Long Term Retention of Gingival Sealing around Titanium Implants with CaCl2 Hydrothermal Treatment: A Rodent Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ti implants and Plates
2.2. Surface Characterization with Thin-Film X-ray Diffractometry (TF-XRD)
2.3. Oral Implantation
2.4. Topical Application of Horseradish Peroxidase
2.5. Tissue Preparation
2.6. HRP Histochemistry
2.7. Immunohistochemistry
2.8. Quantification of HRP Penetration and Down-Growth
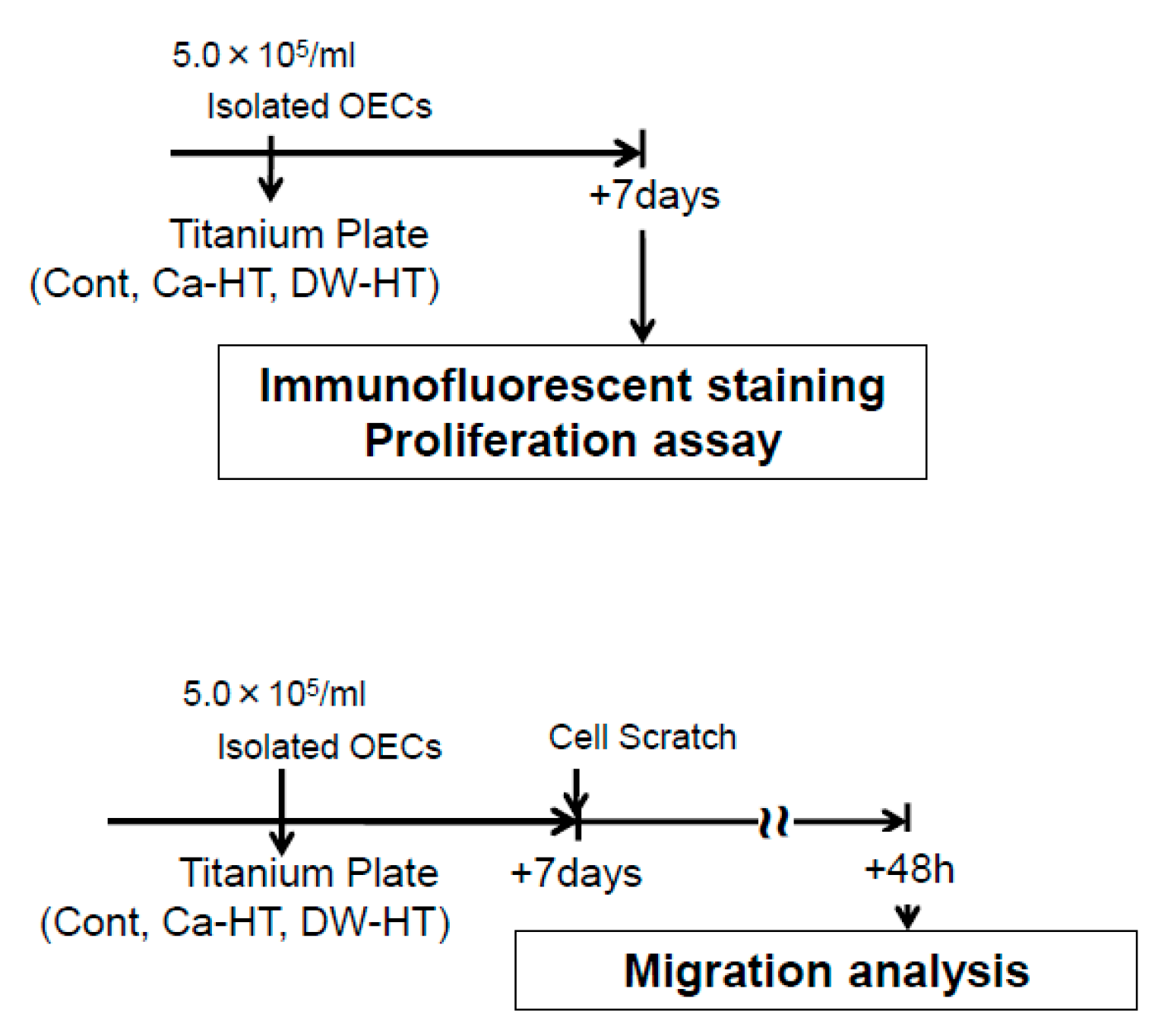
2.9. Cell Culture
2.10. Immunofluorescence Staining for Adhesion Proteins
2.11. Western Blot Analysis
2.12. Proliferation Assay
2.13. Scratch Assay
2.14. Statistical Analysis
3. Results
3.1. Surface Characterization with TF-XRD
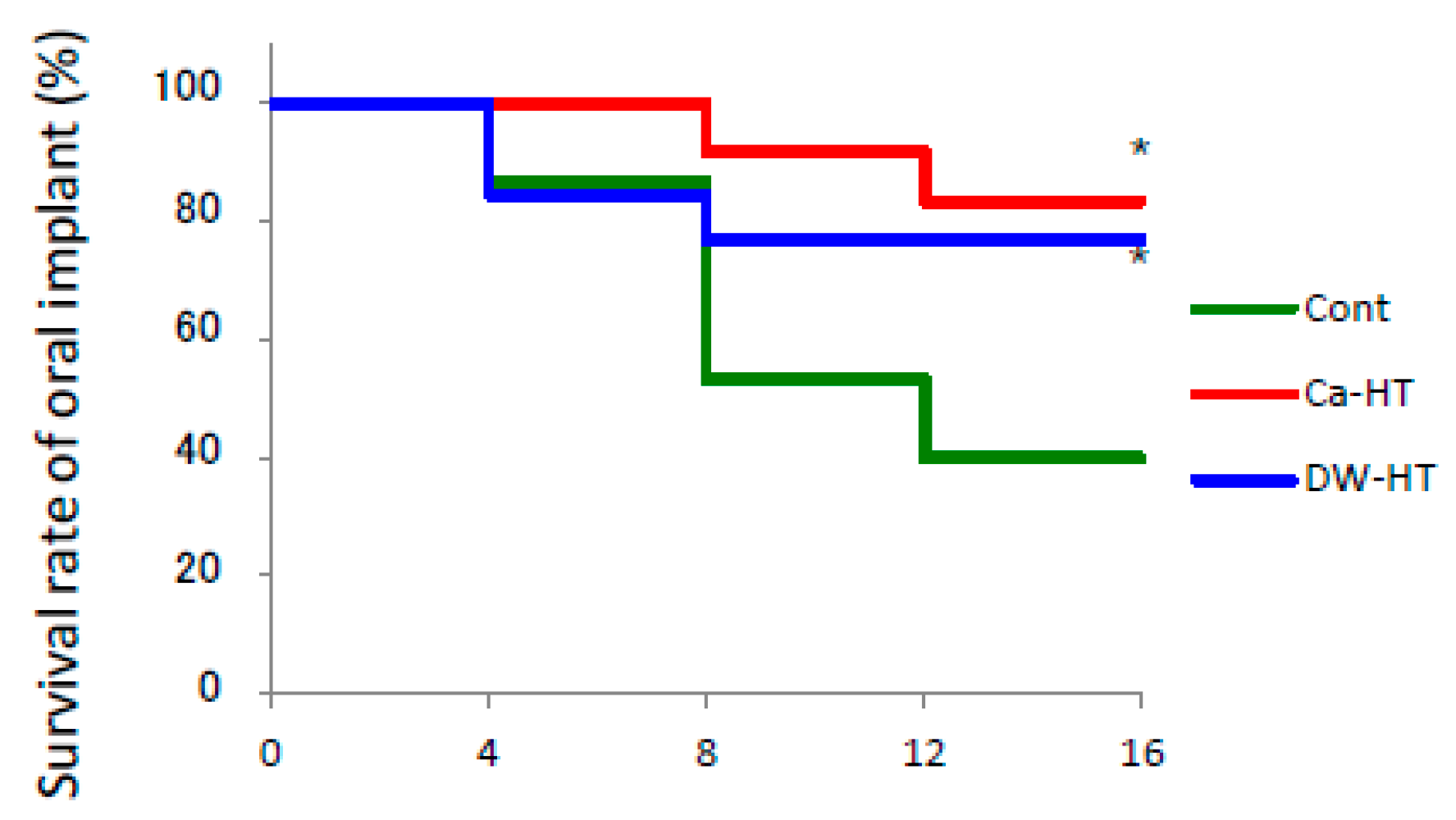
3.2. Implant Survival Rate in Rat Oral Environment.
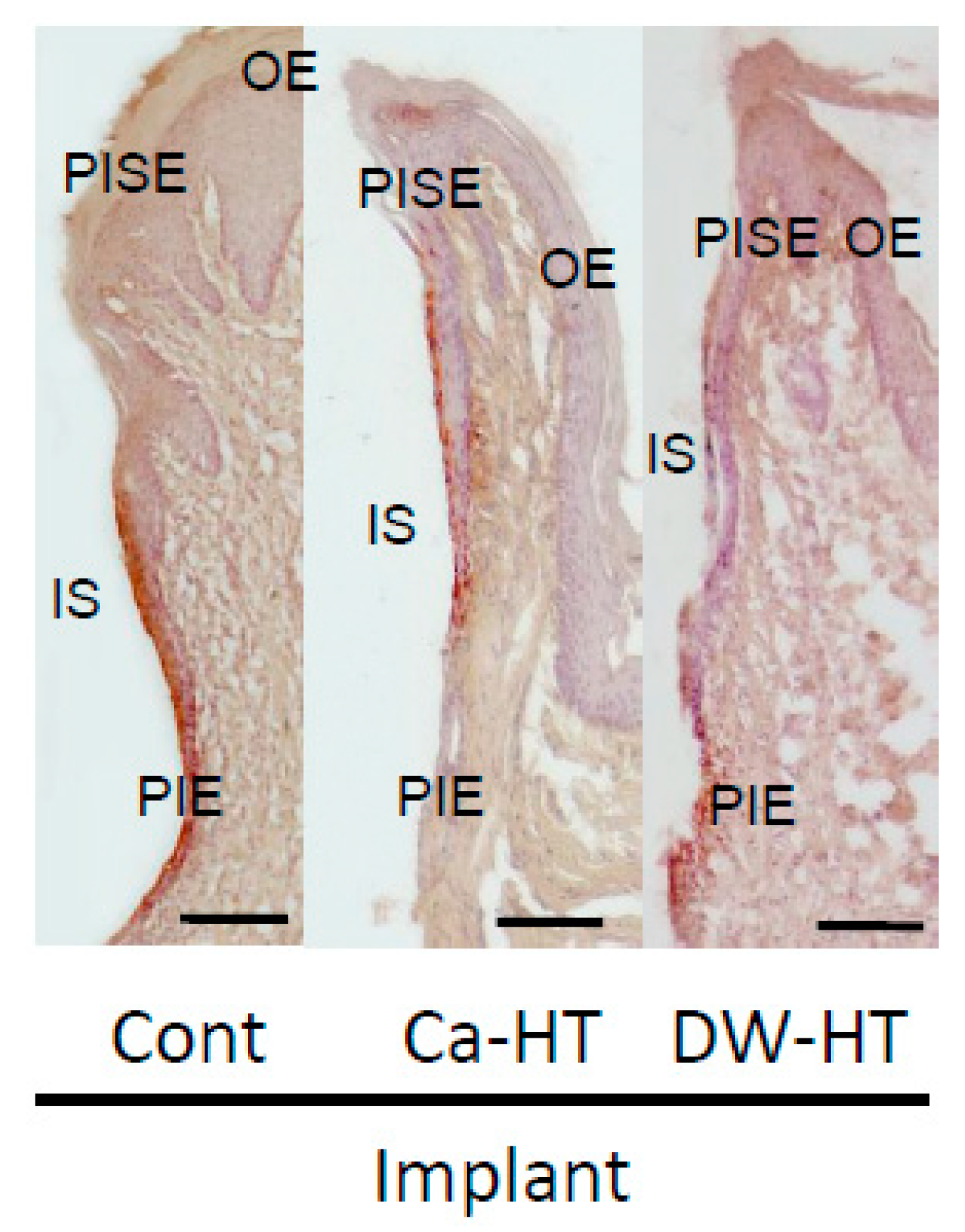
3.3. Down-Growth of PIE and Distribution of Laminin-332 around the Experimental Implants
3.4. Penetration of HRP in the Peri-Implant Soft Tissue
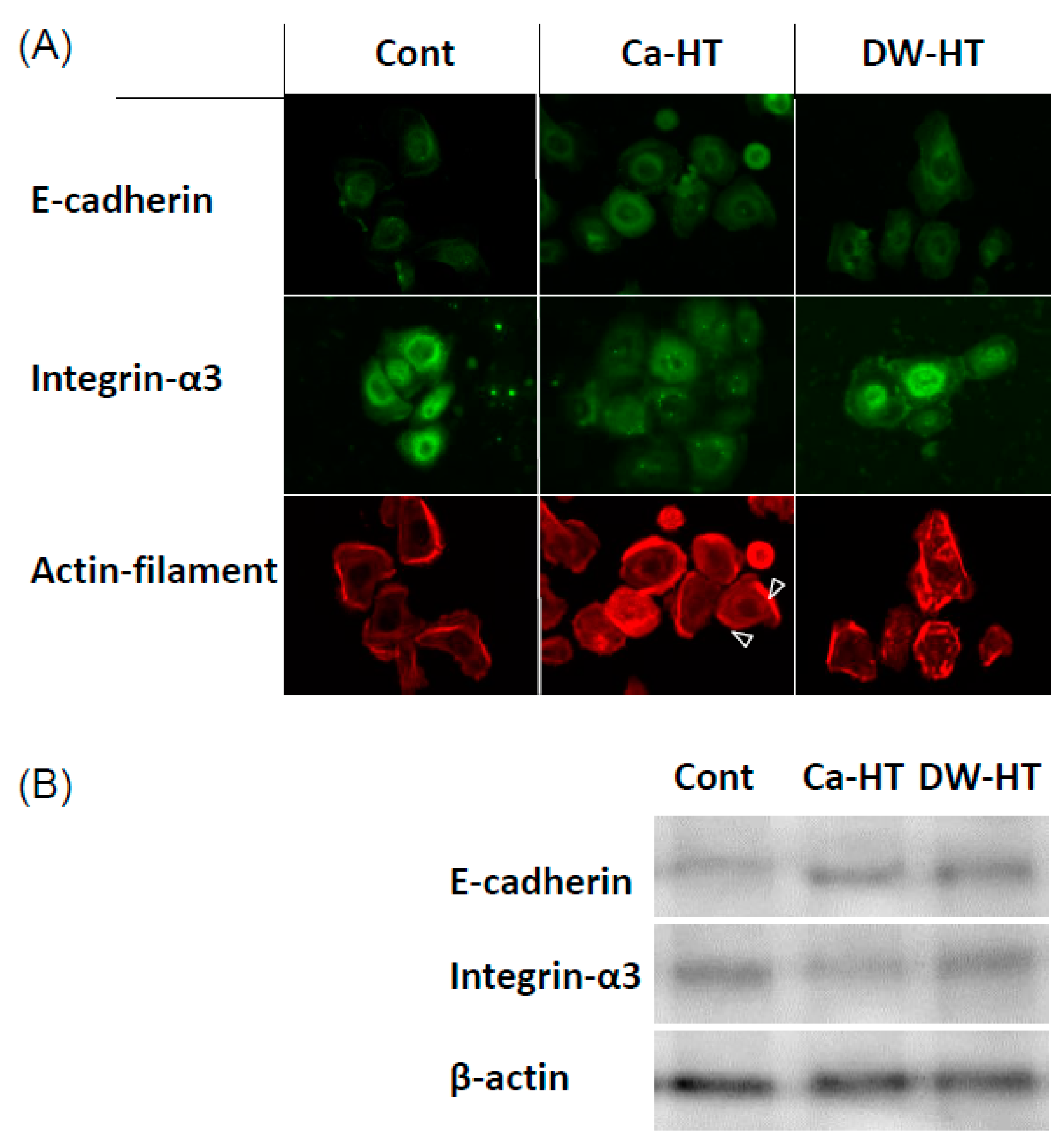
3.5. Expression of E-cadherin, In-α3 and Filamentous Actin in OECs
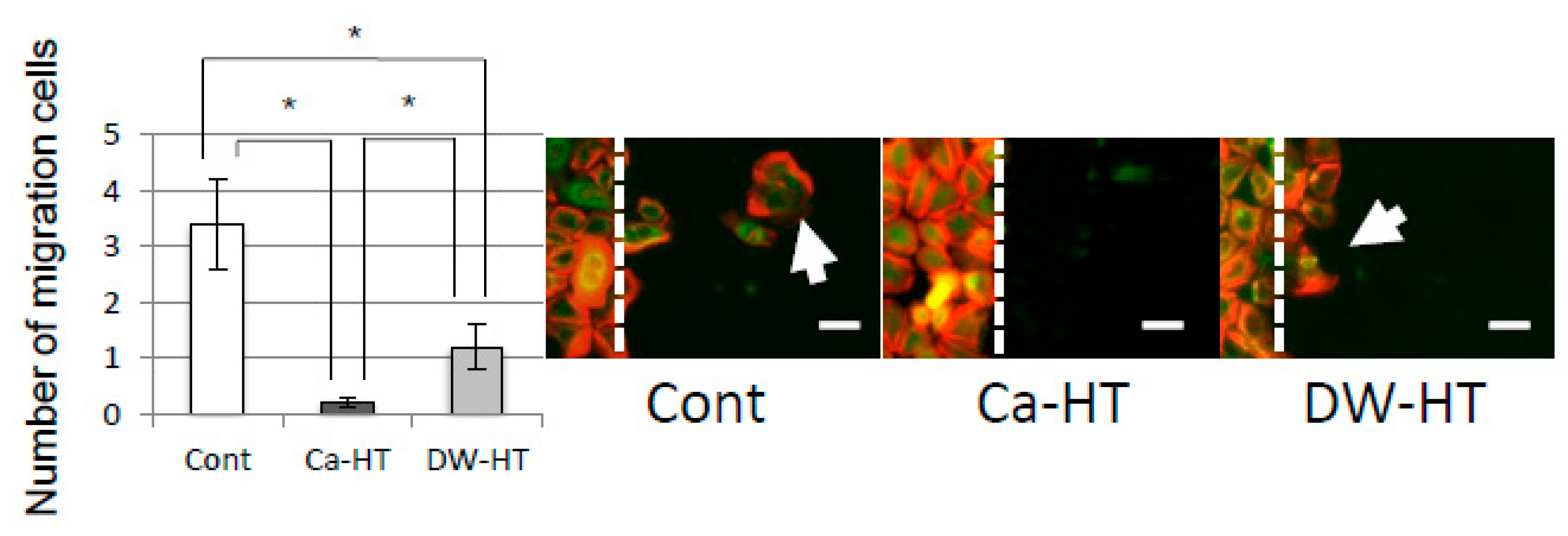
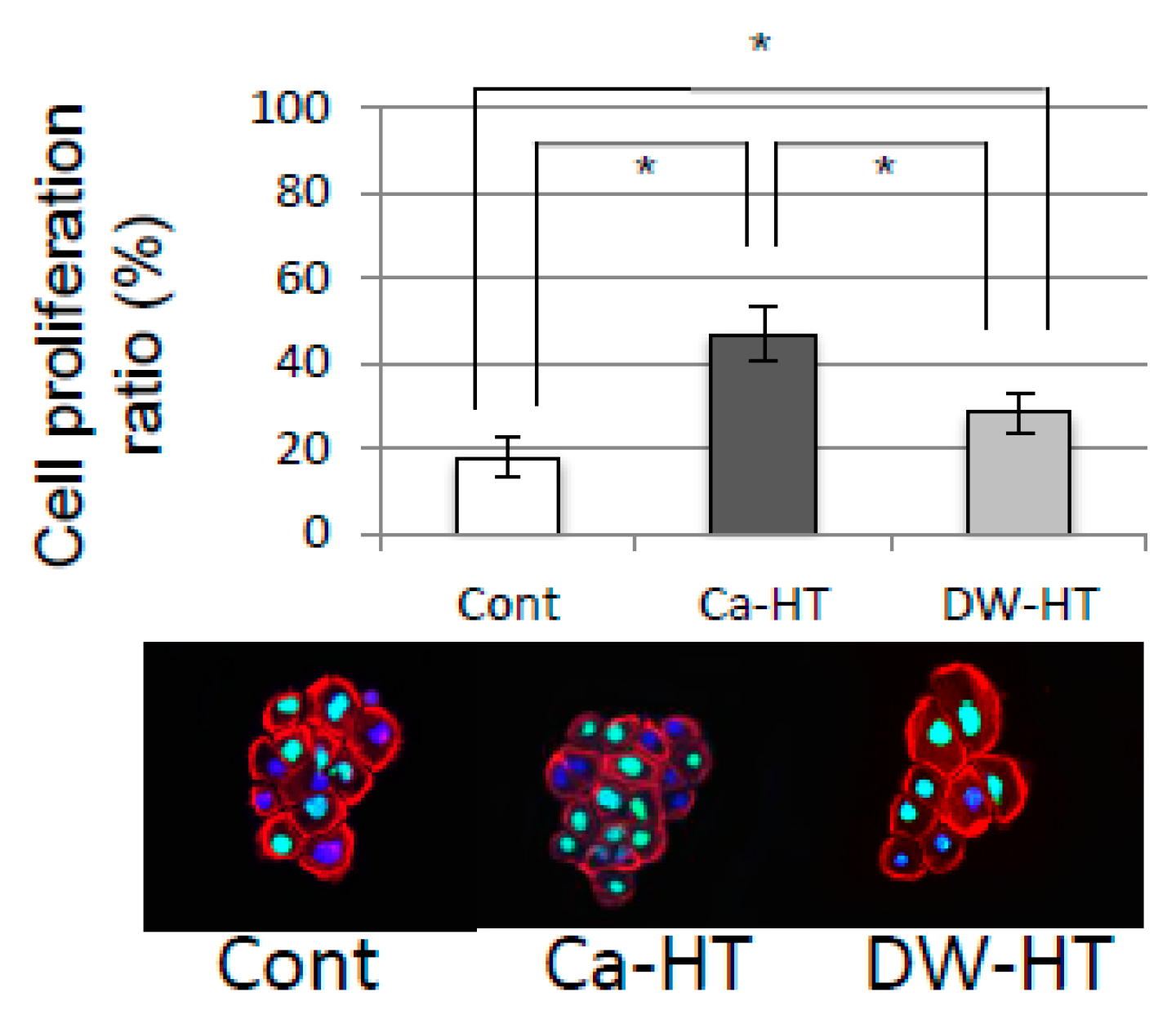
