Inclusion and Exclusion Criteria of Clinical Trials for Insomnia
Abstract
:1. Introduction
2. Experimental Section
2.1. Eligibility Criteria Selection
2.2. Online Survey
2.3. Statistical Analysis
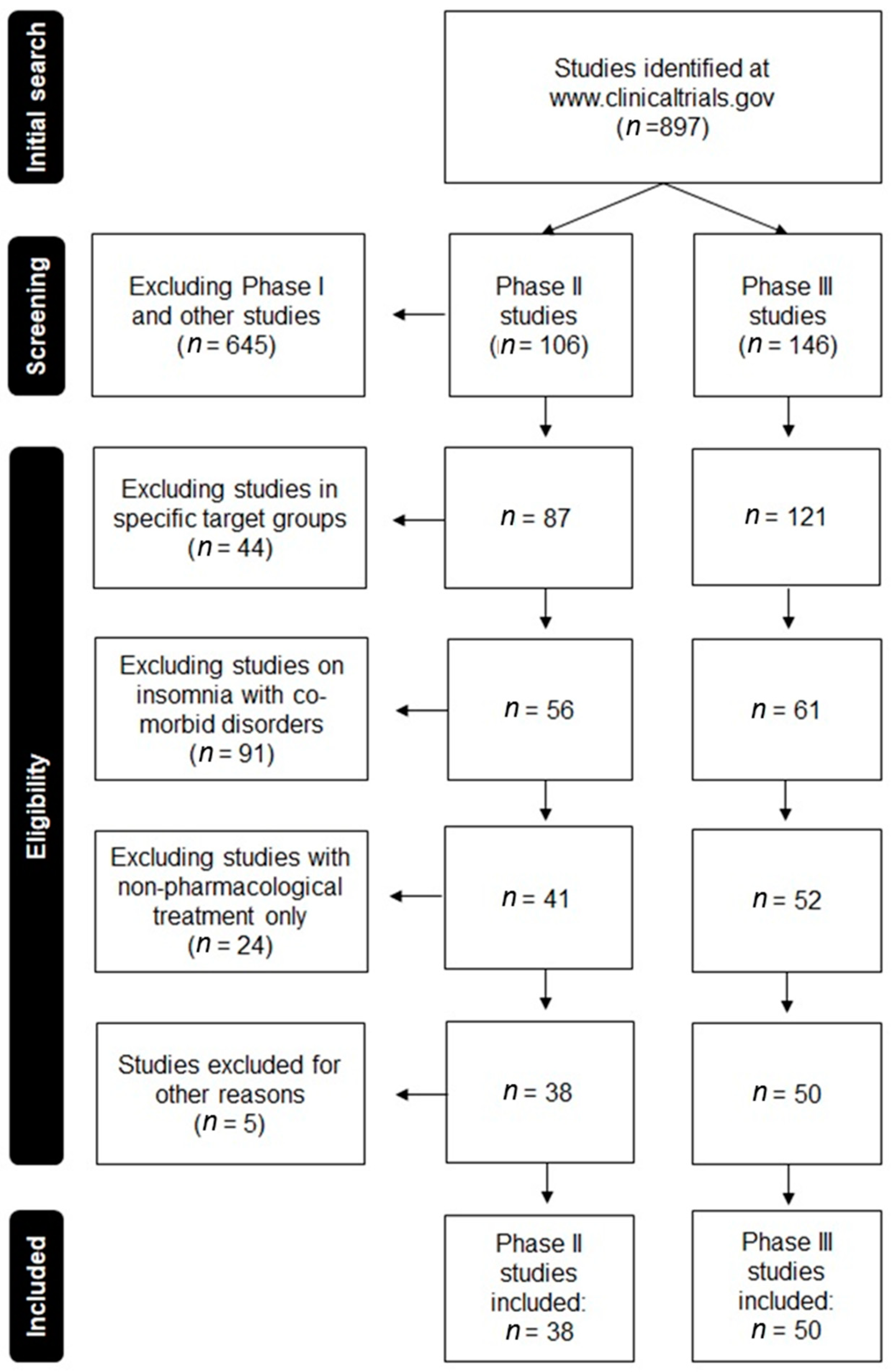
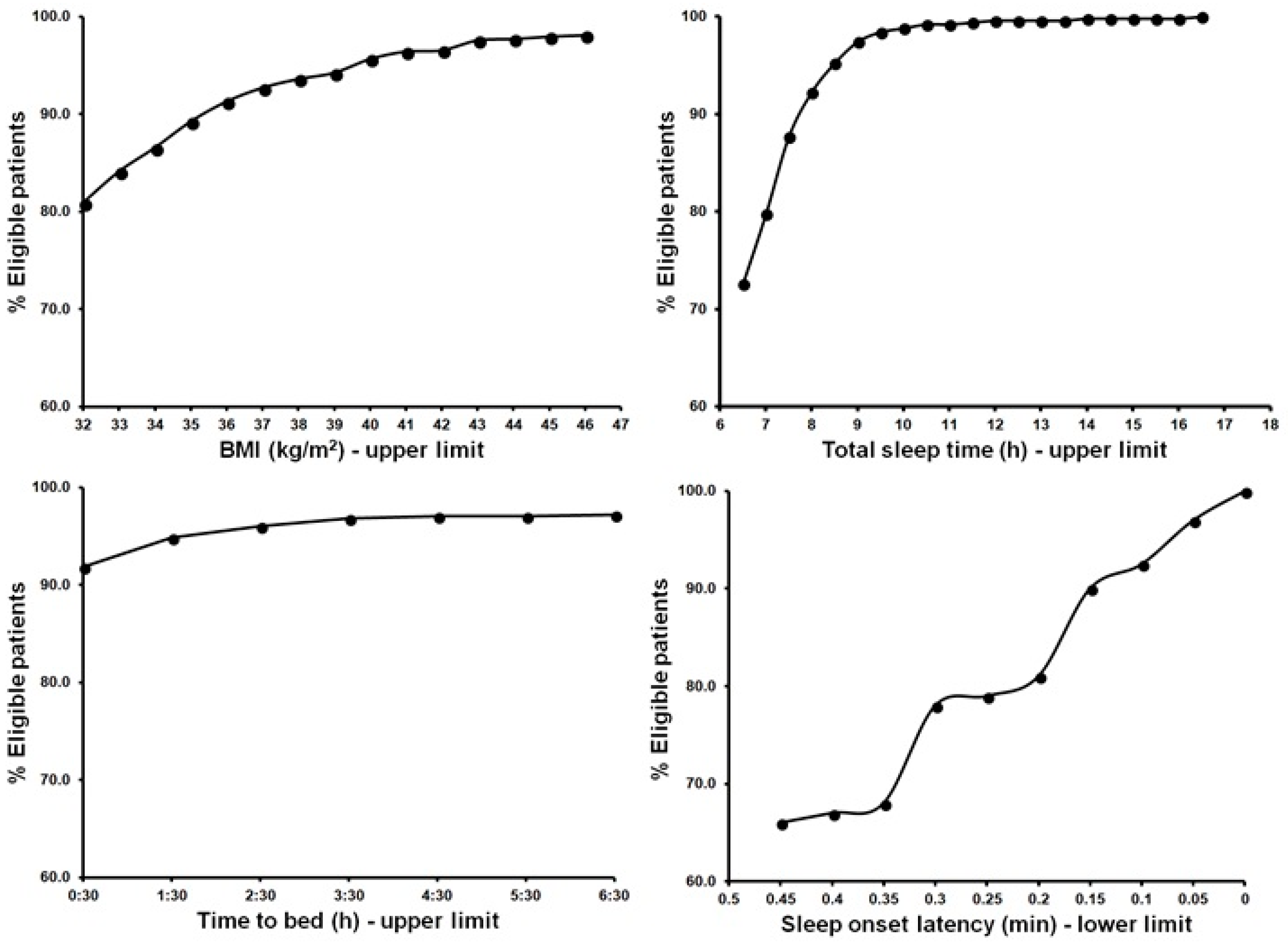
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gross, C.P.; Mallory, R.; Heiat, A.; Krumholz, H.M. Reporting the recruitment process in clinical trials: Who are these patients and how did they get here? Ann. Intern. Med. 2002, 137, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet 2005, 365, 82–93. [Google Scholar] [CrossRef]
- Heffron, T.M. Insomnia Awareness Day Facts and Stats. Tratto Da Sleep Education. Available online: http://www.sleepeducation.org/news/2014/03/10/insomnia-awareness-day-facts-and-stats (accessed on 1 April 2018).
