Type 2 Diabetes Is Associated with a Different Pattern of Serum Polyamines: A Case–Control Study from the PREDIMED-Plus Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Measurements
2.3. Sample Preparation and Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry (UHPLC-MS/MS) Analysis of Serum Polyamine Levels
2.4. Statistical Analysis
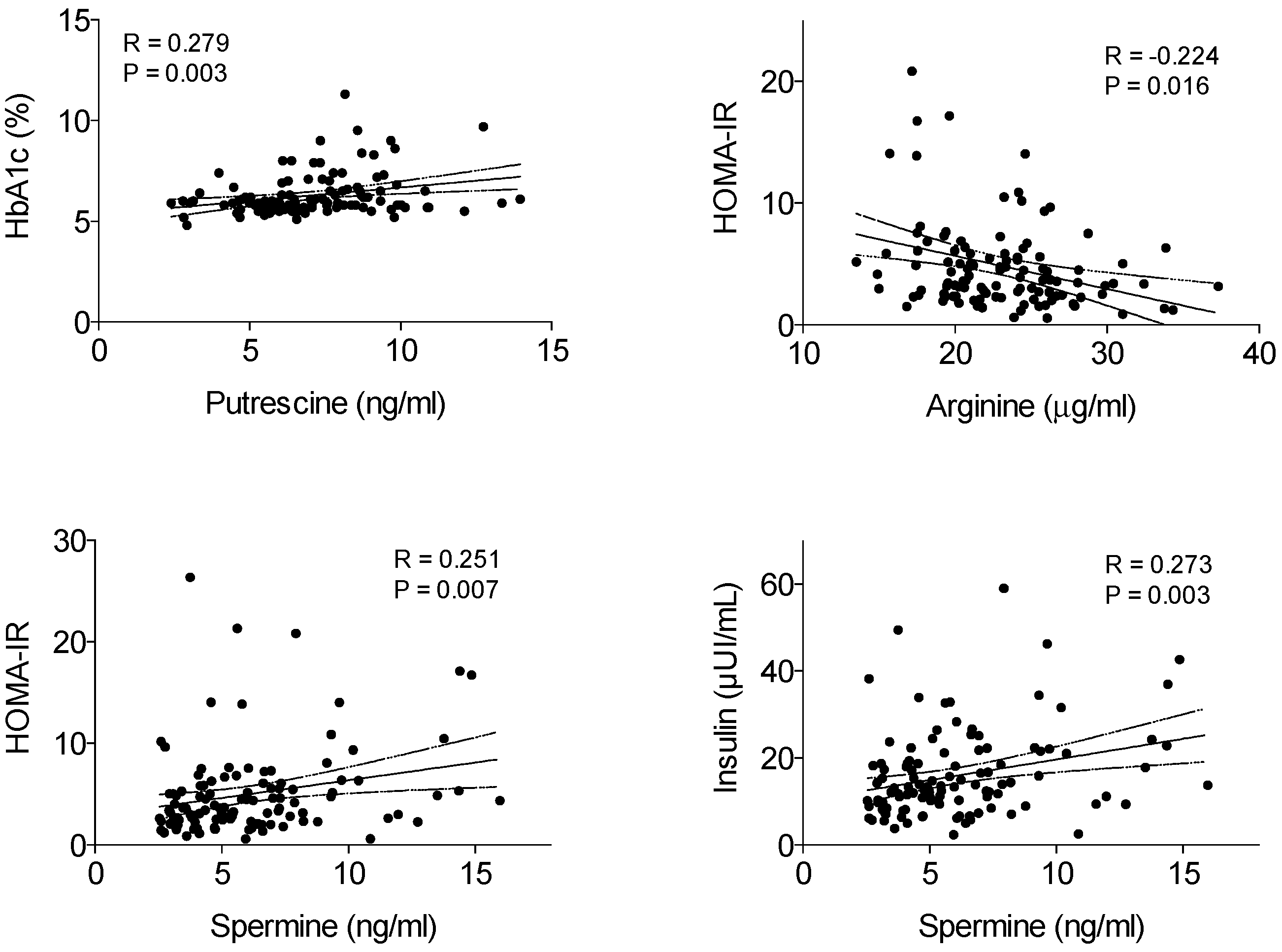
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| DBP | diastolic blood pressure |
| HDL | high-density lipoprotein |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| ODC | ornithine decarboxylase |
| SBP | systolic blood pressure |
| SSAT | spermine/spermidine acetyltransferase |
| T2D | type 2 diabetes |
| UHPLC-MS/MS | ultra-high performance liquid chromatography tandem mass spectrometry. |
References
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Molina, B.; Lambertos, A.; Penafiel, R. Antizyme Inhibitors in Polyamine Metabolism and Beyond: Physiopathological Implications. Med. Sci. 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018. [Google Scholar] [CrossRef]
- Niiranen, K.; Keinanen, T.A.; Pirinen, E.; Heikkinen, S.; Tusa, M.; Fatrai, S.; Suppola, S.; Pietila, M.; Uimari, A.; Laakso, M.; et al. Mice with targeted disruption of spermidine/spermine N1-acetyltransferase gene maintain nearly normal tissue polyamine homeostasis but show signs of insulin resistance upon aging. J. Cell Mol. Med. 2006, 10, 933–945. [Google Scholar] [CrossRef]
- Pirinen, E.; Kuulasmaa, T.; Pietila, M.; Heikkinen, S.; Tusa, M.; Itkonen, P.; Boman, S.; Skommer, J.; Virkamaki, A.; Hohtola, E.; et al. Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism. Mol. Cell Biol. 2007, 27, 4953–4967. [Google Scholar] [CrossRef]
- Cerrada-Gimenez, M.; Tusa, M.; Casellas, A.; Pirinen, E.; Moya, M.; Bosch, F.; Alhonen, L. Altered glucose-stimulated insulin secretion in a mouse line with activated polyamine catabolism. Transgenic Res. 2012, 21, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Kraus, D.; Yang, Q.; Kong, D.; Banks, A.S.; Zhang, L.; Rodgers, J.T.; Pirinen, E.; Pulinilkunnil, T.C.; Gong, F.; Wang, Y.C.; et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 2014, 508, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonhoure, N.; Byrnes, A.; Moir, R.D.; Hodroj, W.; Preitner, F.; Praz, V.; Marcelin, G.; Chua, S.C., Jr.; Martinez-Lopez, N.; Singh, R.; et al. Loss of the RNA polymerase III repressor MAF1 confers obesity resistance. Genes Dev. 2015, 29, 934–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, F.; Zhang, L.; Cao, Y.; Gao, W.; Zhao, C.; Fang, Y.; Zahedi, K.; Soleimani, M.; Lu, X.; Fang, Z.; et al. Spermidine/spermine N1-acetyltransferase-mediated polyamine catabolism regulates beige adipocyte biogenesis. Metabolism 2018, 85, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Jamdar, S.C.; Cao, W.F.; Samaniego, E. Relationship between adipose polyamine concentrations and triacylglycerol synthetic enzymes in lean and obese Zucker rats. Enzym. Protein 1996, 49, 222–230. [Google Scholar] [CrossRef]