3.6. OEC Migration and Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holgers, K.M. Characteristics of the inflammatory process around skin-penetrating titanium implants for aural rehabilitation. Audiology 2000, 39, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Chan, Y.J.; Kung, K.C.; Lee, T.M. Comparison of osseointegration on various implant surfaces after bacterial contamination and cleaning: A rabbit study. Int. J. Oral Maxillofac. Implants 2014, 29, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, J.M.; Small, I.A. Five-year follow-up of mandibular reconstruction with hydroxylapatite and the mandibular staple bone plate. J. Oral Maxillofac. Surg. 1995, 53, 19–22. [Google Scholar] [CrossRef]
- Chehroudi, B.; Gould, T.R.; Brunette, D.M. Titanium-coated micromachined grooves of different dimensions affect epithelial and connective-tissue cells differently in vivo. J. Biomed. Mater. Res. 1990, 24, 1203–1219. [Google Scholar] [CrossRef]
- Paquay, Y.C.; de Ruijter, J.E.; van der Waerden, J.P.; Jansen, J.A. Tissue reaction to Dacron velour and titanium fibre mesh used for anchorage of percutaneous devices. Biomaterials 1996, 17, 1251–1256. [Google Scholar] [CrossRef]
- Winter, G.D. Transcutaneous implants: Reactions of the skin-implant interface. J. Biomed. Mater. Res. 1974, 8, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Qiu, W.Y.; Pan, Q.; Yao, Y.F.; Xing, K.; Lou, M.F. Reactive oxygen species (ROS) are essential