.K. Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis Care Res. (Hoboken) 2011, 63, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Mackenzie, I.S.; Chen, Y.; Struthers, A.D.; MacDonald, T.M. Impact of allopurinol use on urate concentration and cardiovascular outcome. Br. J. Clin. Pharmacol. 2011, 71, 600–607. [Google Scholar] [CrossRef]
- Bredemeier, M.; Lopes, L.M.; Eisenreich, M.A.; Hickmann, S.; Bongiorno, G.K.; d’Avila, R.; Morsch, A.L.; da Silva Stein, F.; Campos, G.G.D. Xanthine oxidase inhibitors for prevention of cardiovascular events: A systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2018, 18, 24. [Google Scholar] [CrossRef]
- Desai, R.J.; Franklin, J.M.; Spoendlin-Allen, J.; Solomon, D.H.; Danaei, G.; Kim, S.C. An evaluation of longitudinal changes in serum urate levels and associated risk of cardio-metabolic events and renal function decline in gout. PLoS ONE 2018, 13, e0193622. [Google Scholar] [CrossRef]
- Pérez-Ruíz, F.; Richette, P.; Stack, A.G.; Gurunath, R.K.; García de Yébenes, M.J.; Carmona, L. Failure to reach uric acid target of <0.36 mmol/L in hyperuricaemia of gout is associated with elevated total and cardiovascular mortality. RMD Open 2019, 5, e001015. [Google Scholar]
- Wu, J.; Lei, G.; Wang, X.; Tang, Y.; Cheng, H.; Jian, G.; Wu, X.; Wang, N. Asymptomatic hyperuricemia and coronary artery disease in elderly patients without comorbidities. Oncotarget 2017, 8, 80688–80699. [Google Scholar] [CrossRef]
- Rajendra, N.S.; Ireland, S.; George, J.; Belch, J.J.; Lang, C.C.; Struthers, A.D. Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris. J. Am. Coll. Cardiol. 2011, 58, 820–828. [Google Scholar] [CrossRef]
- George, J.; Carr, E.; Davies, J.; Belch, J.J.; Struthers, A.D. High-dose allopurinol improves endothelial dysfunction by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 2006, 114, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Segal, M.; Afsar, B.; Kang, D.H.; Rodriguez-Iturbe, B.; Johnson, R.J. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 2013, 99, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Park, S.K.; Lee, I.K.; Johnson, R.J. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005, 16, 3553–3562. [Google Scholar] [CrossRef]
- Halevy, S.; Ghislain, P.D.; Mockenhaupt, M.; Fagot, J.P.; Bouwes Bavinck, J.N.; Sidoroff, A.; Naldi, L.; Dunant, A.; Viboud, C.; Roujeau, J.C.; et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J. Am. Acad. Dermatol. 2008, 58, 25–32. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chen, C.H.; Deng, S.T.; Huang, C.S.; Lin, Y.J.; Chen, Y.J.; Wu, C.Y.; Hung, S.I.; Chung, W.H. Allopurinol Use and Risk of Fatal Hypersensitivity Reactions: A Nationwide Population-Based Study in Taiwan. JAMA Intern. Med. 2015, 175, 1550–1557. [Google Scholar] [CrossRef]
- Keller, S.F.; Lu, N.; Blumenthal, K.G.; Rai, S.K.; Yokose, C.; Choi, J.W.J.; Kim, S.C.; Zhang, Y.; Choi, H.K. Racial/ethnic variation and risk factors for allopurinol associated severe cutaneous adverse reactions: A cohort study. Ann. Rheum. Dis. 2018, 77, 1187–1193. [Google Scholar] [CrossRef]
- Rodríguez-Martin, S.; Martin-Merino, E.; Lerma, V.; Rodríguez-Miguel, A.; González, O.; González-Herrada, C.; Ramírez, E.; de Abajo, F.J.; Bellón, T. Incidence of Stevens-Johnson syndrome/toxic epidermal necrolysis among new users of different individual drugs in a European population: A case-population study. Eur. J. Clin. Pharmacol. 2019, 75, 237–246. [Google Scholar]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 111–127. [Google Scholar]
- Vittinghoff, E.; Glidden, D.V.; Shiboski, S.C.; McCulloch, C.E. Regression Methods in Biostatistics, Linear, Logistic, Survival, and Repeated Measures Models, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 431–467. [Google Scholar]
- De Abajo, F.J.; Gil, M.J.; García Poza, P.; Bryant, V.; Oliva, B.; Timoner, J.; García-Rodríguez, L.A. Risk of nonfatal acute myocardial infarction associated with non-steroidal antiinflammatory drugs, non-narcotic analgesics and other drugs used in osteoarthritis: A nested case-control study. Pharmacoepidemiol. Drug Saf. 2014, 23, 1128–1138. [Google Scholar] [CrossRef]
- García-Poza, P.; de Abajo, F.J.; Gil, M.J.; Chacón, A.; Bryant, V.; García-Rodríguez, L.A. Risk of ischemic stroke associated with non-steroidal anti-inflammatory drugs and paracetamol: A population-based case-control study. J. Thromb. Haemost. 2015, 13, 708–718. [Google Scholar] [CrossRef]
| Cases n = 4697 | Controls n = 18919 | Crude OR * (95% CI) | Adjuster OR ‡ (95% CI) | |
|---|---|---|---|---|
| Age, mean (SD) | 70.0 (±12.9) | 69.9 (±12.6) | - | - |
| Men | 3199 (68.1) | 13503 (71.4) | - | - |
| Visits (last 12 months) | ||||
| Up to 5 | 682 (14.5) | 3819 (20.2) | 1 (ref.) | 1 (ref.) |
| 6–15 | 1883 (40.1) | 8127 (43.0) | 1.35 (1.22–1.49) | 1.21 (1.09–1.34) |
| 16–24 | 1097 (23.4) | 3892 (20.6) | 1.69 (1.51–1.89) | 1.41 (1.25–1.60) |
| ≥25 | 1035 (22.0) | 3081 (16.3) | 2.06 (1.83–2.31) | 1.58 (1.39–1.81) |
| BMI kg/m2 | ||||
| Up to 24.9 | 434 (9.2) | 1765 (9.3) | 1 (ref.) | 1 (ref.) |
| 25–29 30–34 | 2158 (45.9) 1606 (34.2) | 9097 (48.1) 6261 (33.1) | 0.98 (0.87–1.10) 1.05 (0.93–1.18) | 1.01 (0.90–1.14) 1.04 (0.92–1.18) |
| 35–49 | 376 (8.0) | 1413 (7.5) | 1.06 (0.91–1.24) | 0.96 (0.82–1.13) |
| ≥40 | 123 (2.6) | 383 (2.0) | 1.26 (1.00–1.58) | 1.10 (0.86–1.39) |
| Smoking | ||||
| Never smoking | 1499 (31.9) | 6792 (35.9) | 1 (Ref.) | 1 (Ref.) |
| Current smoker | 1281 (27.3) | 3637 (19.2) | 1.45 (1.33–1.58) | 1.43 (1.31–1.57) |
| Past smoker | 394 (8.4) | 1815 (9.6) | 1.03 (0.91–1.17) | 0.98 (0.86–1.11) |
| Unknown | 1523 (32.4) | 6675 (35.3) | Imputed | Imputed |
| CVA | ||||
| Ischemic | 163 (3.5) | 474 (2.5) | 1.42 (1.19–1.71) | 1.06 (0.87–1.29) |
| Hemorrhagic | 17 (0.36) | 68 (0.36) | 1.03 (0.61–1.76) | 0.89 (0.52–1.54) |
| Unspecified | 112 (2.4) | 404 (2.1) | 1.16 (0.93–1.43) | 0.89 (0.71–1.11) |
| TIA | 150 (3.2) | 495 (2.6) | 1.25 (1.03–1.50) | 1.03 (0.84–1.25) |
| Heart failure | 338 (7.2) | 957 (5.1) | 1.45 (1.27–1.66) | 1.22 (1.06–1.41) |
| Angina pectoris § | 660 (14.1) | 1293 (6.8) | 2.28 (2.06–2.52) | 1.69 (1.51–1.90) |
| PAD | 301 (6.4) | 611 (3.2) | 2.16 (1.87–2.50) | 1.54 (1.32–1.79) |
| Hypertension | 3444 (73.3) | 13007 (68.8) | 1.25 (1.16–1.35) | 1.06 (0.97–1.17) |
| Diabetes || | 1669 (35.5) | 4781 (25.3) | 1.63 (1.52–1.75) | 1.37 (1.27–1.47) |
| Dyslipidemia ** | 2847 (60.6) | 9919 (52.4) | 1.39 (1.30–1.49) | 1.12 (1.04–1.20) |
| Rheumatoid arthritis | 39 (0.83) | 128 (0.68) | 1.20 (0.84–1.72) | 1.00 (1.69–1.46) |
| Osteoarthritis | 601 (12.8) | 2298 (12.2) | 1.04 (0.94–1.14) | 1.02 (0.92–1.13) |
| Chronic kidney failure | 441 (9.4) | 1389 (7.3) | 1.32 (1.17–1.48) | 1.08 (0.96–1.22) |
| Current use of Low-dose aspirin | 969 (20.6) | 2826 (14.9) | 1.63 (1.49–1.77) | 1.13 (1.03–1.25) |
| Other antiplatelet drugs | 323 (6.9) | 624 (3.3) | 2.27 (1.97–2.61) | 1.50 (1.28–1.76) |
| Oral anticoagulants | 316 (6.7) | 1405 (7.4) | 0.90 (0.79–1.02) | 0.70 (0.60–0.81) |
| NSAIDs | 501 (10.7) | 2005 (10.6) | 1.04 (0.93–1.17) | 0.94 (0.84–1.07) |
| Colchicine | 67 (1.43) | 220 (1.16) | 1.28 (0.97–1.68) | 1.22 (0.92–1.63) |
| Corticosteroids | 124 (2.6) | 333 (1.8) | 1.55 (1.26–1.91) | 1.38 (1.11–1.72) |
| ACE inhibitors | 1187 (25.3) | 4532 (24.0) | 1.16 (1.07–1.25) | 0.93 (0.85–1.02) |
| ARB | 1089 (23.2) | 3999 (21.1) | 1.18 (1.09–1.28) | 0.93 (0.85–1.03) |
| CCB | 975 (20.8) | 2963 (15.7) | 1.51 (1.38–1.64) | 1.23 (1.12–1.35) |
| Beta-Blockers | 697 (14.8) | 2136 (11.3) | 1.41 (1.28–1.55) | 1.09 (0.98–1.21) |
| Alfa-Blockers | 183 (3.9) | 706 (3.7) | 1.08 (0.91–1.27) | 0.85 (0.71–1.01) |
| Diuretics | 1112 (23.7) | 3937 (20.8) | 1.23 (1.13–1.34) | 1.00 (0.91–1.10)) |
| Cases (%) n = 4697 | Controls (%) n = 18919 | Crude OR † (95% CI) | Adjusted OR ‡ (95% CI) | |
|---|---|---|---|---|
| Recency | ||||
| Non-use | 4060 (86.4) | 16127 (85.2) | 1 (Ref.) | 1 (Ref.) |
| Current | 321 (6.8) | 1417 (7.5) | 0.93 (0.82–1.05) | 0.84 (0.73–0.96) |
| Recent | 144 (3.1) | 619 (3.3) | 0.95 (0.79–1.14) | 0.89 (0.73–1.08) |
| Past | 172 (3.7) | 756 (4.0) | 0.93 (0.78–1.10) | 0.89 (0.75–1.07) |
| Daily dose * | ||||
| <300 mg | 213 (4.5) | 883 (4.7) | 0.98 (0.84–1.15) | 0.90 (0.76–1.05) |
| ≥300 mg | 108 (2.3) | 534 (2.8) | 0.83 (0.68–1.03) | 0.75 (0.60–0.93) |
| Duration * | ||||
| <180 days | 129 (2.8) | 449 (2.4) | 1.17 (0.96–1.43) | 1.13 (0.91–1.39) |
| ≥180 days | 192 (4.1) | 968 (5.1) | 0.81 (0.69–0.95) | 0.71 (0.60–0.84) |
| 180–729 days | 116 (2.5) | 537 (2.8) | 0.88 (0.72–1.08) | 0.76 (0.61–0.94) |
| >729 days | 76 (1.62) | 431 (2.3) | 0.72 (0.57–0.93) | 0.64 (0.50–0.83) |
| p for trend = 0.0001 | ||||
| Daily dose & duration * | ||||
| <300 mg | ||||
| <180 days | 85 (1.81) | 293 (1.55) | 1.18 (0.92–1.50) | 1.15 (0.89–1.48) |
| ≥180 days | 128 (2.7) | 590 (3.1) | 0.88 (0.73–1.07) | 0.77 (0.63–0.94) |
| 180–729 days | 76 (1.62) | 341 (1.80) | 0.90 (0.70–1.16) | 0.77 (0.60–1.01) |
| >729 days | 52 (1.11) | 249 (1.32) | 0.85 (0.63–1.16) | 0.76 (0.55–1.03) |
| ≥300 mg | ||||
| <180 days | 44 (0.94) | 156 (0.82) | 1.16 (0.83–1.62) | 1.14 (0.81–1.59) |
| ≥180 days | 64 (1.36) | 378 (2.0) | 0.70 (0.53–0.91) | 0.61 (0.46–0.81) |
| 180–729 days | 40 (0.85) | 249 (1.32) | 0.84 (0.60–1.19) | 0.72 (0.51–1.03) |
| >729 days | 24 (0.51) | 156 (0.82) | 0.54 (0.35–0.83) | 0.48 (0.31–0.75) |
| Cases (%) n = 4166 | Controls (%) n = 16,594 | Crude OR † (95% CI) | Adjusted OR ‡ (95% CI) | |
|---|---|---|---|---|
| SUA Level | ||||
| <6 mg/dL | 70 (1.68) | 381 (2.3) | 0.74 (0.57–0.96) | 0.70 (0.53–0.91) |
| 6–7 mg/dL | 45 (1.08) | 201 (1.21) | 0.91 (0.66–1.26) | 0.88 (0.63–1.24) |
| >7 mg/dL | 61 (1.46) | 220 (1.33) | 1.12 (0.85–1.50) | 1.03 (0.76–1.40) |
| p for trend = 0.011 | ||||
| SUA level among long-term users * | ||||
| <6 mg/dL | 61 (1.46) | 334 (2.0) | 0.74 (0.56–0.98) | 0.69 (0.51–0.92) |
| 6–7 mg/dL | 35 (0.84) | 172 (1.04) | 0.83 (0.57–1.19) | 0.79 (0.54–1.15) |
| >7 mg/dL | 46 (1.10) | 148 (0.89) | 1.26 (0.90–1.76) | 1.11 (0.78–1.58) |
| p for trend = 0.007 | ||||
| SUA level among long-term users by daily dose * | ||||
| <6 mg/dL | ||||
| <300 mg | 30 (0.72) | 159 (0.96) | 0.76 (0.51–1.12) | 0.69 (0.46–1.04) |
| ≥300 mg | 31 (0.74) | 175 (1.05) | 0.72 (0.49–1.06) | 0.68 (0.45–1.01) |
| 6–7 mg/dL | ||||
| <300 mg | 28 (0.67) | 130 (0.78) | 0.87 (0.58–1.32) | 0.83 (0.54–1.26) |
| ≥300 mg | 7 (0.17) | 42 (0.25) | 0.68 (0.31–1.52) | 0.66 (0.29–1.51) |
| >7 mg/dL | ||||
| <300 mg | 38 (0.91) | 103 (0.62) | 1.50 (1.03–2.18) | 1.31 (0.88–1.93) |
| ≥300 mg | 8 (0.19) | 45 (0.27) | 0.72 (0.34–1.53) | 0.65 (0.30–1.42) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Martín, S.; de Abajo, F.J.; Gil, M.; González-Bermejo, D.; Rodríguez-Miguel, A.; Barreira-Hernández, D.; Mazzucchelli, R.; García-Lledó, A.; García-Rodríguez, L.A. Risk of Acute Myocardial Infarction Among New Users of Allopurinol According to Serum Urate Level: A Nested Case-Control Study. J. Clin. Med. 2019, 8, 2150. https://doi.org/10.3390/jcm8122150
Rodríguez-Martín S, de Abajo FJ, Gil M, González-Bermejo D, Rodríguez-Miguel A, Barreira-Hernández D, Mazzucchelli R, García-Lledó A, García-Rodríguez LA. Risk of Acute Myocardial Infarction Among New Users of Allopurinol According to Serum Urate Level: A Nested Case-Control Study. Journal of Clinical Medicine. 2019; 8(12):2150. https://doi.org/10.3390/jcm8122150
Chicago/Turabian StyleRodríguez-Martín, Sara, Francisco J. de Abajo, Miguel Gil, Diana González-Bermejo, Antonio Rodríguez-Miguel, Diana Barreira-Hernández, Ramón Mazzucchelli, Alberto García-Lledó, and Luis A. García-Rodríguez. 2019. "Risk of Acute Myocardial Infarction Among New Users of Allopurinol According to Serum Urate Level: A Nested Case-Control Study" Journal of Clinical Medicine 8, no. 12: 2150. https://doi.org/10.3390/jcm8122150
APA StyleRodríguez-Martín, S., de Abajo, F. J., Gil, M., González-Bermejo, D., Rodríguez-Miguel, A., Barreira-Hernández, D., Mazzucchelli, R., García-Lledó, A., & García-Rodríguez, L. A. (2019). Risk of Acute Myocardial Infarction Among New Users of Allopurinol According to Serum Urate Level: A Nested Case-Control Study. Journal of Clinical Medicine, 8(12), 2150. https://doi.org/10.3390/jcm8122150