- Yun, K.U.; Ryu, C.S.; Lee, J.Y.; Noh, J.R.; Lee, C.H.; Lee, H.S.; Kang, J.S.; Park, S.K.; Kim, B.H.; Kim, S.K. Hepatic metabolism of sulfur amino acids in db/db mice. Food Chem. Toxicol. 2013, 53, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.C.; Kim, Y.M.; Oh, S.J.; Kim, S.K. Sulfur amino acid metabolism in Zucker diabetic fatty rats. Biochem. Pharmacol. 2015, 96, 256–266. [Google Scholar] [CrossRef]
- Sjoholm, A.; Arkhammar, P.; Berggren, P.O.; Andersson, A. Polyamines in pancreatic islets of obese-hyperglycemic (ob/ob) mice of different ages. Am. J. Physiol. Cell Physiol. 2001, 280, C317–C323. [Google Scholar] [CrossRef]
- Pelantova, H.; Bartova, S.; Anyz, J.; Holubova, M.; Zelezna, B.; Maletinska, L.; Novak, D.; Lacinova, Z.; Sulc, M.; Haluzik, M.; et al. Metabolomic profiling of urinary changes in mice with monosodium glutamate-induced obesity. Anal. Bioanal. Chem. 2016, 408, 567–578. [Google Scholar] [CrossRef]
- Ishii, I.; Ikeguchi, Y.; Mano, H.; Wada, M.; Pegg, A.E.; Shirahata, A. Polyamine metabolism is involved in adipogenesis of 3T3-L1 cells. Amino Acids 2012, 42, 619–626. [Google Scholar] [CrossRef]
- Hyvonen, M.T.; Koponen, T.; Weisell, J.; Pietila, M.; Khomutov, A.R.; Vepsalainen, J.; Alhonen, L.; Keinanen, T.A. Spermidine promotes adipogenesis of 3T3-L1 cells by preventing interaction of ANP32 with HuR and PP2A. Biochem. J. 2013, 453, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, S.; Bercovich, Z.; Feiler, Y.; Keshet, R.; Kahana, C. Dual Regulatory Role of Polyamines in Adipogenesis. J. Biol. Chem. 2015, 290, 27384–27392. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.F.; Barcena, C.; Martinez-Garcia, G.G.; Tamargo-Gomez, I.; Suarez, M.F.; Pietrocola, F.; Castoldi, F.; Esteban, L.; Sierra-Filardi, E.; Boya, P.; et al. Autophagy couteracts weight gain, lipotoxicity and pancreatic beta-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis. 2017, 8, e2970. [Google Scholar] [CrossRef]
- Sadasivan, S.K.; Vasamsetti, B.; Singh, J.; Marikunte, V.V.; Oommen, A.M.; Jagannath, M.R.; Pralhada Rao, R. Exogenous administration of spermine improves glucose utilization and decreases bodyweight in mice. Eur. J. Pharmacol. 2014, 729, 94–99. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, W.; Li, C.; Xie, X.; Li, M.; Bi, Y.; Fang, F.; Du, Y.; Liu, X. Spermidine ameliorates non-alcoholic fatty liver disease through regulating lipid metabolism via AMPK. Biochem. Biophys. Res. Commun. 2018, 505, 93–98. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [Green Version]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher spermidine intake is linked to lower mortality: A prospective population-based study. Am. J. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Bohm, A.; Halama, A.; Meile, T.; Zdichavsky, M.; Lehmann, R.; Weigert, C.; Fritsche, A.; Stefan, N.; Konigsrainer, A.; Haring, H.U.; et al. Metabolic signatures of cultured human adipocytes from metabolically healthy versus unhealthy obese individuals. PLoS ONE 2014, 9, e93148. [Google Scholar] [CrossRef] [PubMed]
