Gout as a Risk Factor for Dry Eye Disease: A Population-Based Cohort Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Data Source
2.2. Patient Selection
2.3. Main Outcome Measurement
2.4. Demographic Variables and Co-Morbidities
2.5. Statistical Analysis
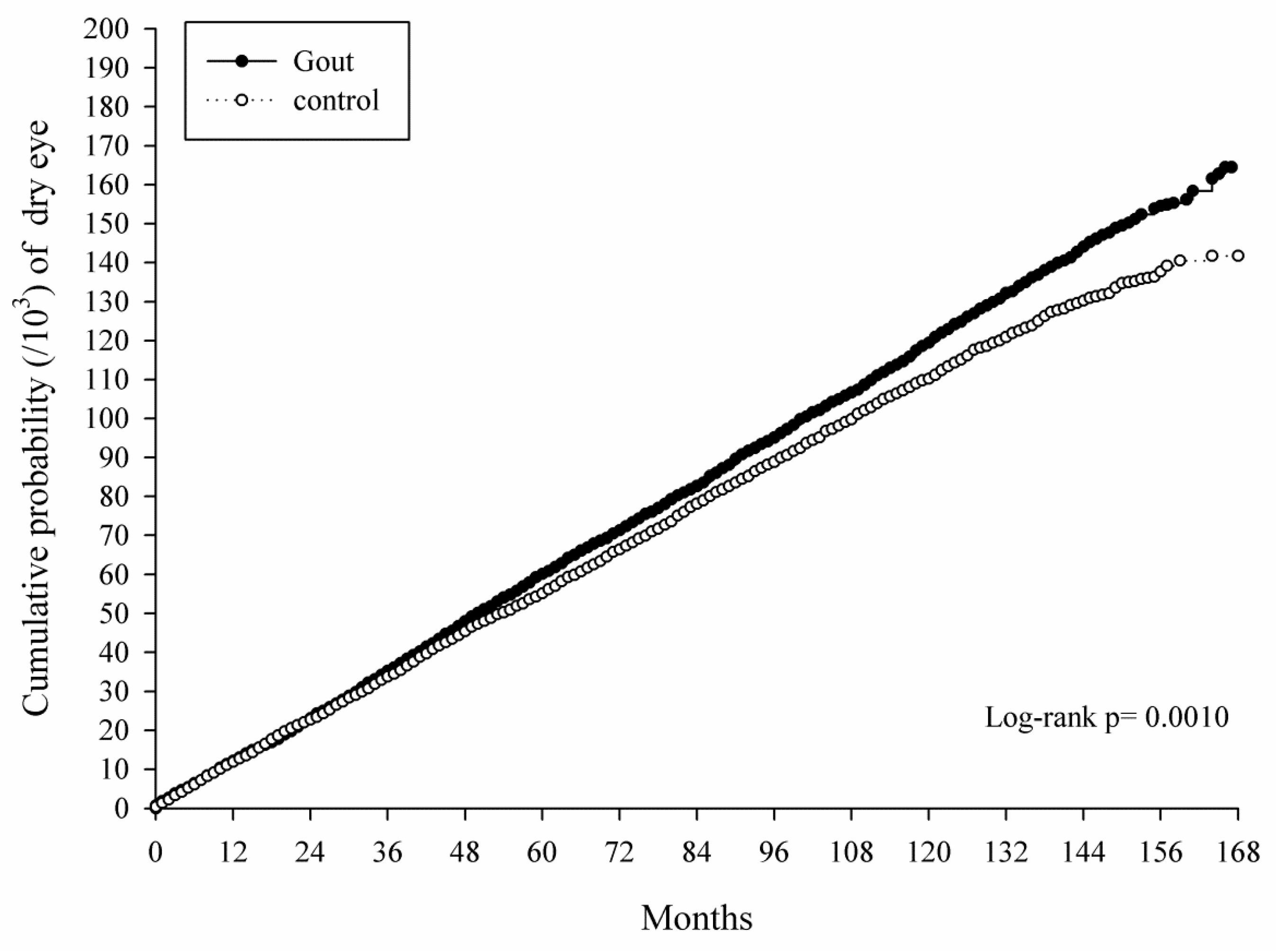
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miljanovic, B.; Dana, R.; Sullivan, D.A.; Schaumberg, D.A. Impact of dry eye syndrome on vision-related quality of life. Am. J. Ophthalmol. 2007, 143, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Labetoulle, M.; Rolando, M.; Messmer, E.M. Understanding symptoms and quality of life in patients with dry eye syndrome. Ocul. Surf. 2016, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Farrand, K.F.; Fridman, M.; Stillman, I.O.; Schaumberg, D.A. Prevalence of diagnosed dry eye disease in the united states among adults aged 18 years and older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; de Paiva, C.S. The pathophysiology of dry eye disease: What we know and future directions for research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Geerling, G.; Kinoshita, S.; Lemp, M.A. Management and therapy of dry eye disease: Report of the management and therapy subcommittee of the international dry eye workshop (2007). Ocul. Surf. 2007, 5, 163–178. [Google Scholar]
- Thulasi, P.; Djalilian, A.R. Update in current diagnostics and therapeutics of dry eye disease. Ophthalmology 2017, 124, S27–S33. [Google Scholar] [CrossRef] [PubMed]
- Generali, E.; Cantarini, L.; Selmi, C. Ocular involvement in systemic autoimmune diseases. Clin. Rev. Allergy Immunol. 2015, 49, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Henrich, C.F.; Ramulu, P.Y.; Akpek, E.K. Association of dry eye and inflammatory systemic diseases in a tertiary care-based sample. Cornea 2014, 33, 819–825. [Google Scholar] [CrossRef]
- Lee, S.Y.; Petznick, A.; Tong, L. Associations of systemic diseases, smoking and contact lens wear with severity of dry eye. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. (Optom.) 2012, 32, 518–526. [Google Scholar] [CrossRef]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- So, A.K.; Martinon, F. Inflammation in gout: Mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Brenner, J.E.; Foster, W.J. Retinal complications of gout: A case report and review of the literature. BMC Ophthalmol. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Yazdanyar, A.; Rizzuti, A.E.; Mechel, E.; Denisova, K.; Lazzaro, D.R. Gout keratitis: A case of peripheral ulcerative keratitis secondary to gout with a review of the literature. Cornea 2018, 37, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Sharon, Y.; Schlesinger, N. Beyond joints: A review of ocular abnormalities in gout and hyperuricemia. Curr. Rheumatol. Rep. 2016, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.E.; Klein, R.; Klein, B.E. Incidence of dry eye in an older population. Arch. Ophthalmol. 2004, 122, 369–373. [Google Scholar] [CrossRef]
