Impact of Sex Differences and Diabetes on Coronary Atherosclerosis and Ischemic Heart Disease
Abstract
:1. Introduction
2. Coronary Artery Disease and Ischemic Heart Disease in Diabetic Women: Defining the Problem
3. Genetic, Epigenetic, and Hormonal Factors Involved in Sex-Specific Effects of Diabetes in CVD
3.1. The Immune System Profile in Women
3.2. Genetics and Epigenetics
3.3. Environmental Factors and Gut Microbiome
3.4. Different Metabolism in Women: Correlation with the Different Asset of Sex Chromosome Genes
3.5. Estrogenes and Oxidative Stress
4. Cellular and Molecular Signaling Pathways Involved in Sex-Specific Effects of Diabetes in CAD
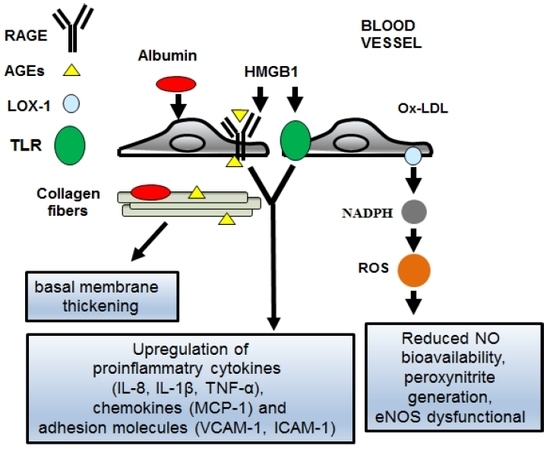
4.1. The AGEs/RAGEs System in Diabetic Women
4.2. The TLR-2 and -4 Signaling Pathways in Diabetic Women
4.3. Oxidative Stress and Ox-LDL in Diabetic Women
5. Non-Invasive Assessment and Biomarkers of Coronary Artery Disease in Diabetic Women
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Heart Network. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Maas, A.H.; Appelman, Y.E. Gender differences in coronary heart disease. Neth. Heart J. 2010, 18, 598–602. [Google Scholar] [CrossRef]
- Crilly, M.A.; Bundred, P.E.; Leckey, L.C.; Johnstone, F.C. Gender bias in the clinical management of women with angina: Another look at the Yentl syndrome. J. Womens Health 2008, 17, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Stouffer, G.A.; Kucharska-Newton, A.; Vaduganathan, M.; Qamar, A.; Matsushita, K.; Kolte, D.; Reynolds, H.R.; Bangalore, S.; Rosamond, W.D.; et al. Fifteen-Year Trends in Management and Outcomes of Non-ST-Segment-Elevation Myocardial Infarction Among Black and White Patients: The ARIC Community Surveillance Study, 2000–2014. J. Am. Heart Assoc. 2018, 7, e010203. [Google Scholar] [CrossRef]
- White, U.A.; Tchoukalova, Y.D. Sex dimorphism and depot differences in adipose tissue function. Biochim. Biophys. Acta 2014, 1842, 377–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijay, V.; Han, T.; Moland, C.L.; Kwekel, J.C.; Fuscoe, J.C.; Desai, V.G. Sexual dimorphism in the expression of mitochondria-related genes in rat heart at different ages. PLoS ONE 2015, 10, e0117047. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Diabetes Atlas, 5th ed.; International Diabetes Federation: Brussels, Belgium, 2011. [Google Scholar]
- Masding, M.G.; Stears, A.J.; Burdge, G.C.; Wootton, S.A.; Sandeman, D.D. Premenopausal advantages in postprandial lipid metabolism are lost in women with type 2 diabetes. Diabetes Care 2003, 26, 3243–3249. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Ren, J.; Kelley, R.O. Cardiac health in women with metabolic syndrome: Clinical aspects and pathophysiology. Obesity 2009, 17, 1114–1123. [Google Scholar] [CrossRef]