- Codoner-Franch, P.; Tavarez-Alonso, S.; Murria-Estal, R.; Herrera-Martin, G.; Alonso-Iglesias, E. Polyamines are increased in obese children and are related to markers of oxidative/nitrosative stress and angiogenesis. J. Clin. Endocrinol. Metab. 2011, 96, 2821–2825. [Google Scholar] [CrossRef] [PubMed]
- Hougaard, D.M.; Nielsen, J.H.; Larsson, L.I. Localization and biosynthesis of polyamines in insulin-producing cells. Biochem. J. 1986, 238, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Sjoholm, A. Role of polyamines in the regulation of proliferation and hormone production by insulin-secreting cells. Am. J. Physiol. 1993, 264, C501–C518. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Buil-Cosiales, P.; Corella, D.; Bullo, M.; Fito, M.; Vioque, J.; Romaguera, D.; Martinez, J.A.; Warnberg, J.; Lopez-Miranda, J.; et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Munger, K.A.; Valdivielso, J.M.; Satriano, J.; Lortie, M.; Blantz, R.C.; Thomson, S.C. Increased expression of ornithine decarboxylase in distal tubules of early diabetic rat kidneys: Are polyamines paracrine hypertrophic factors? Diabetes 2003, 52, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, D.H.; East, L.E. Studies of the insulin-like actions of polyamines on lipid and glucose metabolism in adipose tissue cells. J. Biol. Chem. 1974, 249, 7717–7722. [Google Scholar]
- Pedersen, S.B.; Hougaard, D.M.; Richelsen, B. Polyamines in rat adipocytes: Their localization and their effects on the insulin receptor binding. Mol. Cell Endocrinol. 1989, 62, 161–166. [Google Scholar] [CrossRef]

| Non-Diabetic (n = 70) | T2D (n = 44) | p Value | |
|---|---|---|---|
| Age, years | 64.51 ± 4.46 | 63.75 ± 4.86 | 0.401 |
| Gender, f (%) | 34 (48.6) | 22 (50.0) | - |
| Weight, kg | 88.28 ± 12.83 | 92.29 ± 13.19 | 0.113 |
| Waist, cm | 112.57 ± 9.01 | 116.12 ± 8.66 | 0.039 |
| BMI, mg/kg2 | 32.95 ± 3.38 | 34.23 ± 3.21 | 0.046 |
| SBP, mm Hg | 136.50 ± 13.92 | 141.82 ± 19.12 | 0.114 |
| DBP, mm Hg | 75.36 ± 9.51 | 74.91 ± 9.35 | 0.805 |
| Glucose, mg/dL | 103.43 ± 9.69 | 144.30 ± 34.86 | <0.001 |
| HbA1c, % | 5.74 ± 0.29 | 7.15 ± 1.23 | <0.001 |
| Insulin, mUI/L | 13.17 ± 7.05 | 20.38 ± 12.31 | <0.001 |
| HOMA-IR | 3.42 ± 1.98 | 7.55 ± 5.70 | <0.001 |
| T2D treatment (insulin, metformin, other), n (%) | 0 (0) | 37 (84.1) | - |
| HDL-cholesterol, mg/dL | 47.19 ± 11.19 | 46.11 ± 13.18 | 0.656 |
| Triglycerides, mg/dL | 181.93 ± 70.93 | 186.39 ± 138.78 | 0.844 |
| Non-diabetic (n = 70) | T2D (n = 44) | p Value | |