mediators in epidermal growth factor (EGF)-stimulated corneal epithelial cell proliferation, adhesion, migration, and wound healing. Exp. Eye Res. 2009, 89, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Yanagihara, N.; Imamura, H.; Kaida, M.; Moriwaki, M.; Shiraki, K.; Miki, T. Hepatocyte growth factor stimulates proliferation and migration during wound healing of retinal pigment epithelial cells in vitro. Jpn. J. Ophthalmol. 2003, 47, 268–275. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Ogino, Y.; Moriyama, Y.; Jinno, Y.; Koyano, K. Evaluations of epithelial sealing and peri-implant epithelial down-growth around “step-type” implants. Clin. Oral Implants Res. 2012, 23, 459–466. [Google Scholar] [CrossRef]
- Tabata, L.F.; Assunção, W.G.; Adelino Ricardo Barão, V.; de Sousa, E.A.; Gomes, E.A.; Delben, J.A. Implant platform switching: Biomechanical approach using two-dimensional finite element analysis. J. Craniofac. Surg. 2010, 21, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, A.; Smeets, R.; Hartjen, P.; Heinrich, O.; Heuberger, R.; Heiland, M.; Precht, C.; Cacaci, C. Photofunctionalization and non-thermal plasma activation of titanium surfaces. Clin. Oral Investig. 2018, 22, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, W.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Yamazoe, J.; Kondo, R.; Sakaguchi, M.; Matsuura, Y.; Tsukiyama, Y.; Koyano, K. Effects of CaCl2 hydrothermal treatment of titanium implant surfaces on early epithelial sealing. Coll. Surf. B Biointerfaces 2015, 131, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Okawachi, H.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Sakaguchi, M.; Yamane, K.; Koyano, K. Effect of titanium surface calcium and magnesium on adhesive activity of epithelial-like cells and fibroblasts. Biointerphases 2012, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ayukawa, Y.; Legeros, R.Z.; Matsuya, S.; Koyano, K.; Ishikawa, K. Tissue-response to calcium-bonded titanium surface. J. Biomed. Mater. Res. Part. A 2010, 95, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Shiraiwa, M.; Yamaza, T.; Yoshinari, M.; Kido, M.A.; Ayukawa, Y.; Inoue, T.; Koyano, K.; Tanaka, T. Difference in penetration of horseradish peroxidase tracer as a foreign substance into the peri-implant or junctional epithelium of rat gingivae. Clin. Oral Implants Res. 2002, 13, 243–251. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Yamaza, T.; Tsukiyama, Y.; Koyano, K. Promotive effect of insulin-like growth factor-1 for epithelial sealing to titanium implants. J. Biomed. Mater. Res. Part. A 2013, 101, 2896–2904. [Google Scholar] [CrossRef]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Goto, T.; Kido, M.A.; Kagiya, T.; Mino, S.; Shimono, M.; Tanaka, T. Ultrastructural localization of laminin-5 (gamma2 chain) in the rat peri-implant oral mucosa around a titanium-dental implant by immuno-electron microscopy. Biomaterials 2005, 26, 6280–6287. [Google Scholar] [CrossRef] [PubMed]
- Yamaza, T.; Kido, M.A.; Kiyoshima, T.; Nishimura, Y.; Himeno, M.; Tanaka, T. A fluid-phase endocytotic capacity and intracellular degradation of a foreign protein (horseradish peroxidase) by lysosomal cysteine proteinases in the rat junctional epithelium. J. Periodontal Res. 1997, 32, 651–660. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Goto, T.; Yoshinari, M.; Koyano, K.; Tanaka, T. A study of the initial attachment and subsequent behavior of rat oral epithelial cells cultured on titanium. J. Periodontol. 2002, 73, 852–860. [Google Scholar] [CrossRef]
- Diener, A.; Nebe, B.; Luthen, F.; Becker, P.; Beck, U.; Neumann, H.G.; Rychly, J. Control of focal adhesion dynamics by material surface characteristics. Biomaterials 2005, 26, 383–392. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Schenk-Meuser, K.; Linez, P.; Biehl, V.; Duschner, H.; Breme, J.; Hildebrand, H. Interactions between cells and titanium surfaces. Biomol. Eng. 2002, 19, 243–249. [Google Scholar] [CrossRef]
