Prolonged Mechanical Ventilation Assistance Interacts Synergistically with Carbapenem for Clostridium difficile Infection in Critically Ill Patients
Abstract
:1. Introduction
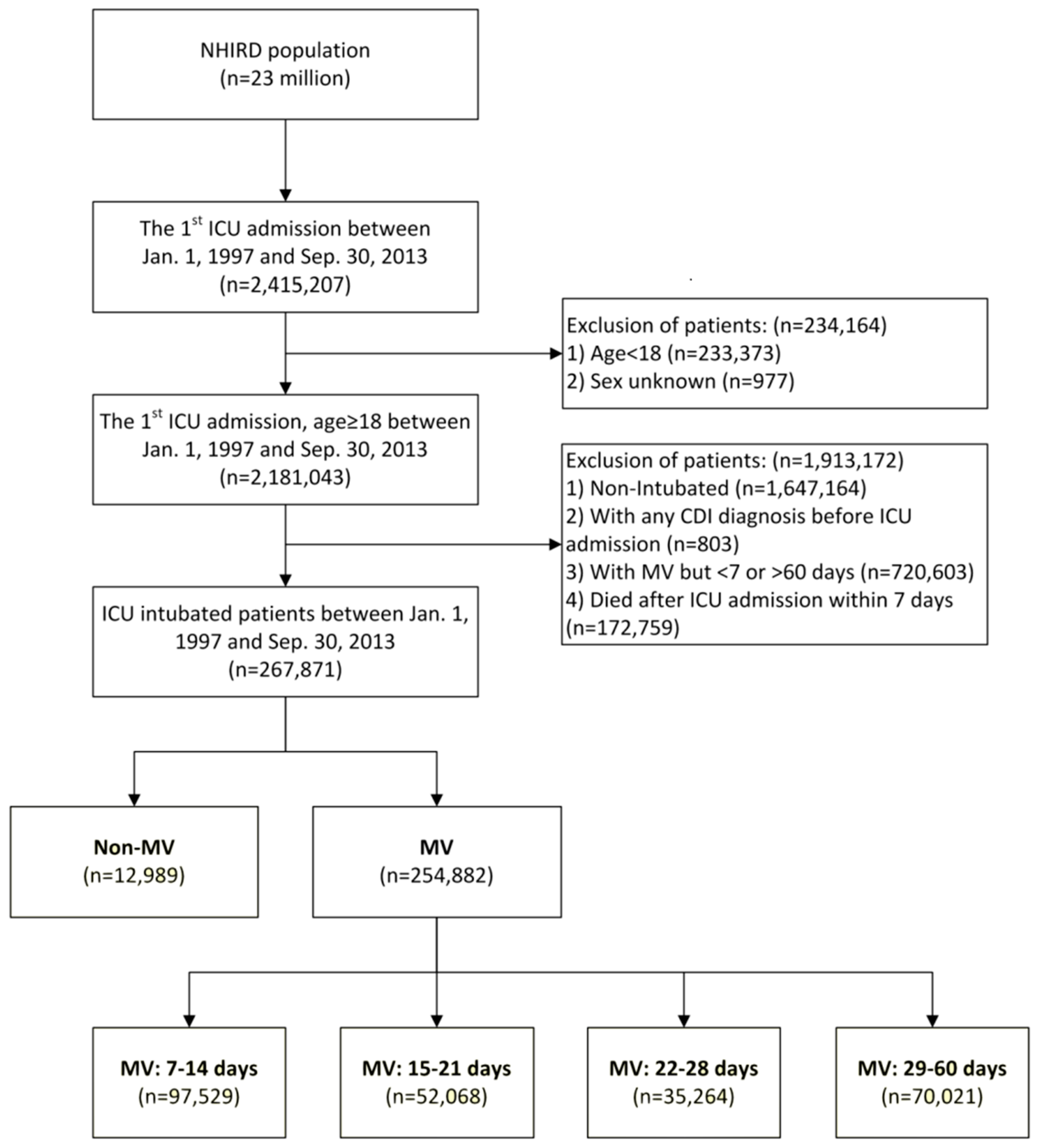
2. Materials and Methods
2.1. Data Source
2.2. Patient Selection
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Analysis of the Risk of Developing CDI with Interactions between MV Support and Carbapenem Therapy
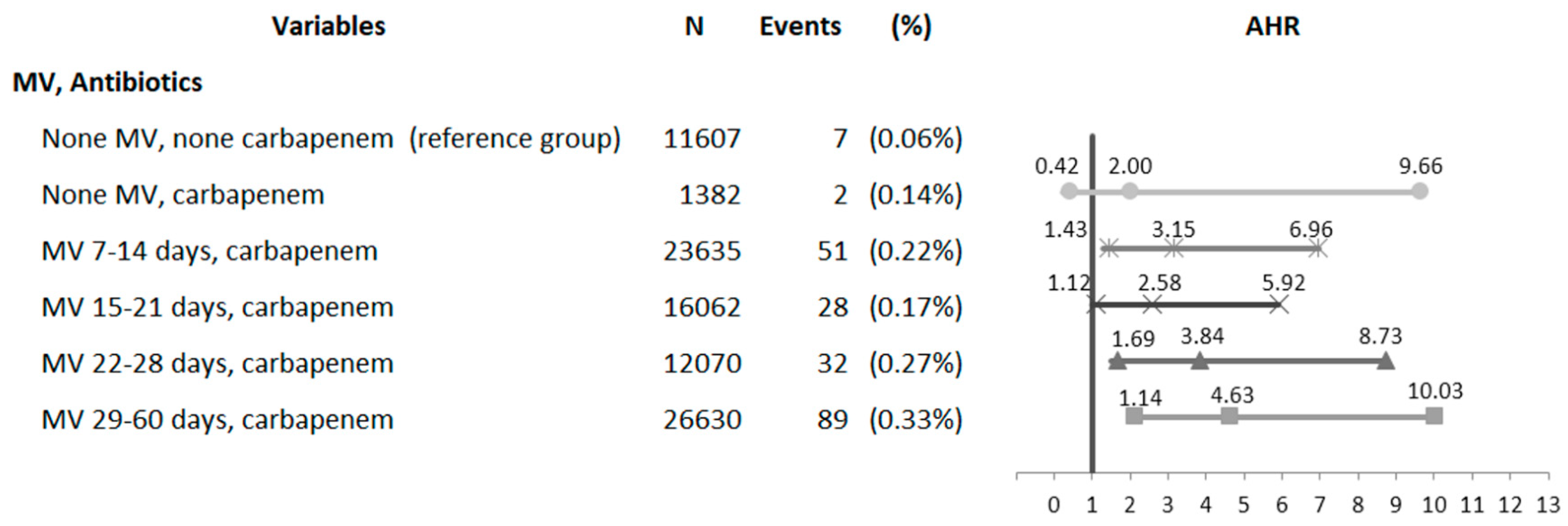
3.3. Analysis of Synergistic Interactions between the Duration of MV Assistance and Carbapenem Therapy for CDI
4. Discussion
Limitations
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CDI | Clostridium difficile infection |
| NICUD | National Intensive Care Unit Database |
| MV | mechanical ventilation |
References
- Beaugerie, L.; Flahault, A.; Barbut, F.; Atlan, P.; Lalande, V.; Cousin, P.; Cadilhac, M.; Petit, J.C.; Study Group. Antibiotic-associated diarrhoea and Clostridium difficile in the community. Aliment. Pharmacol. Ther. 2003, 17, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M. What have we learned about antimicrobial use and the risks for Clostridium difficile -associated diarrhoea? J. Antimicrob. Chemother. 2009, 63, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A.; Chen, L.F.; Sexton, D.J.; Anderson, D.J. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect. Control Hosp. Epidemiol. 2011, 32, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.W.; Heyes, G.; Morrison, P.; Carr, B. The acquisition and outcome of ICU-acquired Clostridium difficile infection in a single centre in the UK. J. Infect. 2008, 57, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.J.; Puzniak, L.A.; Shadel, B.N.; Gillespie, K.N.; Kollef, M.H.; Mundy, L.M. Clostridium difficile in the intensive care unit: Epidemiology, costs, and colonization pressure. Infect. Control Hosp. Epidemiol. 2007, 28, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Lessa, F.C.; Gould, C.V.; McDonald, L.C. Current status of Clostridium difficile infection epidemiology. Clin. Infect. Dis. 2012, 55 (Suppl. 2), S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Loo, V.G.; Bourgault, A.M.; Poirier, L. Host and pathogen factors for Clostridium difficile infection and colonization. N. Engl. J. Med. 2011, 365, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Rupnik, M.; Wilcox, M.H.; Gerding, D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hirota, S.A. The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection. Mol. Immunol. 2015, 63, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Denève, C.; Janoir, C.; Poilane, I.; Fantinato, C.; Collignon, A. A New trends in Clostridium difficile virulence and pathogenesis. Int. J. Antimicrob. Agents 2009, 33 (Suppl. 1), S24–S28. [Google Scholar] [CrossRef]