- Cao, X.L.; Wang, S.B.; Zhong, B.L.; Zhang, L.; Ungvari, G.S.; Ng, C.H.; Li, L.; Chiu, H.F.K.; Lok, G.K.I.; Lu, J.P.; et al. The prevalence of insomnia in the general population in China: A meta-analysis. PLoS ONE 2017, 12, e0170772. [Google Scholar] [CrossRef] [PubMed]
- Van de Straat, V.; Bracke, P. How well does Europe sleep? A cross-national study of sleep problems in European older adults. Int. J. Public Health 2015, 60, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, T.; Verster, J.C.; Koshorek, G.; Withrow, D.; Roth, T. How representative are insomnia clinical trials? Sleep Med. 2018. [Google Scholar] [CrossRef]
- Van, S.H.G.; Toren, A.; Kiss, A. Eligibility criteria of randomized controlled trials published in high-impact general medical journals. JAMA 2007, 297, 1233–1244. [Google Scholar]
- McElroy, L.M.; Ladner, D.P. Defining the study cohort: Inclusion and exclusion criteria. In Success in Academic Surgery: Clinical Trials; Pawlik, T.M., Sosa, J.A., Eds.; Springer: London, NY, USA, 2014; pp. 131–139. [Google Scholar]
- Mackus, M.; Van de Loo, A.J.; Benson, S.; Scholey, A.; Verster, J.C. Consumption of caffeinated beverages and the awareness of their caffeine content among Dutch students. Appetite 2016, 103, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Heiat, A.; Gross, C.P.; Krumholz, H.M. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch. Intern. Med. 2002, 162, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.A.; Biederman, J.; Stewart, E.S.; Mullin, B.; Farrell, C.; Wagner, K.D.; Emslie, G.; Carpenter, D. Impact of comorbidity on treatment response to paroxetine in pediatric obsessive-compulsive disorder: Is the use of exclusion criteria empirically supported in randomized clinical trials? J. Child Adolesc. Psychopharmacol. 2003, 13, S19–S29. [Google Scholar] [CrossRef] [PubMed]
- Merkatz, R.B.; Temple, R.; Sobel, S.; Feiden, K.; Kessler, D.A. Working group on women in clinical trials. Women in clinical trials of new drugs—A change in Food and Drug Administration policy. N. Engl. J. Med. 1993, 329, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Roehrs, T. Insomnia: Epidemiology, characteristics, and consequences. Clin. Cornerstone 2003, 5, 5–15. [Google Scholar] [CrossRef]
- Verster, J.C.; Pandi, P.S.; Streiner, D. Sleep and Quality of Life in Clinical Medicine; Springer: London, UK, 2008. [Google Scholar]
- Cherubini, A.; Oristrell, J.; Pla, X.; Ruggiero, C.; Ferretti, R.; Diestre, G.; Clarfield, A.M.; Crome, P.; Hertogh, C.; Lesauskaite, V.; et al. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch. Intern. Med. 2011, 171, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Statista. Distribution of Facebook users worldwide as op April 2018, by age and gender. Available online: https://www.statista.com/statistics/376128/facebook-global-user-age-distribution/ (accessed on 12 April 2018).