- Alshamrani, A.A.; Almousa, A.S.; Almulhim, A.A.; Alafaleq, A.A.; Alosaimi, M.B.; Alqahtani, A.M.; Almulhem, A.M.; Alshamrani, M.A.; Alhallafi, A.H.; Alqahtani, I.Z.; et al. Prevalence and risk factors of dry eye symptoms in a saudi arabian population. Middle East Afr. J. Ophthalmol. 2017, 24, 67–73. [Google Scholar] [CrossRef]
- Moss, S.E.; Klein, R.; Klein, B.E. Prevalence of and risk factors for dry eye syndrome. Arch. Ophthalmol. 2000, 118, 1264–1268. [Google Scholar] [CrossRef]
- Chia, E.M.; Mitchell, P.; Rochtchina, E.; Lee, A.J.; Maroun, R.; Wang, J.J. Prevalence and associations of dry eye syndrome in an older population: The blue mountains eye study. Clin. Exp. Ophthalmol. 2003, 31, 229–232. [Google Scholar] [CrossRef]
- Moss, S.E.; Klein, R.; Klein, B.E. Long-term incidence of dry eye in an older population. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2008, 85, 668–674. [Google Scholar] [CrossRef]
- Uchino, M.; Nishiwaki, Y.; Michikawa, T.; Shirakawa, K.; Kuwahara, E.; Yamada, M.; Dogru, M.; Schaumberg, D.A.; Kawakita, T.; Takebayashi, T.; et al. Prevalence and risk factors of dry eye disease in japan: Koumi study. Ophthalmology 2011, 118, 2361–2367. [Google Scholar] [CrossRef]
- Rymal, E.; Rizzolo, D. Gout: A comprehensive review. JAAPA 2014, 27, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, U.; Head, S.J.; Angelini, G.D.; Blackstone, E.H. Statistical primer: Propensity score matching and its alternatives. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2018, 53, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B.; Thomas, N. Matching using estimated propensity scores: Relating theory to practice. Biometrics 1996, 52, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, W.; Davidson, R.S.; Durairaj, V.D.; Gelston, C.D. Dry eye syndrome: An update in office management. Am. J. Med. 2011, 124, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Titiyal, J.S.; Falera, R.C.; Kaur, M.; Sharma, V.; Sharma, N. Prevalence and risk factors of dry eye disease in north India: Ocular surface disease index-based cross-sectional hospital study. Indian J. Ophthalmol. 2018, 66, 207–211. [Google Scholar] [PubMed]
- Ahn, J.H.; Choi, Y.H.; Paik, H.J.; Kim, M.K.; Wee, W.R.; Kim, D.H. Sex differences in the effect of aging on dry eye disease. Clin. Interv. Aging 2017, 12, 1331–1338. [Google Scholar] [CrossRef]