- De Rosa, S.; Chiefari, E.; Salerno, N.; Ventura, V.; D’Ascoli, G.L.; Arcidiacono, B.; Ambrosio, G.; Bilotta, F.L.; Torella, D.; Foti, D.; et al. HMGA1 is a novel candidate gene for myocardial infarction susceptibility. Int. J. Cardiol. 2017, 227, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.L.; Cohn, B.A.; Wingard, D.L.; Edelstein, S.L. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA 1991, 265, 627–631. [Google Scholar] [CrossRef]
- Barrett-Connor, E.; Bush, T.L. Estrogen and coronary heart disease in women. JAMA 1991, 265, 1861–1867. [Google Scholar] [CrossRef]
- Manson, J.E.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Krolewski, A.S.; Rosner, B.; Arky, R.A.; Speizer, F.E.; Hennekens, C.H. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 1991, 151, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Juutilainen, A.; Kortelainen, S.; Lehto, S.; Ronnemaa, T.; Pyorala, K.; Laakso, M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 2004, 27, 2898–2904. [Google Scholar] [CrossRef]
- Huxley, R.; Barzi, F.; Woodward, M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. BMJ 2006, 332, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kiencke, S.; Handschin, R.; von Dahlen, R.; Muser, J.; Brunner-Larocca, H.P.; Schumann, J.; Felix, B.; Berneis, K.; Rickenbacher, P. Pre-clinical diabetic cardiomyopathy: Prevalence, screening, and outcome. Eur. J. Heart Fail. 2010, 12, 951–957. [Google Scholar] [CrossRef]
- Suys, B.E.; Katier, N.; Rooman, R.P.; Matthys, D.; Op De Beeck, L.; Du Caju, M.V.; De Wolf, D. Female children and adolescents with type 1 diabetes have more pronounced early echocardiographic signs of diabetic cardiomyopathy. Diabetes Care 2004, 27, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Peters, S.A.; Mishra, G.D.; Woodward, M. Cardiovascular disease risk in type 1 diabetes—Authors’ reply. Lancet Diabetes Endocrinol. 2015, 3, 317. [Google Scholar] [CrossRef]
- Huxley, R.R.; Peters, S.A.; Mishra, G.D.; Woodward, M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 198–206. [Google Scholar] [CrossRef]
- Peters, S.; Smit, E. Pan-HER inhibition in EGFR wild-type non-small-cell lung cancer. Lancet Oncol. 2014, 15, 1289–1290. [Google Scholar] [CrossRef]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar] [CrossRef]
- Peters, S.A.; Huxley, R.R.; Sattar, N.; Woodward, M. Sex Differences in the Excess Risk of Cardiovascular Diseases Associated with Type 2 Diabetes: Potential Explanations and Clinical Implications. Curr. Cardiovasc. Risk Rep. 2015, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Diabetes Audit–2012–2013 Report 1: Care Processes and Treatment Targets, Health and Social Care Information Centre 2014; NHS Digital: Leeds, UK, 2014.