- Takeichi, M.; Okada, T.S. Roles of magnesium and calcium ions in cell-to-substrate adhesion. Exp. Cell Res. 1972, 74, 51–60. [Google Scholar] [CrossRef]
- Klagsbrun, M.; Baird, A. A dual receptor system is required for basic fibroblast growth factor activity. Cell 1991, 67, 229–231. [Google Scholar] [CrossRef]
- Kan, M.; Wang, F.; To, B.; Gabriel, J.L.; McKeehan, W.L. Divalent cations and heparin/heparan sulfate cooperate to control assembly and activity of the fibroblast growth factor receptor complex. J. Biol. Chem. 1996, 271, 26143–26148. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Fagerholm, P.; Kim, H.J. Effect of leukocytes on corneal cellular proliferation and wound healing. Invest. Ophthalmol. Vis. Sci. 1999, 40, 575–581. [Google Scholar]
- Albrektsson, T.; Becker, W.; Coli, P.; Jemt, T.; Molne, J.; Sennerby, L. Bone loss around oral and orthopedic implants: An immunologically based condition. Clin. Implant Dent. Relat. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Jemt, T.; Molne, J.; Tengvall, P.; Wennerberg, A. On inflammation-immunological balance theory-A critical apprehension of disease concepts around implants: Mucositis and marginal bone loss may represent normal conditions and not necessarily a state of disease. Clin. Implant Dent. Relat. Res. 2019, 21, 183–189. [Google Scholar] [CrossRef]