- Lo Vecchio, A.; Zacur, G.M. Clostridium difficile infection: An update on epidemiology, risk factors, and therapeutic options. Curr. Opin. Gastroenterol. 2012, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, T.M. New perspectives in Clostridium difficile disease pathogenesis. Infect. Dis. Clin. N. Am. 2015, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Péchiné, S.; Collignon, A. Immune responses induced by Clostridium difficile. Anaerobe 2016, 41, 68–78. [Google Scholar] [CrossRef] [PubMed]
- El Feghaly, R.E.; Stauber, J.L.; Deych, E.; Gonzalez, C.; Tarr, P.I.; Haslam, D.B. Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. Clin. Infect. Dis. 2013, 56, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K. The host immune response to Clostridium difficile infection. Ther. Adv. Infect. Dis. 2013, 1, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.; Petri, W.A., Jr. Immune responses to Clostridium difficile infection. Trends Mol. Med. 2012, 18, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Ricard, J.D.; Dreyfuss, D.; Saumon, G. Ventilator-induced lung injury. Curr. Opin. Crit. Care 2002, 8, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slutsky, A.S.; Tremblay, L.N. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am. J. Respir. Crit. Care Med. 1998, 157 Pt 1, 1721–1725. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Giunta, F.; Suter, P.M.; Slutsky, A.S. Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA 2000, 284, 43–44. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, D.S.; Liles, W.C.; Altemeier, W.A.; Dhanireddy, S. Mechanical ventilation interacts with endotoxemia to induce extrapulmonary organ dysfunction. Crit. Care 2006, 10, R136. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.E.; Barbar, S.D. Toll-like receptors: A link between mechanical ventilation, innate immunity and lung injury? Intens. Care Med. 2010, 36, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Dubberke, E.R.; Reske, K.A.; Yan, Y.; Olsen, M.A.; McDonald, L.C.; Fraser, V.J. Clostridium difficile—associated disease in a setting of endemicity: Identification of novel risk factors. Clin. Infect. Dis. 2007, 45, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Nathanson, B.H.; Sadigov, S.; Higgins, T.L.; Kollef, M.H.; Shorr, A.F. Epidemiology and outcomes of Clostridium difficile-associated disease among patients on prolonged acute mechanical ventilation. Chest 2009, 136, 752–758. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Owings, M.; Jernigan, D.B. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg. Infect. Dis. 2006, 12, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.; Valiquette, L.; Alary, M.E.; Villemure, P.; Pelletier, A.; Forget, K.; Pépin, K.; Chouinard, D. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: A changing pattern of disease severity. CMAJ 2004, 171, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Hung, Y.P.; Lin, H.J.; Tsai, P.J. Clostridium difficile infections in medical intensive care units of a medical center in southern Taiwan: Variable seasonality and disease severity. PLoS ONE 2016, 11, e0160760. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.A.; Young-Xu, Y.; Stwalley, D.; Kelly, C.P.; Gerding, D.N.; Saeed, M.J.; Mahé, C.; Dubberke, E.R. The burden of Clostridium difficile infection: Estimates of the incidence of CDI from U.S. Administrative databases. BMC Infect. Dis. 2016, 16, 177. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.H.; Gerding, D.N.; Johnson, S. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society of healthcare epidemiology of America (SHEA) and the infectious diseases society of america (IDSA). Infect. Control Hosp. Epidemiol. 2010, 31, 431–455. [Google Scholar] [CrossRef] [PubMed]
- Jump, R.L. Clostridium difficile infection in older adults. Aging Health 2013, 9, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin. Microbiol. Infect. 2012, 18 (Suppl. 6), 21–27. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chron. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Dubberke, E.R.; Reske, K.A. Evaluation of Clostridium difficile-associated disease pressure as a risk factor for C difficile-associated disease. Arch. Intern. Med. 2007, 167, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.S. Staphylococcus aureus bacteremia in older adults: Predictors of 7-day mortality and infection with a methicillin-resistant strain. Infect. Control Hosp. Epidemiol. 2006, 27, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Zavascki, A.P.; Barth, A.L.; Fernandes, J.F.; Moro, A.L.; Gonçalves, A.L.; Goldani, L.Z. Reappraisal of Pseudomonas aeruginosa hospital-acquired pneumonia mortality in the era of metallo-beta-lactamase-mediated multidrug resistance: A prospective observational study. Crit. Care 2006, 10, R114. [Google Scholar] [CrossRef] [PubMed]
- Loss, S.H.; de Oliveira, R.P.; Maccari, J.G.; Savi, A.; Boniatti, M.M.; Hetzel, M.P. The reality of patients requiring prolonged mechanical ventilation: A multicenter study. Rev. Bras. Ter. Intens. 2015, 27, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Estenssoro, E.; Reina, R.; Canales, H.S. The distinct clinical profile of chronically critically ill patients: A cohort study. Crit. Care 2006, 10, R89. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.E.; Cox, C.E.; Hope, A.A. Chronic critical illness. Am. J. Respir. Crit. Care Med. 2010, 182, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Rumbak, M.J. Pneumonia in patients who require prolonged mechanical ventilation. Microbes Infect. 2005, 7, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Kalb, T.H.; Lorin, S. Infection in the chronically critically ill: Unique risk profile in a newly defined population. Crit. Care Clin. 2002, 18, 529–552. [Google Scholar] [CrossRef]