- Beckman, J.A.; Paneni, F.; Cosentino, F.; Creager, M.A. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Eur. Heart J. 2013, 34, 2444–2452. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.W.; Orchard, T.J. Oxidized lipids in insulin-dependent diabetes mellitus: A sex-diabetes interaction? Metab. Clin. Exp. 1994, 43, 1196–1200. [Google Scholar] [CrossRef]
- Howard, B.V.; Cowan, L.D.; Go, O.; Welty, T.K.; Robbins, D.C.; Lee, E.T. Adverse effects of diabetes on multiple cardiovascular disease risk factors in women. The Strong Heart Study. Diabetes Care 1998, 21, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.W.; Heywood, D.M.; Grant, P.J. Sex differences in coagulation and fibrinolysis in white subjects with non-insulin-dependent diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Ossei-Gerning, N.; Wilson, I.J.; Grant, P.J. Sex differences in coagulation and fibrinolysis in subjects with coronary artery disease. Thromb. Haemost. 1998, 79, 736–740. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Paradisi, G.; Cronin, J.; Crowde, K.; Hempfling, A.; Hook, G.; Baron, A.D. Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation 2000, 101, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Papacosta, O.; Lawlor, D.A.; Whincup, P.H.; Lowe, G.D.; Ebrahim, S.; Sattar, N. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012, 55, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Perex-Llamas, F.; Fuente, T.; Zamora, S.; Tebar, F.J. Anthropometric, computed tomography and fat cell data in an obese population: Relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur. J. Endocrinol. 2000, 143, 657–666. [Google Scholar] [CrossRef]
- Donahue, R.P.; Rejman, K.; Rafalson, L.B.; Dmochowski, J.; Stranges, S.; Trevisan, M. Sex differences in endothelial function markers before conversion to pre-diabetes: Does the clock start ticking earlier among women? The Western New York Study. Diabetes Care 2007, 30, 354–359. [Google Scholar] [CrossRef]
- Haffner, S.M.; Miettinen, H.; Stern, M.P. Relatively more atherogenic coronary heart disease risk factors in prediabetic women than in prediabetic men. Diabetologia 1997, 40, 711–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugger, H.; Abel, E.D. Rodent models of diabetic cardiomyopathy. Dis. Models Mech. 2009, 2, 454–466. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, A.; Sekhon, H.K.; Kaur, G. Sex hormones and immune dimorphism. Sci. World J. 2014, 2014, 159150. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Accardi, G.; Candore, G. Probiotics and Prebiotics: Health Promotion by Immune Modulation in the Elderly; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Perez-Llamas, F.; Baraza, J.C.; Garcia-Prieto, M.D.; Fardy, P.S.; Tebar, F.J.; Zamora, S. Body fat distribution in pre-and post-menopausal women: Metabolic and anthropometric variables. J. Nutr. Health Aging 2002, 6, 123–126. [Google Scholar] [PubMed]
- Lima, R.; Wofford, M.; Reckelhoff, J.F. Hypertension in postmenopausal women. Curr. Hypertens. Rep. 2012, 14, 254–260. [Google Scholar] [CrossRef]
- Link, J.C.; Chen, X.; Arnold, A.P.; Reue, K. Metabolic impact of sex chromosomes. Adipocyte 2013, 2, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Link, J.C.; Reue, K. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu. Rev. Nutr. 2017, 37, 225–245. [Google Scholar] [CrossRef]
- Zore, T.; Palafox, M.; Reue, K. Sex differences in obesity, lipid metabolism, and inflammation-A role for the sex chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Dehghan, P.; Gargari, B.P.; Jafar-Abadi, M.A.; Aliasgharzadeh, A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized-controlled clinical trial. Int. J. Food Sci. Nutr. 2014, 65, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.; Vich Vila, A.; Garmaeva, S.; Jankipersadsing, S.A.; Imhann, F.; Collij, V.; Bonder, M.J.; Jiang, X.; Gurry, T.; Alm, E.J.; et al. Analysis of 1135 gut metagenomes identifies sex-specific resistome profiles. Gut Microbes 2018, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, R.; Sylvia, K.E.; Klein, S.L.; Forster, S.C.; Plebanski, M.; Eri, R.; Flanagan, K.L. The microgenderome revealed: Sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin. Immunopathol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Aravindhan, V.; Madhumitha, H. Metainflammation in Diabetic Coronary Artery Disease: Emerging Role of Innate and Adaptive Immune Responses. J. Diabetes Res. 2016, 2016, 6264149. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Monticone, R.E.; Lakatta, E.G. Proinflammation of aging central arteries: A mini-review. Gerontology 2014, 60, 519–529. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.W.; Im, J.A.; Hwang, H.J. Monocyte chemoattractant protein-1 is related to metabolic syndrome and homocysteine in subjects without clinically significant atherosclerotic cardiovascular disease. Scand. J. Clin. Lab. Investig. 2011, 71, 1–6. [Google Scholar] [CrossRef]
- Jia, S.J.; Niu, P.P.; Cong, J.Z.; Zhang, B.K.; Zhao, M. TLR4 signaling: A potential therapeutic target in ischemic coronary artery disease. Int. Immunopharmacol. 2014, 23, 54–59. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Ruvolo, G.; Lio, D.; Madonna, R. Toll-like receptor-4 signaling pathway in aorta aging and diseases: “its double nature”. J. Mol. Cell. Cardiol. 2017, 110, 38–53. [Google Scholar] [CrossRef]
- Yamagishi, S.; Fukami, K.; Matsui, T. Crosstalk between advanced glycation end products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications. Cardiovasc. Diabetol. 2015, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Balistreri, C.R.; Geng, Y.J.; De Caterina, R. Diabetic microangiopathy: Pathogenetic insights and novel therapeutic approaches. Vasc. Pharmacol. 2017, 90, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Orsatti, C.L.; Petri Nahas, E.A.; Nahas-Neto, J.; Orsatti, F.L.; Giorgi, V.I.; Witkin, S.S. Evaluation of Toll-Like receptor 2 and 4 RNA expression and the cytokine profile in postmenopausal women with metabolic syndrome. PLoS ONE 2014, 9, e109259. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Bonfigli, A.R.; Boemi, M.; Olivieri, F.; Ceriello, A.; Genovese, S.; Franceschi, C.; Spazzafumo, L.; Fabietti, P.; Candore, G.; et al. Evidences of +896 A/G TLR4 polymorphism as an indicative of prevalence of complications in T2DM patients. Med. Inflamm. 2014, 2014, 973139. [Google Scholar] [CrossRef] [PubMed]
- Matthan, N.R.; Jalbert, S.M.; Barrett, P.H.; Dolnikowski, G.G.; Schaefer, E.J.; Lichtenstein, A.H. Gender-specific differences in the kinetics of nonfasting TRL, IDL, and LDL apolipoprotein B-100 in men and premenopausal women. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1838–1843. [Google Scholar] [CrossRef]
- Magkos, F.; Patterson, B.W.; Mohammed, B.S.; Klein, S.; Mittendorfer, B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J. Clin. Endocrinol. Metab. 2007, 92, 1311–1318. [Google Scholar] [CrossRef]
- Hewitt, K.N.; Boon, W.C.; Murata, Y.; Jones, M.E.; Simpson, E.R. The aromatase knockout mouse presents with a sexually dimorphic disruption to cholesterol homeostasis. Endocrinology 2003, 144, 3895–3903. [Google Scholar] [CrossRef]
- Sakurai, K.; Sawamura, T. Stress and vascular responses: Endothelial dysfunction via lectin-like oxidized low-density lipoprotein receptor-1: Close relationships with oxidative stress. J. Pharmacol. Sci. 2003, 91, 182–186. [Google Scholar] [CrossRef]
- Mehta, J.L.; Chen, J.; Hermonat, P.L.; Romeo, F.; Novelli, G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): A critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 2006, 69, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Kork, F.; Jankowski, V.; Just, A.R.; Pfeilschifter, J.; Tepel, M.; Zidek, W.; Jankowski, J. Oxidized low-density lipoprotein in postmenopausal women. J. Hypertens. 2014, 32, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, T.E.; Kume, N.; Mitsuoka, H.; Phares, D.A.; Hagberg, J.M. Elevated soluble lectin-like oxidized LDL receptor-1 (sLOX-1) levels in obese postmenopausal women. Obesity 2008, 16, 1454–1456. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, A.; Spiller, L.; Wolke, C.; Lendeckel, U.; Weinert, S.; Hoffmann, J.; Bornfleth, P.; Kutschka, I.; Gardemann, A.; Isermann, B.; et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp. Biol. Med. 2017, 242, 1412–1423. [Google Scholar] [CrossRef]
- Kume, N.; Murase, T.; Moriwaki, H.; Aoyama, T.; Sawamura, T.; Masaki, T.; Kita, T. Inducible expression of lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ. Res. 1998, 83, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Nagase, M.; Fujita, T.; Narumiya, S.; Masaki, T.; Sawamura, T. Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: Possible role of LOX-1 ligand and AGE. Biochem. Biophys. Res. Commun. 2001, 287, 962–968. [Google Scholar] [CrossRef]
- Garrido-Sanchez, L.; Cardona, F.; Garcia-Fuentes, E.; Rojo-Martinez, G.; Gomez-Zumaquero, J.M.; Picon, M.J.; Soriguer, F.; Tinahones, F.J. Anti-oxidized low-density lipoprotein antibody levels are associated with the development of type 2 diabetes mellitus. Eur. J. Clin. Investig. 2008, 38, 615–621. [Google Scholar] [CrossRef]
- Cominacini, L.; Rigoni, A.; Pasini, A.F.; Garbin, U.; Davoli, A.; Campagnola, M.; Pastorino, A.M.; Lo Cascio, V.; Sawamura, T. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J. Biol. Chem. 2001, 276, 13750–13755. [Google Scholar] [CrossRef]
- Bertoluci, M.C.; Ce, G.V.; da Silva, A.M.; Wainstein, M.V.; Boff, W.; Punales, M. Endothelial dysfunction as a predictor of cardiovascular disease in type 1 diabetes. World J. Diabetes 2015, 6, 679–692. [Google Scholar] [CrossRef]
- Meischke, H.; Larsen, M.P.; Eisenberg, M.S. Gender differences in reported symptoms for acute myocardial infarction: Impact on prehospital delay time interval. Am. J. Emerg. Med. 1998, 16, 363–366. [Google Scholar] [CrossRef]
- Khan, N.A.; Daskalopoulou, S.S.; Karp, I.; Eisenberg, M.J.; Pelletier, R.; Tsadok, M.A.; Dasgupta, K.; Norris, C.M.; Pilote, L.; Genesis Praxy Team. Sex differences in acute coronary syndrome symptom presentation in young patients. JAMA Intern. Med. 2013, 173, 1863–1871. [Google Scholar] [PubMed]
- Shah, A.S.; Griffiths, M.; Lee, K.K.; McAllister, D.A.; Hunter, A.L.; Ferry, A.V.; Cruikshank, A.; Reid, A.; Stoddart, M.; Strachan, F.; et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: Prospective cohort study. BMJ 2015, 350, g7873. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.I. The puzzle of aspirin and sex. N. Engl. J. Med. 2005, 352, 1366–1368. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.; Borhani, N.; Furberg, C.; Gardin, J.; Manolio, T.; O’Leary, D.; Psaty, B.; Robbins, J. Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am. J. Epidemiol. 1994, 139, 1164–1179. [Google Scholar] [CrossRef]
- Karam, N.; Marijon, E.; Bougouin, W.; Spaulding, C.; Jouven, X. [Sudden cardiac death: Are women different?]. Annales de Cardiologie et D’angeiologie. 2016, 65, 390–394. [Google Scholar] [CrossRef]
- Berthome, P.; Tixier, R.; Briand, J.; Geoffroy, O.; Babuty, D.; Mansourati, J.; Jesel, L.; Dupuis, J.M.; Bru, P.; Kyndt, F.; et al. Clinical presentation and follow-up of women affected by Brugada syndrome. Heart Rhythm 2018. [Google Scholar] [CrossRef] [PubMed]
- Catalan, M.; Herreras, Z.; Pinyol, M.; Sala-Vila, A.; Amor, A.J.; de Groot, E.; Gilabert, R.; Ros, E.; Ortega, E. Prevalence by sex of preclinical carotid atherosclerosis in newly diagnosed type 2 diabetes. Nutrition, metabolism, and cardiovascular diseases. NMCD 2015, 25, 742–748. [Google Scholar]
- Reynolds, H.R.; Hausvater, A.; Carney, K. Test Selection for Women with Suspected Stable Ischemic Heart Disease. J. Womens Health 2018, 27, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Pathak, L.A.; Shirodkar, S.; Ruparelia, R.; Rajebahadur, J. Coronary artery disease in women. Indian Heart J. 2017, 69, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Irace, C.; De Rosa, S.; Tripolino, C.; Ambrosio, G.; Covello, C.; Abramo, E.; Carallo, C.; Mongiardo, A.; Spaccarotella, C.; Torella, D.; et al. Delayed flow-mediated vasodilation and critical coronary stenosis. J. Investig. Med. 2018, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Criqui, M.H.; McClelland, R.L.; Allison, M.A.; McDermott, M.M.; Goff, D.C., Jr.; Manolio, T.A. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: The Multi-Ethnic Study of Atherosclerosis (MESA). J. Vasc. Surg. 2007, 45, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, Y.; Chikamori, T.; Hida, S.; Tanaka, H.; Shiba, C.; Usui, Y.; Hatano, T.; Yamashina, A. Importance of the ankle-brachial pressure index in the diagnosis of coronary artery disease in women with diabetes without anginal pain. Circ. J. 2011, 75, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Gongora, M.C.; Gopal, A.; Callister, T.Q.; Budoff, M.; Shaw, L.J. Coronary artery calcium to predict all-cause mortality in elderly men and women. J. Am. Coll. Cardiol. 2008, 52, 17–23. [Google Scholar] [CrossRef]
- Wackers, F.J.; Young, L.H.; Inzucchi, S.E.; Chyun, D.A.; Davey, J.A.; Barrett, E.J.; Taillefer, R.; Wittlin, S.D.; Heller, G.V.; Filipchuk, N.; et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: The DIAD study. Diabetes Care 2004, 27, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.S.; Kang, X.; Hayes, S.W.; Friedman, J.D.; Cohen, I.; Abidov, A.; Shaw, L.J.; Amanullah, A.M.; Germano, G.; Hachamovitch, R. Adenosine myocardial perfusion single-photon emission computed tomography in women compared with men. Impact of diabetes mellitus on incremental prognostic value and effect on patient management. J. Am. Coll. Cardiol. 2003, 41, 1125–1133. [Google Scholar] [CrossRef]
- Mielke, C.H.; Shields, J.P.; Broemeling, L.D. Coronary artery calcium, coronary artery disease, and diabetes. Diabetes Res. Clin. Pract. 2001, 53, 55–61. [Google Scholar] [CrossRef]
- Schindler, T.H.; Zhang, X.L.; Vincenti, G.; Mhiri, L.; Lerch, R.; Schelbert, H.R. Role of PET in the evaluation and understanding of coronary physiology. J. Nucl. Cardiol. 2007, 14, 589–603. [Google Scholar] [CrossRef] [Green Version]
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef]
- Bengel, F.M.; Higuchi, T.; Javadi, M.S.; Lautamaki, R. Cardiac positron emission tomography. J. Am. Coll. Cardiol. 2009, 54, 1–15. [Google Scholar] [CrossRef]
- Camici, P.G.; Rimoldi, O.E. The clinical value of myocardial blood flow measurement. J. Nucl. Med. 2009, 50, 1076–1087. [Google Scholar] [CrossRef]
