Portal Vein Embolization: Impact of Chemotherapy and Genetic Mutations
Abstract
:1. Introduction
2. Materials and Methods
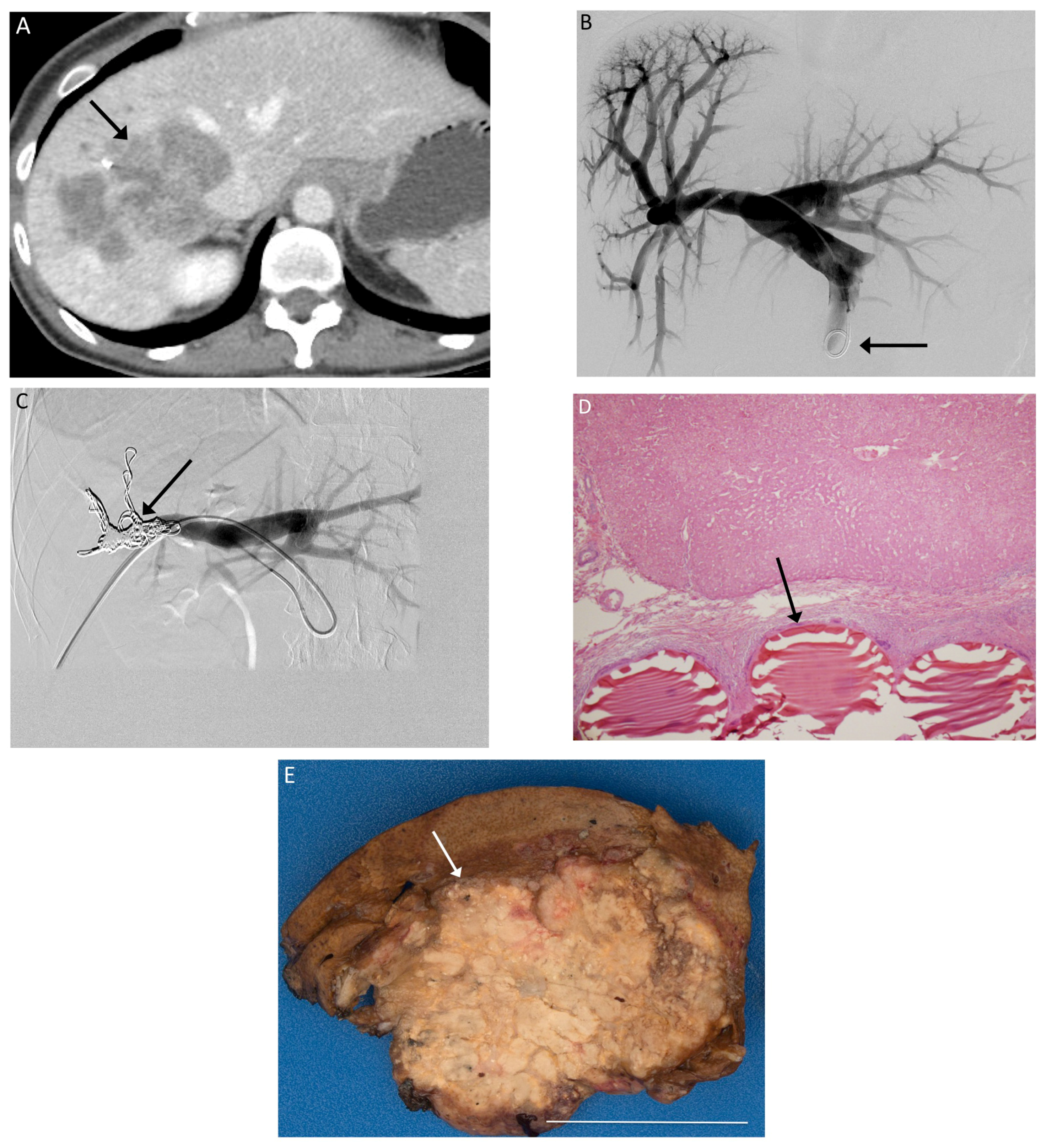
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Azoulay, D.; