Validation and Adaptation of the Multidimensional Prognostic Index in an Older Australian Cohort
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Original MPI
2.3. Adapted MPI
2.3.1. Medications with Anticholinergic Effects
2.3.2. RUDAS
2.3.3. Adapted MPI Models
- MPI with number of medications domain substituted with the ARS score. Cut-off points were applied to the ARS score with 0 points for patients not on any anticholinergics, 0.5 points for patients on 1–2 anticholinergics, and 1 point for patients taking >2 anticholinergics.
- MPI with SPMSQ domain substituted with the RUDAS score. Cut-off points were applied to the RUDAS score with 0 points for patients who had a RUDAS score >25 points, 0.5 points for patients who had a RUDAS score between 25 and 17 points, and 1 point for patients who had a RUDAS score < 17 points.
- MPI with both the number of medications and SPMSQ domains substituted with ARS and RUDAS scores using the above cut-off points.
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Study Patient Characteristics
3.2. Validation of Original MPI—12-Month All-Cause Mortality
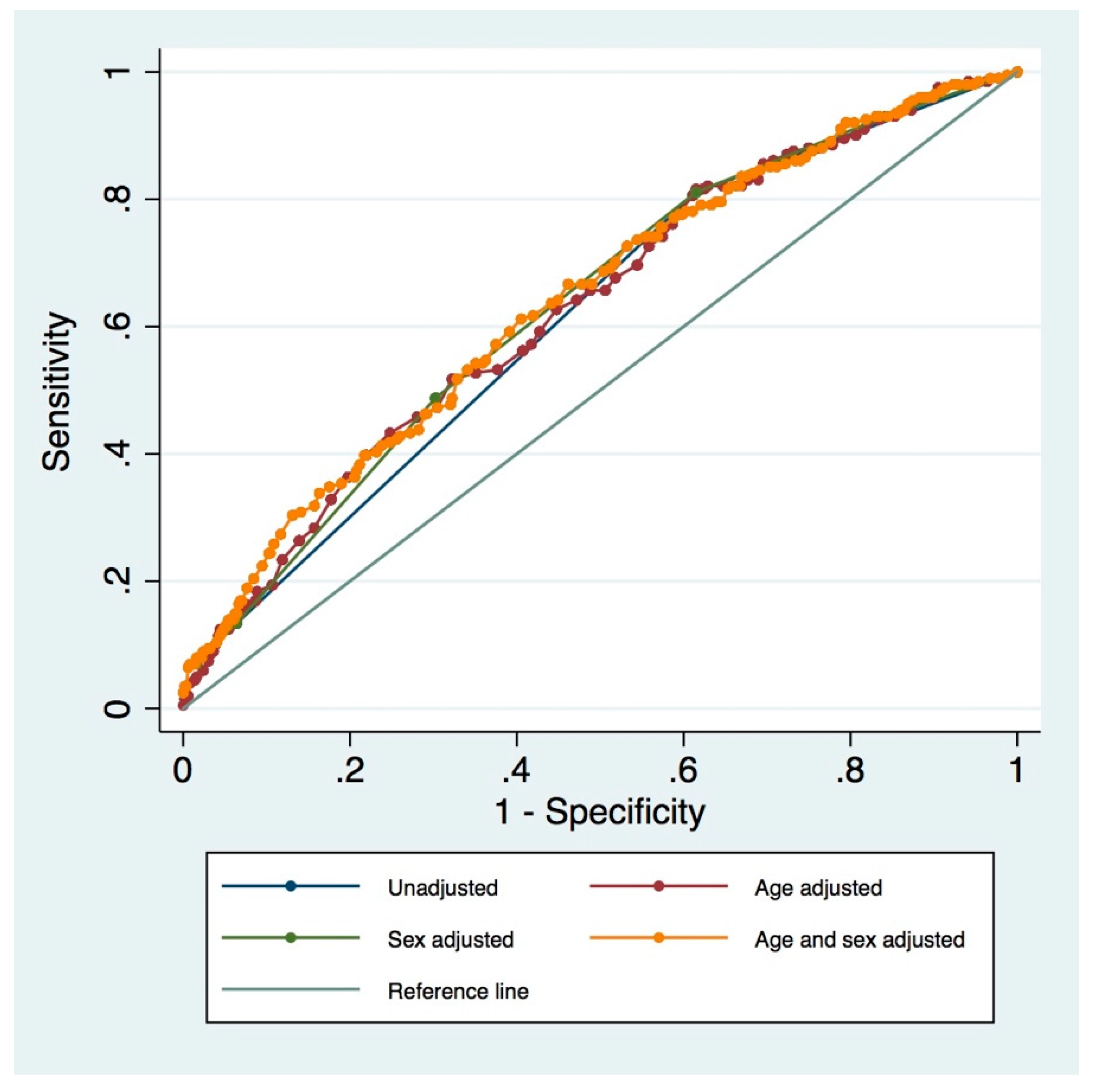
3.3. Adapted MPI
3.4. MPI and Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Health and Aging. Available online: https://www.who.int/ageing/publications/global_health.pdf?ua=1 (accessed on 12 February 2016).
- Begg, S.J. Health in a ‘post-transition’Australia: Adding years to life or life to years? Aust. Health Rev. 2014, 38, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nepal, B.; Brown, L. Projection of older Australians with a history of midlife obesity and overweight 2010–2050. Obesity 2013, 21, 2579–2581. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. A brief history of geriatrics. J. Gerontol. 2004, 59, 1132–1152. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index: A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged: The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 1996, 54, S59–S65. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Pilotto, A.; Ferrucci, L.; Franceschi, M.; D’Ambrosio, L.P.; Scarcelli, C.; Cascavilla, L.; Paris, F.; Placentino, G.; Seripa, D.; Dallapiccola, B. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuv. Res. 2008, 11, 151–161. [Google Scholar] [CrossRef]
- Pilotto, A.; Addante, F.; D’Onofrio, G.; Sancarlo, D.; Ferrucci, L. The Comprehensive Geriatric Assessment and the multidimensional approach. A new look at the older patient with gastroenterological disorders. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Pilotto, A.; Addante, F.; Ferrucci, L.; Leandro, G.; D’Onofrio, G.; Corritore, M.; Niro, V.; Scarcelli, C.; Dallapiccola, B.; Franceschi, M. The multidimensional prognostic index predicts short-and long-term mortality in hospitalized geriatric patients with pneumonia. J. Gerontol. 2009, 64, 880–887. [Google Scholar] [CrossRef]
- Pilotto, A.; Addante, F.; Franceschi, M.; Leandro, G.; Rengo, G.; D’ambrosio, P.; Longo, M.G.; Rengo, F.; Pellegrini, F.; Dallapiccola, B. Multidimensional Prognostic Index based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circ. Heart Fail. 2010, 3, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; D’Onofrio, G.; Panza, F.; Copetti, M.; Cascavilla, L.; Paris, F.; Pellegrini, F.; Seripa, D.; Ferrucci, L. Treatment of late-life major depressive disorder with selective serotonin reuptake inhibitors improves the multidimensional prognostic index. J. Clin. Psychopharmacol. 2012, 32, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Ferrucci, L.; Scarcelli, C.; Niro, V.; Di Mario, F.; Seripa, D.; Andriulli, A.; Leandro, G.; Franceschi, M. Usefulness of the comprehensive geriatric assessment in older patients with upper gastrointestinal bleeding: A two-year follow-up study. Dig. Dis. 2007, 25, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Rengo, F.; Marchionni, N.; Sancarlo, D.; Fontana, A.; Panza, F.; Ferrucci, L.; FIRI-SIGG Study Group. Comparing the prognostic accuracy for all-cause mortality of frailty instruments: A multicentre 1-year follow-up in hospitalized older patients. PLoS ONE 2012, 7, e29090. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Addante, F.; Copetti, M.; Paroni, G.; Fontana, A.; Sancarlo, D.; Pellegrini, F.; Ferrucci, L.; Pilotto, A. Identification of a metabolic signature for multidimensional impairment and mortality risk in hospitalized older patients. Aging Cell 2013, 12, 459–466. [Google Scholar] [CrossRef]
- Sancarlo, D.; D’Onofrio, G.; Franceschi, M.; Scarcelli, C.; Niro, V.; Addante, F.; Copetti, M.; Ferrucci, L.; Fontana, L.; Pilotto, A. Validation of a Modified-Multidimensional Prognostic Index (m-MPI) including the Mini Nutritional Assessment Short-Form (MNA-SF) for the prediction of one-year mortality in hospitalized elderly patients. J. Nutr. Health Aging 2011, 15, 169–173. [Google Scholar] [CrossRef]
- Sancarlo, D.; Pilotto, A.; Panza, F.; Copetti, M.; Longo, M.G.; D’Ambrosio, P.; D’Onofrio, G.; Ferrucci, L.; Pilotto, A. A Multidimensional Prognostic Index (MPI) based on a comprehensive geriatric assessment predicts short-and long-term all-cause mortality in older hospitalized patients with transient ischemic attack. J. Neurol. 2012, 259, 670–678. [Google Scholar] [CrossRef]
- Pilotto, A.; Sancarlo, D.; Aucella, F.; Fontana, A.; Addante, F.; Copetti, M.; Panza, F.; Strippoli, G.F.; Ferrucci, L. Addition of the multidimensional prognostic index to the estimated glomerular filtration rate improves prediction of long-term all-cause mortality in older patients with chronic kidney disease. Rejuv. Res. 2012, 15, 82–88. [Google Scholar] [CrossRef]
- Pilotto, A.; Gallina, P.; Fontana, A.; Sancarlo, D.; Bazzano, S.; Copetti, M.; Maggi, S.; Paroni, G.; Marcato, F.; Pellegrini, F. Development and validation of a Multidimensional Prognostic Index for mortality based on a standardized Multidimensional Assessment Schedule (MPI-SVaMA) in community-dwelling older subjects. J. Am. Med. Dir. Assoc. 2013, 14, 287–292. [Google Scholar] [CrossRef]
- Volpato, S.; Bazzano, S.; Fontana, A.; Ferrucci, L.; Pilotto, A. Multidimensional prognostic index predicts mortality and length of stay during hospitalization in the older patients: A multicenter prospective study. J. Gerontol. 2014, 70, 325–331. [Google Scholar] [CrossRef]
- Brunello, A.; Fontana, A.; Zafferri, V.; Panza, F.; Fiduccia, P.; Basso, U.; Copetti, M.; Lonardi, S.; Roma, A.; Falci, C. Development of an oncological-multidimensional prognostic index (Onco-MPI) for mortality prediction in older cancer patients. J. Cancer Res. Clin. Oncol. 2016, 142, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Onofrio, G.; Sancarlo, D.; Addante, F.; Ciccone, F.; Cascavilla, L.; Paris, F.; Elia, A.C.; Nuzzaci, C.; Picoco, M.; Greco, A. A pilot randomized controlled trial evaluating an integrated treatment of rivastigmine transdermal patch and cognitive stimulation in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2015, 30, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Giantin, V.; Valentini, E.; Iasevoli, M.; Falci, C.; Siviero, P.; De Luca, E.; Maggi, S.; Martella, B.; Orrù, G.; Crepaldi, G. Does the Multidimensional Prognostic Index (MPI), based on a Comprehensive Geriatric Assessment (CGA), predict mortality in cancer patients? Results of a prospective observational trial. J. Geriatr. Oncol. 2013, 4, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Sancarlo, D.; Panza, F.; Paris, F.; D’Onofrio, G.; Cascavilla, L.; Addante, F.; Seripa, D.; Solfrizzi, V.; Dallapiccola, B. The Multidimensional Prognostic Index (MPI), based on a comprehensive geriatric assessment predicts short-and long-term mortality in hospitalized older patients with dementia. J. Alzheimers Dis. 2009, 18, 191–199. [Google Scholar] [CrossRef]
- Pilotto, A.; Sancarlo, D.; Pellegrini, F.; Rengo, F.; Marchionni, N.; Volpato, S.; Ferrucci, L. The Multidimensional Prognostic Index predicts in-hospital length of stay in older patients: A multicentre prospective study. Age Ageing 2016, 45, 90–96. [Google Scholar] [CrossRef]
- Pilotto, A.; Gallina, P.; Panza, F.; Copetti, M.; Cella, A.; Cruz-Jentoft, A.; Daragjati, J.; Ferrucci, L.; Maggi, S.; Mattace-Raso, F. Relation of statin use and mortality in community-dwelling frail older patients with coronary artery disease. Am. J. Cardiol. 2016, 118, 1624–1630. [Google Scholar] [CrossRef]
- Pilotto, A.; Gallina, P.; Copetti, M.; Pilotto, A.; Marcato, F.; Mello, A.M.; Simonato, M.; Logroscino, G.; Padovani, A.; Ferrucci, L. Warfarin Treatment and All-Cause Mortality in Community-Dwelling Older Adults with Atrial Fibrillation: A Retrospective Observational Study. J. Am. Geriatr. Soc. 2016, 64, 1416–1424. [Google Scholar] [CrossRef]
- Pilotto, A.; Panza, F.; Copetti, M.; Simonato, M.; Sancarlo, D.; Gallina, P.; Strandberg, T.; MPI_AGE Project Investigators. Statin treatment and mortality in community-dwelling frail older patients with diabetes mellitus: A retrospective observational study. PLoS ONE 2015, 10, e0130946. [Google Scholar] [CrossRef]
- Pilotto, A.; Giancristofaro, R.A.; Panza, F.; Daragjati, J.; Prete, C.; Polidori, M.; Cella, A.; Maggi, S. Role of anti-dementia drugs and multidimensional impairment on mortality rates in frail multimorbid older patients with dementia: Results from the European MPI_AGE project. Eur. Geriatr. Med. 2015, 6, S20. [Google Scholar] [CrossRef]
- Bureau, M.-L.; Liuu, E.; Christiaens, L.; Pilotto, A.; Mergy, J.; Bellarbre, F.; Ingrand, P.; Paccalin, M.; Cruz-Jentoft, A.; Maggi, S. Using a multidimensional prognostic index (MPI) based on comprehensive geriatric assessment (CGA) to predict mortality in elderly undergoing transcatheter aortic valve implantation. Int. J. Cardiol. 2017, 236, 381–386. [Google Scholar] [CrossRef]
- Angleman, S.B.; Santoni, G.; Pilotto, A.; Fratiglioni, L.; Welmer, A.-K.; MPI_AGE Project Investigators. Multidimensional prognostic index in association with future mortality and number of hospital days in a population-based sample of older adults: Results of the EU funded MPI_AGE project. PLoS ONE 2015, 10, e0133789. [Google Scholar] [CrossRef] [PubMed]
- Routledge, P.A.; O’mahony, M.; Woodhouse, K. Adverse drug reactions in elderly patients. Br. J. Clin. Pharm. 2004, 57, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Brahma, D.K.; Wahlang, J.B.; Marak, M.D.; Sangma, M.C. Adverse drug reactions in the elderly. J. Pharm. Pharm. 2013, 4, 91. [Google Scholar] [CrossRef]
- Hilmer, S.N.; Mager, D.E.; Simonsick, E.M.; Cao, Y.; Ling, S.M.; Windham, B.G.; Harris, T.B.; Hanlon, J.T.; Rubin, S.M.; Shorr, R.I. A drug burden index to define the functional burden of medications in older people. Arch. Intern. Med. 2007, 167, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; van Munster, B.C.; Woodman, R.J.; de Rooij, S.E. Measures of anticholinergic drug exposure, serum anticholinergic activity, and all-cause postdischarge mortality in older hospitalized patients with hip fractures. Am. J. Geriatr. Psychiatry 2013, 21, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Lowry, E.; Woodman, R.J.; Soiza, R.L.; Hilmer, S.N.; Mangoni, A.A. Drug burden index, physical function, and adverse outcomes in older hospitalized patients. J. Clin. Pharmacol. 2012, 52, 1584–1591. [Google Scholar] [CrossRef]