- Kelly, C.P.; Becker, S.; Linevsky, J.K.; Joshi, M.A.; O’Keane, J.C.; Dickey, B.F.; LaMont, J.T.; Pothoulakis, C. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J. Clin. Investig. 1994, 93, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Mikelsaar, R.H.; Salminen, S.; Mikelsaar, M. Bacterial translocation, intestinal microflora and morphological changes of intestinal mucosa in experimental models of Clostridium difficile infection. J. Med. Microbiol. 1998, 47, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Pothoulakis, C.; LaMont, J.T.; Carlson, S.; Madara, J.L. C. difficile toxin A increases intestinal permeability and induces Cl-secretion. Am. J. Physiol. 1990, 259, G165–G172. [Google Scholar] [CrossRef] [PubMed]
- Koyner, J.L.; Murray, P.T. Mechanical ventilation and the kidney. Blood Purif. 2010, 29, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Guery, B.P.; Welsh, D.A.; Viget, N.B.; Robriquet, L.; Fialdes, P.; Mason, C.M.; Beaucaire, G.; Bagby, G.J.; Neviere, R. Ventilation-induced lung injury is associated with an increase in gut permeability. Shock 2003, 19, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Quílez, M.E.; López-Aguilar, J.; Blanch, L. Organ crosstalk during acute lung injury, acute respiratory distress syndrome, and mechanical ventilation. Curr. Opin. Crit. Care 2012, 18, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Altemeier, W.A.; Matute-Bello, G.; Gharib, S.A.; Glenny, R.W.; Martin, T.R.; Liles, W.C. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J. Immunol. 2005, 175, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, D.; Soler, P.; Saumon, G. Mechanical ventilation-induced pulmonary edema. Interaction with previous lung alterations. Am. J. Respir. Crit. Care Med. 1995, 151, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Cowardin, C.A.; Petri, W.A., Jr. Host recognition of Clostridium difficile and the innate immune response. Anaerobe 2014, 30, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.V.; Kuehne, S.A.; Minton, N.P.; Allan, E.; Bajaj-Elliott, M. Clostridium difficile-mediated effects on human intestinal epithelia: Modelling host-pathogen interactions in a vertical diffusion chamber. Anaerobe 2016, 37, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Barbar, S.D.; Pauchard, L.A.; Bruyère, R.; Bruillard, C.; Hayez, D. Mechanical ventilation alters the development of Staphylococcus aureus pneumonia in rabbit. PLoS ONE 2016, 11, e0158799. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total (n = 267,871) | MV (n = 254,882) | Non-MV (n = 12,989) | p |