- Kuo, C.F.; Grainge, M.J.; Zhang, W.; Doherty, M. Global epidemiology of gout: Prevalence, incidence and risk factors. Nat. Rev. Rheumatol. 2015, 11, 649–662. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, C.L.; Tsai, Y.Y.; Kao, C.H. Association between glaucoma medication usage and dry eye in taiwan. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2015, 92, e227–e232. [Google Scholar] [CrossRef]
- Wong, A.B.C.; Wang, M.T.M.; Liu, K.; Prime, Z.J.; Danesh-Meyer, H.V.; Craig, J.P. Exploring topical anti-glaucoma medication effects on the ocular surface in the context of the current understanding of dry eye. Ocul. Surf. 2018, 16, 289–293. [Google Scholar] [CrossRef]
- Leibovitch, I.; Alster, Y.; Lazar, M.; Langevitz, P.; Livneh, A.; Loewenstein, A. Corneal wound healing in a patient treated with colchicine for familial Mediterranean Fever (FMF). Rheumatology 2003, 42, 1021–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terkeltaub, R. What makes gouty inflammation so variable? BMC Med. 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, M.K.; Mah, F.S. Inflammation in dry eye disease: How do we break the cycle? Ophthalmology 2017, 124, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Irkec, M.; Messmer, E.M.; Benitez-Del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical impact of inflammation in dry eye disease: Proceedings of the odissey group meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New perspectives on dry eye definition and diagnosis: A consensus report by the asia dry eye society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Chhadva, P.; Goldhardt, R.; Galor, A. Meibomian gland disease: The role of gland dysfunction in dry eye disease. Ophthalmology 2017, 124, S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chen, H.T.; Chen, H.C.; Chen, Y.T.; Hwang, Y.H.; Sun, C.C.; Hsiao, C.H.; Ma, D.H.; Wu, W.C.; Lai, C.C. Asymptomatic meibomian gland dysfunction and cardiovascular disease risk factors in a middle-aged population in Taiwan—A cross-sectional analysis. Sci. Rep. 2017, 7, 4935. [Google Scholar] [CrossRef]
- Lee, L.; Garrett, Q.; Flanagan, J.; Chakrabarti, S.; Papas, E. Genetic factors and molecular mechanisms in dry eye disease. Ocul. Surf. 2018, 16, 206–217. [Google Scholar] [CrossRef]
- Yen, J.C.; Hsu, C.A.; Li, Y.C.; Hsu, M.H. The prevalence of dry eye syndrome’s and the likelihood to develop sjogren’s syndrome in Taiwan: A population-based study. Int. J. Environ. Res. Public Health 2015, 12, 7647–7655. [Google Scholar] [CrossRef]

| Variable | Gout n = 32,164 | Control n = 32,164 | p Value | ASD |