- Reeder, C.E.; Franklin, M.; Bramley, T.J. Current landscape of insomnia in managed care. Am. J. Manag. Care 2007, 13, S112–S116. [Google Scholar] [PubMed]
- Theorell-Haglöw, J.; Miller, C.B.; Bartlett, D.J.; Yee, B.J.; Openshaw, H.D.; Grunstein, R.R. Gender differences in obstructive sleep apnoea, insomnia and restless legs syndrome in adults—What do we know? A clinical update. Sleep Med. Rev. 2018, 38, 28–38. [Google Scholar] [CrossRef] [PubMed]
| Frequency | Criterion | Type and Range | Justification |
|---|---|---|---|
| 69 | Pregnant or lactating (females) | Exclusion if yes | Strong |
| 40 | Nightshift or rotating shift work | Exclusion if yes | Strong |
| 32 | Sleep disorder other than insomnia | Exclusion if yes | Strong |
| 28 | Medication affecting sleep | Exclusion if yes | Strong |
| 24 | Total sleep time (h) | Inclusion if <6.5 | Strong |
| 23 | Sleep onset latency (min) | Inclusion if >30 or >45 | Strong |
| 19 | Time in bed (h) | Inclusion if 6.5–9 | Strong |
| 18 | Use of caffeine (mg) | Exclusion if >600 | Strong |
| 16 | Habitual bedtime (h) | Inclusion if 21:00–00:30 | Strong |
| 15 | Wake after sleep onset (min) * | Inclusion if >60 | Strong |
| 14 | Number of naps per week * | Inclusion if >3 | Strong |
| 8 | Apnoea-hypopnea index * | Exclusion if >10 | Strong |
| 7 | Periodic leg movement with arousal index * | Exclusion if >10 | Strong |
| 7 | Insomnia severity index * | Inclusion if >14 | Strong |
| 7 | Willing to have a fixed bedtime, remain in bed for 8 h * | Inclusion if yes | Strong |
| 6 | Willing to comply with RCT restrictions and clinic visits * | Inclusion if yes | Strong |
| 6 | Willing to complete surveys at home, access to phone * | Inclusion if yes | Strong |
| 4 | Positive urine drug screen * | Exclusion if yes | Strong |
| 2 | Pittsburgh Sleep Quality Index * | Inclusion if >4 | Strong |
| 1 | Sleep efficiency (%) * | Inclusion if <85 | Strong |
| 2 | Positive alcohol breathalyser test * | Exclusion if yes | Strong |
| 1 | Multivariable Apnoea risk index * | Exclusion if >0.5 | Strong |
| 1 | Epworth Sleepiness Scale score * | Inclusion if <9 | Strong |
| 56 | Diagnosed with primary insomnia | Inclusion if yes | Potential |
| 38 | History of alcohol or drug abuse | Exclusion if yes | Potential |
| 27 | Use of prescription drugs or clinically significant drugs | Exclusion if yes | Potential |
| 21 | Alcohol consumption (units per day) | Exclusion if >2 | Potential |
| 16 | Use of contraception (pre-menopausal females) | Exclusion if no | Potential |
| 3 | Haematology deviating from normal range * | Exclusion if yes | Potential |
| 2 | Creatine clearance (mL/min) * | Exclusion if <30 | Potential |
| 2 | AST/ALT (UNL) * | Exclusion if >2 | Potential |
| 1 | Bilirubin (UNL) * | Exclusion if >1.5 | Potential |
| 1 | Not euthyroid as evident by normal TSH * | Exclusion if yes | Potential |
| 1 | Glomerular filtration rate (mL/min) * | Exclusion if <30 | Potential |
| 74 | Clinically significant psychiatric, neurological, or medical disorders | Exclusion if yes | Poor |
| 60 | Age (years) | Inclusion if 18–65 | Poor |
| 37 | Body mass index (kg/m2) | Inclusion if 18–32 | Poor |
| 24 | History of significant neurological disorder | Exclusion if yes | Poor |
| 23 | Tobacco use | Exclusion if yes | Poor |
| 19 | History of sleep disorder other than insomnia | Exclusion if yes | Poor |
| 6 | ECG parameters outside of specified range * | Exclusion if yes | Poor |
| 5 | Outpatient * | Inclusion if yes | Poor |
| 4 | History of cancer | Exclusion if yes | Poor |
| 3 | Systolic blood pressure (mm Hg) * | Exclusion if >150 | Poor |
| 3 | Heart rate (bpm) * | Exclusion if >100 | Poor |