|---|---|---|---|---|
| Age (years): mean ± SD | 68.8 ± 16.7 | 69.1 ± 16.5 | 63.7 ± 19.6 | <0.0001 |
| 18–64 | 87,675 (32.73) | 82,120 (32.22) | 5555 (42.77) | <0.0001 |
| 65–74 | 61,383 (22.92) | 58,616 (23.00) | 2767 (21.30) | |
| ≥75 | 118,813 (44.35) | 114,146 (44.78) | 4667 (35.93) | |
| Gender | ||||
| Female | 102,217 (38.16) | 96,574 (37.89) | 5643 (43.44) | <0.0001 |
| Male | 165,654 (61.84) | 158,308 (62.11) | 7346 (56.56) | |
| Carbapenem in ICU † | 79,779 (29.78) | 78,397 (30.76) | 1382 (10.64) | <0.0001 |
| ICU duration (days): median (IQR) | 10 (6–17) | 11 (6–17) | 2 (1–5) | <0.0001 |
| Comorbidities | ||||
| Congestive heart failure | 15,687 (5.86) | 15,037 (5.90) | 650 (5.00) | <0.0001 |
| Cerebrovascular accident | 27,633 (10.32) | 26,331 (10.33) | 1302 (10.02) | 0.2621 |
| Chronic obstructive pulmonary disease | 43,935 (16.40) | 42,181 (16.55) | 1754 (13.50) | <0.0001 |
| Liver disease | 20,097 (7.50) | 18,935 (7.43) | 1162 (8.95) | <0.0001 |
| Chronic kidney disease | 12,706 (4.74) | 12,110 (4.75) | 596 (4.59) | 0.3948 |
| CCI: mean ± SD | 1.9 ± 2.2 | 1.9 ± 2.2 | 1.7 ± 2.1 | <0.0001 |
| 0 | 85,527 (31.93) | 80,483 (31.58) | 5044 (38.83) | <0.0001 |
| 1–2 | 104,476 (39.00) | 99,834 (39.17) | 4642 (35.74) | |
| ≥3 | 77,868 (29.07) | 74,565 (29.25) | 3303 (25.43) | |
| Hospital mortality | 90,918 (33.94) | 86,642 (33.99) | 4276 (32.92) | 0.0118 |
| 1-year mortality | 157,846 (58.93) | 151,002 (59.24) | 6844 (52.69) | <0.0001 |
| Clostridium difficile infection ‡ | 435 (0.16) | 426 (0.17) | 9 (0.07) | 0.0069 |
| Variables | Total | Events | (%) | AHR * | (95% CI) | p |
|---|---|---|---|---|---|---|
| Age (years): mean ± SD | ||||||
| 18–64 | 87,675 | 104 | (0.12%) | 1.00 | (Reference) | - |
| 65–74 | 61,383 | 100 | (0.16%) | 1.20 | (0.91–1.59) | 0.2001 |
| ≥75 | 118,813 | 231 | (0.19%) | 1.43 | (1.12–1.83) | 0.0041 |
| Gender | ||||||
| Female | 102,217 | 172 | (0.17%) | 1.00 | (Reference) | - |
| Male | 165,654 | 263 | (0.16%) | 0.95 | (0.78–1.16) | 0.6235 |
| MV Support Duration (days) | ||||||
| None | 12,989 | 9 | (0.07%) | 1.00 | (Reference) | - |
| 7–14 | 97,529 | 126 | (0.13%) | 1.63 | (0.83–3.20) | 0.1595 |
| 15–21 | 52,068 | 67 | (0.13%) | 1.52 | (0.76–3.06) | 0.2377 |
| 22–28 | 35,264 | 73 | (0.21%) | 2.34 | (1.17–4.68) | 0.0168 |
| 29–60 | 70,021 | 160 | (0.23%) | 2.39 | (1.21–4.69) | 0.0117 |
| Carbapenem Therapy Duration (days) | ||||||
| None | 188,092 | 233 | (0.12%) | 1.00 | (Reference) | - |
| 1–7 | 3678 | 11 | (0.30%) | 2.02 | (1.10–3.10) | 0.0227 |