- Ganz, P.; Vita, J.A. Testing endothelial vasomotor function: Nitric oxide, a multipotent molecule. Circulation 2003, 108, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Naya, M.; Tsukamoto, T.; Morita, K.; Katoh, C.; Furumoto, T.; Fujii, S.; Tamaki, N.; Tsutsui, H. Olmesartan, but not amlodipine, improves endothelium-dependent coronary dilation in hypertensive patients. J. Am. Coll. Cardiol. 2007, 50, 1144–1149. [Google Scholar] [CrossRef]
- Sato, H.; Fujimoto, S.; Kogure, Y.; Daida, H. Feasibility of Macrophage Plaque Imaging Using Novel Ultrasmall Superparamagnetic Iron Oxide in Dual Energy CT. Eur. J. Radiol. Open 2018, 5, 87–91. [Google Scholar] [CrossRef]
- Sampson, U.K.; Dorbala, S.; Limaye, A.; Kwong, R.; Di Carli, M.F. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J. Am. Coll. Cardiol. 2007, 49, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Bateman, T.M.; Heller, G.V.; McGhie, A.I.; Friedman, J.D.; Case, J.A.; Bryngelson, J.R.; Hertenstein, G.K.; Moutray, K.L.; Reid, K.; Cullom, S.J. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: Comparison with ECG-gated Tc-99m sestamibi SPECT. J. Nucl. Cardiol. 2006, 13, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Marchesseau, S.; Seneviratna, A.; Sjoholm, A.T.; Qin, D.L.; Ho, J.X.M.; Hausenloy, D.J.; Townsend, D.W.; Richards, A.M.; Totman, J.J.; Chan, M.Y.Y.; et al. Hybrid PET/CT and PET/MRI imaging of vulnerable coronary plaque and myocardial scar tissue in acute myocardial infarction. J. Nucl. Cardiol. 2018, 25, 2001–2011. [Google Scholar] [CrossRef]
- Senders, M.L.; Hernot, S.; Carlucci, G.; van de Voort, J.C.; Fay, F.; Calcagno, C.; Tang, J.; Alaarg, A.; Zhao, Y.; Ishino, S.; et al. Nanobody-Facilitated Multiparametric PET/MRI Phenotyping of Atherosclerosis. JACC Cardiovasc. Imaging 2018. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef] [Green Version]
- Larsen, J.R.; Brekke, M.; Bergengen, L.; Sandvik, L.; Arnesen, H.; Hanssen, K.F.; Dahl-Jorgensen, K. Mean HbA1c over 18 years predicts carotid intima media thickness in women with type 1 diabetes. Diabetologia 2005, 48, 776–779. [Google Scholar] [CrossRef]
- Pithova, P.; Stechova, K.; Pitha, J.; Lanska, V.; Kvapil, M. Determinants of preclinical atherosclerosis are different in type 1 and type 2 diabetic women. Physiol. Res. 2016, 65, 219–228. [Google Scholar]
- Woodard, G.A.; Brooks, M.M.; Barinas-Mitchell, E.; Mackey, R.H.; Matthews, K.A.; Sutton-Tyrrell, K. Lipids, menopause, and early atherosclerosis in Study of Women’s Health Across the Nation Heart women. Menopause 2011, 18, 376–384. [Google Scholar] [CrossRef]
- Polotsky, H.N.; Polotsky, A.J. Metabolic implications of menopause. Semin. Reprod. Med. 2010, 28, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Inigo, L.; Navarro-Gonzalez, D.; Fernandez-Montero, A.; Pastrana-Delgado, J.; Martinez, J.A. The TyG index may predict the development of cardiovascular events. Eur. J. Clin. Investig. 2016, 46, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Kazani, M.V.; Armeni, E.; Georgiopoulos, G.; Tampakis, K.; Rizos, D.; Augoulea, A.; Kaparos, G.; Alexandrou, A.; Stamatelopoulos, K. The TyG Index as a Marker of Subclinical Atherosclerosis and Arterial Stiffness in Lean and Overweight Postmenopausal Women. Heart Lung Circ. 2018, 27, 716–724. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Arcidiacono, B.; Chiefari, E.; Brunetti, A.; Indolfi, C.; Foti, D.P. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front. Endocrinol. 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Prior, S.L.; Tang, T.S.; Bain, S.C.; Hurel, S.J.; Humphries, S.E.; Stephens, J.W. Association between the rs4880 superoxide dismutase 2 (C>T) gene variant and coronary heart disease in diabetes mellitus. Diabetes Res. Clin. Pract. 2010, 90, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, J.; Fernandez-Real, J.M.; Gutierrez, C.; Zamora, A.; Simon, I.; Bardaji, A.; Ricart, W.; Richart, C. A polymorphism in the promoter of the tumor necrosis factor-alpha gene (-308) is associated with coronary heart disease in type 2 diabetic patients. Atherosclerosis 2003, 167, 257–264. [Google Scholar] [CrossRef]