- Chan, E.P.; Mhawi, A.; Clode, P.; Saunders, M.; Filgueira, L. Effects of titanium(iv) ions on human monocyte-derived dendritic cells. Metallomics 2009, 1, 166–174. [Google Scholar] [CrossRef]
- Wu, T.; Tang, M. The inflammatory response to silver and titanium dioxide nanoparticles in the central nervous system. Nanomedicine 2018, 13, 233–249. [Google Scholar] [CrossRef]
- Fraker, A.C. Corrosion of metallic implants and prosthetic devices. In Metals Handbook, 9th ed.; ASM International: Metals Park, OH, USA, 1987; Volume 13. [Google Scholar]
- Williams, D.F. Titanium and titanium alloys. In Biocompatibility of Clinic Implant Materials; Williams, D.F., Ed.; CRC Press: Boca Raton, FL, USA, 1981. [Google Scholar]
- Ektessabi, A.M.; Otsuka, T.; Tsuboi, Y.; Yokoyama, K.; Albrektsson, T.; Sennerby, L.; Johansson, C. Application of micro beam PIXE to detection of titanium ion release from dental and orthopaedic implants. Int. J. PIXE 1994, 4, 81–91. [Google Scholar] [CrossRef]
- Woodman, J.L.; Jacobs, J.J.; Galante, J.O.; Urban, R.M. Metal ion release from titanium-based prosthetic segmental replacements of long bones in baboons: A long-term study. J. Orthop. Res. 1984, 1, 421–430. [Google Scholar] [CrossRef] [PubMed]



| Material | Surface | Surface roughness (Ra, μm) | Dimension | |
|---|---|---|---|---|