|---|---|---|---|---|
| Age at baseline, Mean ± SD | 54.86 ± 17.02 | 56.80 ± 16.76 | <0.0001 | 0.1148 |
| <40 | 6502 (20.22%) | 5493 (17.08%) | ||
| 40–59 | 12,108 (37.64%) | 11,366 (35.34%) | ||
| ≥60 | 13,554 (42.14%) | 15,305 (47.58%) | ||
| Sex | 0.1667 | 0.0806 | ||
| Female | 9839 (30.59%) | 10,001 (31.09%) | ||
| Male | 22,325 (69.41%) | 22,163 (68.91%) | ||
| Urbanization | 0.5120 | 0.0091 | ||
| Urban | 18,894 (58.74%) | 18,960 (58.95%) | ||
| Sub-urban | 9750 (30.31%) | 9775 (30.39%) | ||
| Rural | 3520 (10.94%) | 3429 (10.66%) | ||
| Low-income | 229 (0.71%) | 194 (0.60%) | 0.0878 | 0.0135 |
| Co-morbidities | ||||
| Keratopathy | 1348 (4.19%) | 1168 (3.63%) | 0.0003 | 0.0289 |
| AMD | 320 (0.99%) | 349 (1.09%) | 0.2597 | 0.0089 |
| Glaucoma | 954 (2.97%) | 855 (2.66%) | 0.0182 | 0.0186 |
| Cataract | 4182 (13.00%) | 4357 (13.55%) | 0.0420 | 0.0160 |
| Uveitis | 165 (0.51%) | 143 (0.44%) | 0.2089 | 0.0099 |
| Hypertension | 15,837 (49.24%) | 17,080 (53.10%) | <0.0001 | 0.0774 |
| DM | 8836 (27.47%) | 9465 (29.43%) | <0.0001 | 0.0434 |
| Ischemic heart diseases | 2318 (7.21%) | 2364 (7.35%) | 0.4851 | 0.0055 |
| Hyperlipidemia | 13,302 (41.36%) | 13,374 (41.58%) | 0.5645 | 0.0045 |
| Congestive heart failure | 2772 (8.62%) | 1903 (5.92%) | <0.0001 | 0.1042 |
| Peripheral vascular disease | 1503 (4.67%) | 1127 (3.50%) | <0.0001 | 0.0591 |
| Cerebrovascular disease | 3917 (12.18%) | 4223 (13.13%) | 0.0003 | 0.0286 |
| Dementia | 619 (1.92%) | 646 (2.01%) | 0.4433 | 0.0061 |
| Chronic pulmonary disease | 7930 (24.65%) | 6358 (19.77%) | <0.0001 | 0.1178 |
| Rheumatic disease | 1603 (4.98%) | 439 (1.36%) | <0.0001 | 0.2075 |
| Peptic ulcer disease | 9066 (28.19%) | 6132 (19.06%) | <0.0001 | 0.2160 |
| Liver disease | 11,990 (37.28%) | 6177 (19.20%) | <0.0001 | 0.4098 |
| Hemiplegia or paraplegia | 621 (1.93%) | 670 (2.08%) | 0.1683 | 0.0109 |
| Renal disease | 4507 (14.01%) | 1930 (6.00%) | <0.0001 | 0.2694 |
| Malignancy | 2060 (6.40%) | 1551 (4.82%) | <0.0001 | 0.0688 |
| Variable | Gout, n = 32,164 | Control, n = 32,164 |
|---|---|---|
| Dry eye | ||
| Follow up person months | 2,766,859 | 2,730,647 |
| Event | 2913 | 2631 |
| Incidence rate * (95% CI) | 105.28 (101.53–109.17) | 96.35 (92.74–100.11) |
| Crude HR (95% CI) | 1.093 (1.036–1.152) | Reference + |
| aHR (95% CI) | 1.065 (1.009–1.126) | Reference |
| Variable | Crude HR (95% CI) | aHR (95% CI) |
|---|---|---|
| Exposure of gout (Ref: Non) | ||
| Yes | 1.093 (1.036–1.152) | 1.065 (1.009–1.126) |
| Age at baseline (Ref: <40) | ||
| 40–59 | 2.828 (2.531–3.159) | 2.204 (1.965–2.471) |
| ≥60 | 4.687 (4.209–5.218) | 2.886 (2.560–3.254) |
| Sex (Ref: Female) | ||