| 8–14 | 10,616 | 15 | (0.14%) | 1.19 | (0.72–2.01) | 0.5117 |
| ≥15 | 65,485 | 176 | (0.27%) | 1.88 | (1.54–2.30) | <.0001 |
| Comorbidities | ||||||
| CHF | 15,687 | 35 | (0.22%) | 1.14 | (0.80–1.63) | 0.4676 |
| CVA | 27,633 | 53 | (0.19%) | 0.93 | (0.69–1.25) | 0.6116 |
| COPD | 43,935 | 79 | (0.18%) | 0.88 | (0.68–1.14) | 0.3378 |
| Liver disease | 20,097 | 34 | (0.17%) | 1.05 | (0.73–1.51) | 0.7896 |
| CKD | 12,706 | 33 | (0.26%) | 1.38 | (0.95–2.02) | 0.0902 |
| CCI | ||||||
| 0 | 85,527 | 89 | (0.10%) | 1.00 | (Reference) | - |
| 1–2 | 104,476 | 192 | (0.18%) | 1.62 | (1.24–2.10) | 0.0004 |
| ≥3 | 77,868 | 154 | (0.20%) | 1.65 | (1.22–2.24) | 0.0012 |
| Variables | n | Events | (%) | AHR * | (95% CI) | p |
|---|---|---|---|---|---|---|
| Carbapenem | ||||||
| No | 188,092 | 233 | (0.12%) | 1.00 | (Reference) | - |
| Yes | 79,779 | 202 | (0.25%) | 1.92 | (1.59–2.32) | <0.0001 |
| Mechanical Ventilation | ||||||
| No | 12,989 | 9 | (0.07%) | 1.00 | (Reference) | - |
| Yes | 254,882 | 426 | (0.17%) | 2.19 | (1.13–4.24) | 0.0199 |
| MV Support Duration (days) | ||||||
| None | 12,989 | 9 | (0.07%) | 1.00 | (Reference) | - |
| 7–14 | 97,529 | 126 | (0.13%) | 1.76 | (0.90–3.46) | 0.1013 |
| 15–21 | 52,068 | 67 | (0.13%) | 1.73 | (0.86–3.48) | 0.1219 |
| 22–28 | 35,264 | 73 | (0.21%) | 2.72 | (1.36–5.44) | 0.0046 |
| 29–60 | 70,021 | 160 | (0.23%) | 2.85 | (1.46–5.58) | 0.0023 |
| MV, Carbapenem | ||||||
| No MV, no carbapenem | 11,607 | 7 | (0.06%) | 1.00 | (Reference) | - |
| No MV, carbapenem | 1382 | 2 | (0.14%) | 1.99 | (0.41–9.60) | 0.3898 |
| MV, no carbapenem | 176,485 | 226 | (0.13%) | 1.94 | (0.91–4.11) | 0.0857 |
| MV, carbapenem | 78,397 | 200 | (0.26%) | 3.64 | (1.71–7.75) | 0.0008 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, S.-R.; Lai, C.-C.; Ho, C.-H.; Chen, C.-M.; Chao, C.-M.; Wang, J.-J.; Cheng, K.-C. Prolonged Mechanical Ventilation Assistance Interacts Synergistically with Carbapenem for Clostridium difficile Infection in Critically Ill Patients. J. Clin. Med. 2018, 7, 224. https://doi.org/10.3390/jcm7080224
Chiang S-R, Lai C-C, Ho C-H, Chen C-M, Chao C-M, Wang J-J, Cheng K-C. Prolonged Mechanical Ventilation Assistance Interacts Synergistically with Carbapenem for Clostridium difficile Infection in Critically Ill Patients. Journal of Clinical Medicine. 2018; 7(8):224. https://doi.org/10.3390/jcm7080224
Chicago/Turabian StyleChiang, Shyh-Ren, Chih-Cheng Lai, Chung-Han Ho, Chin-Ming Chen, Chien-Ming Chao, Jhi-Joung Wang, and Kuo-Chen Cheng. 2018. "Prolonged Mechanical Ventilation Assistance Interacts Synergistically with Carbapenem for Clostridium difficile Infection in Critically Ill Patients" Journal of Clinical Medicine 7, no. 8: 224. https://doi.org/10.3390/jcm7080224