- Lowry, E.; Woodman, R.J.; Soiza, R.L.; Mangoni, A.A. Associations between the anticholinergic risk scale score and physical function: Potential implications for adverse outcomes in older hospitalized patients. J. Am. Med. Dir. Assoc. 2011, 12, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.L.; Salow, M.J.; Angelini, M.C.; McGlinchey, R.E. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch. Intern. Med. 2008, 168, 508–513. [Google Scholar] [CrossRef]
- Basic, D.; Khoo, A.; Conforti, D.; Rowland, J.; Vrantsidis, F.; LoGiudice, D.; Hill, K.; Harry, J.; Lucero, K.; Prowse, R. Rowland Universal Dementia Assessment Scale, Mini-Mental State Examination and General Practitioner Assessment of Cognition in a multicultural cohort of community-dwelling older persons with early dementia. Aust. Psychol. 2009, 44, 40–53. [Google Scholar] [CrossRef]
- Lawton, M.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Nurs. Res. 1970, 19, 278. [Google Scholar] [CrossRef]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Bliss, M.R.; McLaren, R.; Exton-Smith, A.N. Mattresses for preventing pressure sores in geriatric patients. Mon. Bull. Minist. Health Public Health Lab. Serv. 1966, 25, 238. [Google Scholar] [PubMed]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y. Mini Nutritional Assessment: A practical assessment tool for grading the nutritional state of elderly patients. Facts Res. Geyontol. 1994, 4, 15–59. [Google Scholar]
- Storey, J.E.; Rowland, J.T.; Conforti, D.A.; Dickson, H.G. The Rowland universal dementia assessment scale (RUDAS): A multicultural cognitive assessment scale. Int. Psychogeriatr. 2004, 16, 13–31. [Google Scholar] [CrossRef]
- Casey, P.; Cross, W.; Mart, M.W.S.; Baldwin, C.; Riddell, K.; Dārziņš, P. Hospital discharge data under-reports delirium occurrence: Results from a point prevalence survey of delirium in a major Australian health service. Intern. Med. J. 2019, 49, 338–344. [Google Scholar] [CrossRef]
- Veronese, N.; Siri, G.; Cella, A.; Daragjati, J.; Cruz-Jentoft, A.J.; Polidori, M.C.; Mattace-Raso, F.; Paccalin, M.; Topinkova, E.; Greco, A. Older women are frailer, but less often die then men: A prospective study of older hospitalized people. Maturitas 2019, 128, 81–86. [Google Scholar] [CrossRef]
- Bostock, C.V.; Soiza, R.L.; Mangoni, A.A. Association between prescribing of antimuscarinic drugs and antimuscarinic adverse effects in older people. Expert Rev. Clin. Pharm. 2010, 3, 441–452. [Google Scholar] [CrossRef]
- Ruxton, K.; Woodman, R.J.; Mangoni, A.A. Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: A systematic review and meta-analysis. Br. J. Clin. Pharm. 2015, 80, 209–220. [Google Scholar] [CrossRef]
| Mild Risk | Moderate Risk | Severe Risk | p-Value | |
|---|---|---|---|---|
| Characteristics | 0.0–0.33 | 0.34–0.66 | 0.67–1.0 | |
| Patients, n (%) | 229 (32.86) | 409 (58.68) | 59 (8.46) | |
| Women | 92 (40.17) | 220 (53.79) | 37 (62.71) | <0.001 |
| Men | 137 (59.83) | 189 (46.21) | 22 (37.29) | |
| MPI score | ||||
| Score range | 0.063–0.313 | 0.375–0.625 | 0.688–0.875 | |
| Median (IQR) | 0.250 (0.250–0.313) | 0.438 (0.438–0.500) | 0.688 (0.688–0.750) | |
| Age | ||||
| Range | 65–96 | 65–101 | 67–102 | |
| Median (IQR) | 75 (70–82) | 82 (74–87) | 86 (80–92) | <0.001 |
| Fall, n (%) | 4 (1.75) | 11 (2.69) | 5 (8.47) | 0.021 |
| Delirium, n (%) | 14 (6.11) | 34 (8.31) | 19 (32.20) | <0.001 |