- Chan, K.H.; Huang, Y.T.; Meng, Q.; Wu, C.; Reiner, A.; Sobel, E.M.; Tinker, L.; Lusis, A.J.; Yang, X.; Liu, S. Shared molecular pathways and gene networks for cardiovascular disease and type 2 diabetes mellitus in women across diverse ethnicities. Circulation. Cardiovasc. Genet. 2014, 7, 911–919. [Google Scholar] [CrossRef] [PubMed]


| Mechanisms and Environmental Factors | Risk for Diabetes in Women |
|---|---|
| A lower proportions and numbers of CD8+ T cells [36] | ↑ |
| Viral infections [37] | ↑ |
| High expression of HLA-DR3 and DR4 alleles [37] | ↑ |
| Decreased DNA methylation of X-chromosome-related genes [37] | ↑ |
| Sex chromosome instability [37] | ↑ |
| Escape from X-chromosome inactivation [37] | ↑ |
| High expression of Receptors for estrogens in the heart and high oxidative stress [38] | ↑ |
| Pathogens, xenobiotics and smoking [37] | ↑ |
| Different metabolism: High accumulation vs. low mobilization of adipose tissue, and altered distribution of body fat associated with not only sex hormones, but also with sex chromosome complement [39,40,41,42,43] | ↑ |
| Microbiome [37,44,45] | ↑ |
| Sex Differences Premenopausal Women vs. Men | Effect of Diabetes Premenopausal Women vs. Men | |
|---|---|---|
| Cardiovascular risk | ↓ | ↑ |
| Fatal CAD risk | ↓ | ↑ |
| PAD risk | ↑ | ↑ |
| Propensity to treat | ↓ | ↓ |
| Delayed diagnosis | ↑ | ↑ |
| CD4+/CD8+ T cells ratio | ↑ | ~ |
| HLA-DR3 and DR4 alleles expression | ↑ | ~ |
| DNA methylation of X-chromosome-related genes | ↓ | ~ |
| Sex chromosome instability | ↓ | ~ |
| Escape from X-chromosome inactivation | ↓ | ~ |
| Activation of immune/pro-inflammatory innate pathways | ↑ | ↑ |
| Different metabolism: High accumulation vs. low mobilization of adipose tissue | ↑ | ~ |
| Oxidative stress | ↓ | ↑ |
| Intestinal microbiome | ↑ | ↑ |
| AGEs/RAGE activation | ~ | ↑ |
| TLR-2 and -4 activation | ~ | ↑ |
| LDL production | ↑ | ↑ |
| LDL oxidation | ↓ | ↑ |
| Ox-LDL/LOX-1 binding | ↓ | ↑ |
| Foam cell formation | ↓ | ↑ |
| Atypical angina | ↑ | ↑ |
| Responsiveness to antiplatelet drugs | ↓ | ↓ |
| Incidence of ABI <0.90 | ↑ | ↑ |
| Ischemia area at cardiac stress imaging | ↓ | ↑ |
| Prevalence and severity of CAC | ~ | ~ |
| IMT | ↓ | ↑ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madonna, R.; Balistreri, C.R.; De Rosa, S.; Muscoli, S.; Selvaggio, S.; Selvaggio, G.; Ferdinandy, P.; De Caterina, R. Impact of Sex Differences and Diabetes on Coronary Atherosclerosis and Ischemic Heart Disease. J. Clin. Med. 2019, 8, 98. https://doi.org/10.3390/jcm8010098
Madonna R, Balistreri CR, De Rosa S, Muscoli S, Selvaggio S, Selvaggio G, Ferdinandy P, De Caterina R. Impact of Sex Differences and Diabetes on Coronary Atherosclerosis and Ischemic Heart Disease. Journal of Clinical Medicine. 2019; 8(1):98. https://doi.org/10.3390/jcm8010098
Chicago/Turabian StyleMadonna, Rosalinda, Carmela Rita Balistreri, Salvatore De Rosa, Saverio Muscoli, Stefano Selvaggio, Giancarlo Selvaggio, Péter Ferdinandy, and Raffaele De Caterina. 2019. "Impact of Sex Differences and Diabetes on Coronary Atherosclerosis and Ischemic Heart Disease" Journal of Clinical Medicine 8, no. 1: 98. https://doi.org/10.3390/jcm8010098