| 2 | QT interval (msec) * | Exclusion if >450 | Poor |
| 1 | 2nd or 3rd degree atrioventricular block * | Exclusion if yes | Poor |
| Criterion | Justification | % Excluded |
|---|---|---|
| Medication affecting sleep | Strong | 43.8 |
| Time in bed | Strong | 39.4 |
| Sleep onset latency (45 min) | Strong | 34.1 |
| Total sleep time | Strong | 27.4 |
| Sleep onset latency (30 min) | Strong | 22.2 |
| Nightshift or rotating shift work | Strong | 18.1 |
| Sleep disorder other than insomnia | Strong | 8.9 |
| Habitual bedtime | Strong | 8.1 |
| Use of caffeine | Strong | 5.0 |
| Pregnant or lactating (females) | Strong | 1.4 |
| Diagnosed with primary insomnia | Potential | 75.4 |
| Use of contraception (pre-menopausal females) | Potential | 52.5 |
| Use of prescription drugs or clinically significant drugs | Potential | 40.6 |
| Alcohol intake | Potential | 1.9 |
| History of alcohol or drug abuse | Potential | 3.0 |
| Clinically significant psychiatric, neurological, or medical disorders | Poor | 39.0 |
| Tobacco use | Poor | 34.8 |
| BMI | Poor | 19.1 |
| History of cancer | Poor | 3.5 |
| Age | Poor | 2.1 |
| History of significant neurological disorder | Poor | 1.3 |
| Criterion | Frequency | Eligible (n) | Patients (%) |
|---|---|---|---|
| Clinically significant psychiatric, neurological, or medical disorders | 74 | 309 | 59.5 |
| Pregnant or lactating (females) | 69 | 303 | 58.4 |
| Age | 60 | 298 | 57.4 |
| Diagnosed with primary insomnia | 56 | 69 | 13.3 |
| Nightshift or rotating shift work | 40 | 52 | 10.0 |
| History of alcohol or drug abuse | 38 | 48 | 9.2 |
| BMI | 37 | 39 | 7.5 |
| Sleep disorder other than insomnia | 32 | 36 | 6.9 |
| Medication affecting sleep | 28 | 10 | 1.9 |
| Use of prescription or clinically significant drugs | 27 | 8 | 1.5 |
| Total sleep time | 24 | 7 | 1.3 |
| History of significant neurological disorder | 24 | 7 | 1.3 |
| Tobacco use | 23 | 5 | 1.0 |
| Sleep onset latency (30 min) | 23 | 4 | 0.8 |
| Sleep onset latency (45 min) | 23 | 4 | 0.8 |
| Alcohol intake | 21 | 4 | 0.8 |
| Time in bed | 19 | 4 | 0.8 |
| Use of caffeine | 18 | 4 | 0.8 |
| Use of contraception (pre-menopausal females) | 16 | 2 | 0.4 |
| Habitual bedtime | 16 | 2 | 0.4 |
| History of cancer | 4 | 2 | 0.4 |
| Criterion | % Eligible | Impact (%) |
|---|---|---|
| All criteria included | 0.4 | - |
| Diagnosed with primary insomnia | 1.3 | +0.9 |
| Use of contraception (pre-menopausal females) | 0.8 | +0.4 |
| Use of drugs that affect sleep | 0.8 | +0.4 |
| Tobacco use | 0.6 | +0.2 |
| Body mass index | 0.6 | +0.2 |
| Criterion Justification | Included | Excluded |
|---|---|---|
| Strong | 53 (10.2%) | 466 (89.8%) |
| Potential | 26 (5.0%) | 493 (95.0%) |
| Poor | 171 (32.9%) | 348 (67.1%) |
| Strong + Potential | 3 (0.6%) | 516 (99.4%) |
| Poor + Potential | 9 (1.7%) | 510 (98.3%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huls, H.; Abdulahad, S.; Mackus, M.; Van de Loo, A.J.A.E.; Roehrs, T.; Roth, T.; Verster, J.C. Inclusion and Exclusion Criteria of Clinical Trials for Insomnia. J. Clin. Med. 2018, 7, 206. https://doi.org/10.3390/jcm7080206
Huls H, Abdulahad S, Mackus M, Van de Loo AJAE, Roehrs T, Roth T, Verster JC. Inclusion and Exclusion Criteria of Clinical Trials for Insomnia. Journal of Clinical Medicine. 2018; 7(8):206. https://doi.org/10.3390/jcm7080206
Chicago/Turabian StyleHuls, Hendrikje, Smedra Abdulahad, Marlou Mackus, Aurora J. A. E. Van de Loo, Timothy Roehrs, Thomas Roth, and Joris C. Verster. 2018. "Inclusion and Exclusion Criteria of Clinical Trials for Insomnia" Journal of Clinical Medicine 7, no. 8: 206. https://doi.org/10.3390/jcm7080206