| LOS (days), median (IQR) | 5 (3–8) | 6 (4–11) | 11 (5–18) | 0.001 |
| Mortality, n (%) | ||||
| In-hospital | 1 (0.44) | 16 (3.91) | 8 (13.56) | <0.001 |
| 1-month | 3 (1.31) | 20 (4.89) | 12 (20.34) | <0.001 |
| 3-month | 7 (3.06) | 42 (10.27) | 16 (27.12) | <0.001 |
| 6-month | 22 (9.61) | 95 (23.23) | 20 (33.90) | <0.001 |
| 12-month | 38 (16.59) | 136 (33.25) | 27 (45.76) | <0.001 |
| Re-admission rate, % | ||||
| 1-month | 11.35 | 15.65 | 8.47 | 0.157 |
| 3-month | 23.58 | 33.25 | 27.12 | 0.022 |
| 6-month | 35.37 | 45.72 | 38.98 | 0.008 |
| Unadjusted OR | Adjusted OR * | |||||
|---|---|---|---|---|---|---|
| Variable | ||||||
| Six-month mortality | ||||||
| Mild | Reference group | |||||
| Moderate | 2.85 | 1.73–4.67 | <0.0001 | 2.92 | 1.75–4.87 | <0.0001 |
| Severe | 4.83 | 2.41–9.67 | <0.0001 | 4.88 | 2.34–10.20 | <0.0001 |
| Three-month mortality | ||||||
| Mild | Reference group | |||||
| Moderate | 3.63 | 1.60–8.22 | 0.002 | 3.56 | 1.55–8.20 | 0.003 |
| Severe | 11.80 | 4.58–30.40 | <0.0001 | 11.20 | 4.14–30.32 | <0.0001 |
| One-month mortality | ||||||
| Mild | Reference group | |||||
| Moderate | 3.87 | 1.14–13.18 | 0.030 | 3.91 | 1.13–13.55 | 0.031 |
| Severe | 19.23 | 5.22–70.83 | <0.0001 | 19.43 | 4.96–76.06 | <0.0001 |
| In-hospital mortality | ||||||
| Mild | Reference group | |||||
| Moderate | 9.28 | 1.22–70.46 | 0.031 | 9.19 | 1.19–70.74 | 0.033 |
| Severe | 35.76 | 4.38–292.33 | 0.001 | 34.46 | 4.02–295.78 | 0.001 |
| In-hospital delirium | ||||||
| Mild | Reference group | |||||
| Moderate | 1.39 | 0.73–2.65 | 0.314 | 1.21 | 0.62–2.37 | 0.573 |
| Severe | 7.29 | 3.38–15.73 | <0.0001 | 5.68 | 2.48–13.01 | <0.0001 |
| Unadjusted IRR | Adjusted IRR * | |||||
| IRR | 95% CI | p-value | IRR | 95% CI | p-value | |
| Re-admission rate, one month | ||||||
| Mild | Reference group | |||||
| Moderate | 1.29 | 0.86–1.94 | 0.223 | 1.31 | 0.86–2.00 | 0.215 |
| Severe | 0.59 | 0.23–1.51 | 0.269 | 0.60 | 0.23–1.58 | 0.304 |
| Re-admission rate, three months | ||||||
| Mild | Reference group | |||||
| Moderate | 1.46 | 1.14–1.88 | 0.003 | 1.52 | 1.17–1.97 | 0.002 |
| Severe | 0.88 | 0.53–1.44 | 0.608 | 0.95 | 0.57–1.59 | 0.844 |
| Re-admission rate, six months | ||||||
| Mild | Reference group | |||||
| Moderate | 1.59 | 1.31–1.93 | <0.0001 | 1.69 | 1.39–2.07 | <0.0001 |
| Severe | 1.04 | 0.72–1.50 | 0.830 | 1.18 | 0.81–1.72 | 0.379 |
| In-hospital falls | ||||||
| Mild | Reference group | |||||
| Moderate | 1.24 | 0.40–3.85 | 0.706 | 1.04 | 0.32–3.33 | 0.949 |
| Severe | 2.24 | 0.56–8.95 | 0.254 | 1.69 | 0.40–7.18 | 0.478 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryant, K.; Sorich, M.J.; Woodman, R.J.; Mangoni, A.A. Validation and Adaptation of the Multidimensional Prognostic Index in an Older Australian Cohort. J. Clin. Med. 2019, 8, 1820. https://doi.org/10.3390/jcm8111820
Bryant K, Sorich MJ, Woodman RJ, Mangoni AA. Validation and Adaptation of the Multidimensional Prognostic Index in an Older Australian Cohort. Journal of Clinical Medicine. 2019; 8(11):1820. https://doi.org/10.3390/jcm8111820
Chicago/Turabian StyleBryant, Kimberley, Michael J. Sorich, Richard J. Woodman, and Arduino A. Mangoni. 2019. "Validation and Adaptation of the Multidimensional Prognostic Index in an Older Australian Cohort" Journal of Clinical Medicine 8, no. 11: 1820. https://doi.org/10.3390/jcm8111820