| Implant for animal study | Pure titanium Japan Industrial Standards Class 1 (equivalent for ASTM grade I) | Machined | 0.438 | screw shape 2 mm in diameter 4.5 mm in length (2.5 mm transmucosal + 2 mm intrabony) |
| Disk for culture study | Pure titanium Japan Industrial Standards Class 1 (equivalent for ASTM grade I) | Mirror-polished | 0.20–0.22 | 15 mm × 1 mm (diameter × thickness) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayukawa, Y.; Oshiro, W.; Atsuta, I.; Furuhashi, A.; Kondo, R.; Jinno, Y.; Koyano, K. Long Term Retention of Gingival Sealing around Titanium Implants with CaCl2 Hydrothermal Treatment: A Rodent Study. J. Clin. Med. 2019, 8, 1560. https://doi.org/10.3390/jcm8101560
Ayukawa Y, Oshiro W, Atsuta I, Furuhashi A, Kondo R, Jinno Y, Koyano K. Long Term Retention of Gingival Sealing around Titanium Implants with CaCl2 Hydrothermal Treatment: A Rodent Study. Journal of Clinical Medicine. 2019; 8(10):1560. https://doi.org/10.3390/jcm8101560
Chicago/Turabian StyleAyukawa, Yasunori, Wakana Oshiro, Ikiru Atsuta, Akihiro Furuhashi, Ryosuke Kondo, Yohei Jinno, and Kiyoshi Koyano. 2019. "Long Term Retention of Gingival Sealing around Titanium Implants with CaCl2 Hydrothermal Treatment: A Rodent Study" Journal of Clinical Medicine 8, no. 10: 1560. https://doi.org/10.3390/jcm8101560