| Male | 0.436 (0.414–0.460) | 0.564 (0.534–0.596) |
| Urbanization (Ref: Urban) | ||
| Sub-urban | 0.924 (0.871–0.981) | 0.907 (0.855–0.963) |
| Rural | 0.851 (0.776–0.933) | 0.734 (0.669–0.806) |
| Co-morbidities | ||
| Keratopathy | 2.059 (1.851–2.291) | 1.578 (1.416–1.758) |
| AMD | 2.487 (2.048–3.020) | 1.406 (1.154–1.713) |
| Glaucoma | 2.547 (2.272–2.856) | 1.531 (1.359–1.724) |
| Cataract | 2.562 (2.409–2.726) | 1.531 (1.427–1.643) |
| Uveitis | 1.939 (1.441–2.609) | 1.084 (0.801–1.467) |
| Hypertension | 1.691 (1.603–1.784) | 1.032 (0.972–1.097) |
| DM | 1.472 (1.392–1.556) | 0.991 (0.934–1.051) |
| Ischemic heart diseases | 1.573 (1.440–1.718) | 1.101 (1.005–1.206) |
| Hyperlipidemia | 1.647 (1.562–1.736) | 1.161 (1.097–1.228) |
| Congestive heart failure | 1.358 (1.228–1.501) | 0.868 (0.782–0.964) |
| Peripheral vascular disease | 1.602 (1.427–1.797) | 1.137 (1.012–1.279) |
| Cerebrovascular disease | 1.350 (1.250–1.459) | 0.949 (0.874–1.030) |
| Dementia | 1.004 (0.784–1.284) | 0.690 (0.538–0.887) |
| Chronic pulmonary disease | 1.579 (1.490–1.673) | 1.197 (1.127–1.272) |
| Rheumatic disease | 1.693 (1.508–1.902) | 1.331 (1.183–1.498) |
| Peptic ulcer disease | 1.644 (1.555–1.739) | 1.268 (1.196–1.345) |
| Liver disease | 1.247 (1.180–1.319) | 1.137 (1.072–1.205) |
| Hemiplegia or paraplegia | 1.058 (0.868–1.291) | 0.871 (0.711–1.067) |
| Renal disease | 1.302 (1.197–1.417) | 0.989 (0.907–1.079) |
| Malignancy | 1.547 (1.390–1.721) | 1.144 (1.027–1.274) |
| Variable | 0–56 Months | 56–112 Months | 112–168 Months |
|---|---|---|---|
| Incidence * in control | 95.47 (90.65–100.55) | 102.58 (96.22–109.36) | 93.60 (83.55–104.85) |
| Incidence in gout | 102.00 (97.02–107.22) | 109.7 (103.17–116.64) | 119.78 (108.5–132.22) |
| aHR (95% CI) | 1.034 0.959–1.114) | 1.057 (0.964–1.159) | 1.270 (1.087–1.485) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Chen, H.-C.; Sun, C.-C.; Lin, H.-Y.; Lu, K.-H.; Huang, J.-Y.; Yeh, C.-B.; Yang, S.-F. Gout as a Risk Factor for Dry Eye Disease: A Population-Based Cohort Study. J. Clin. Med. 2019, 8, 62. https://doi.org/10.3390/jcm8010062
Lee C-Y, Chen H-C, Sun C-C, Lin H-Y, Lu K-H, Huang J-Y, Yeh C-B, Yang S-F. Gout as a Risk Factor for Dry Eye Disease: A Population-Based Cohort Study. Journal of Clinical Medicine. 2019; 8(1):62. https://doi.org/10.3390/jcm8010062
Chicago/Turabian StyleLee, Chia-Yi, Hung-Chi Chen, Chi-Chin Sun, Hung-Yu Lin, Ko-Hsiu Lu, Jing-Yang Huang, Chao-Bin Yeh, and Shun-Fa Yang. 2019. "Gout as a Risk Factor for Dry Eye Disease: A Population-Based Cohort Study" Journal of Clinical Medicine 8, no. 1: 62. https://doi.org/10.3390/jcm8010062