|---|---|---|---|
| Arginine, μg/mL | 23.77 ± 4.54 | 21.99 ± 4.86 | 0.038 |
| Ornithine, μg/mL | 26.08 ± 5.59 | 23.47 ± 6.56 | 0.028 |
| Putrescine, ng/mL | 6.73 ± 2.34 | 7.54 ± 1.98 | 0.010 |
| Spermidine, ng/mL | 21.82 ± 9.23 | 21.09 ± 9.54 | 0.617 |
| Spermine, ng/mL | 5.65 ± 2.61 | 6.79 ± 3.44 | 0.067 |
| Acetylputrescine, ng/mL | 3.89 ± 1.33 | 3.86 ± 1.27 | 0.818 |
| N1-Acetylspermidine, ng/mL | 19.69 ± 6.39 | 19.78 ± 6.23 | 0.903 |
| N8-Acetylspermidine, ng/mL | 9.22 ± 2.15 | 8.88 ± 2.32 | 0.284 |
| N1,N8-Diacetylspermidine, ng/mL | 0.19 ± 0.12 | 0.24 ± 0.15 | 0.126 |
| N1-Acetylspermine, ng/mL | 0.60 ± 0.32 | 0.73 ± 0.36 | 0.053 |
| N1,N12-Diacetylspermine, ng/mL | 0.277 ± 0.22 | 0.29 ± 0.12 | 0.054 |
| HbA1c (R = 0.350; R2 = 0.122) | ||||
|---|---|---|---|---|
| β | Standard Error | 95 % (CI) | p Value | |
| Gender | −0.169 | 0.193 | −0.552–0.214 | 0.383 |
| Age (years) | −0.038 | 0.021 | −0.080–0.004 | 0.076 |
| Putrescine (ng/mL) | 0.137 | 0.042 | 0.056–0.223 | 0.001 |
| HOMA-IR (R = 0.305; R2 = 0.093) | ||||
|---|---|---|---|---|
| β | Standard Error | 95 % (CI) | p Value | |
| Gender | −0.046 | 0.815 | −1.662–1.570 | 0.955 |
| Age (years) | −0.178 | 0.090 | −0.356–0.000 | 0.049 |
| Spermine (ng/mL) | 0.291 | 0.135 | 0.023–0.559 | 0.034 |
| OR | 95% CI | p Value | |
|---|---|---|---|
| Gender | 1.190 | 0.499–2.838 | 0.694 |
| Age (years) | 0.974 | 0.886-–1.070 | 0.576 |
| BMI (kg/m2) | 1.098 | 0.968–1.246 | 0.144 |
| Putrescine (ng/mL) | 1.251 | 1.032–1.517 | 0.022 |
| Spermidine (ng/mL) | 0.952 | 0.904–1.003 | 0.066 |
| Spermine (ng/mL) | 1.205 | 1.026–1.416 | 0.023 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Garcia, J.C.; Delpino-Rius, A.; Samarra, I.; Castellano-Castillo, D.; Muñoz-Garach, A.; Bernal-Lopez, M.R.; Queipo-Ortuño, M.I.; Cardona, F.; Ramos-Molina, B.; Tinahones, F.J. Type 2 Diabetes Is Associated with a Different Pattern of Serum Polyamines: A Case–Control Study from the PREDIMED-Plus Trial. J. Clin. Med. 2019, 8, 71. https://doi.org/10.3390/jcm8010071
Fernandez-Garcia JC, Delpino-Rius A, Samarra I, Castellano-Castillo D, Muñoz-Garach A, Bernal-Lopez MR, Queipo-Ortuño MI, Cardona F, Ramos-Molina B, Tinahones FJ. Type 2 Diabetes Is Associated with a Different Pattern of Serum Polyamines: A Case–Control Study from the PREDIMED-Plus Trial. Journal of Clinical Medicine. 2019; 8(1):71. https://doi.org/10.3390/jcm8010071
Chicago/Turabian StyleFernandez-Garcia, Jose C., Antoni Delpino-Rius, Iris Samarra, Daniel Castellano-Castillo, Araceli Muñoz-Garach, Maria R. Bernal-Lopez, Maria I. Queipo-Ortuño, Fernando Cardona, Bruno Ramos-Molina, and Francisco J. Tinahones. 2019. "Type 2 Diabetes Is Associated with a Different Pattern of Serum Polyamines: A Case–Control Study from the PREDIMED-Plus Trial" Journal of Clinical Medicine 8, no. 1: 71. https://doi.org/10.3390/jcm8010071






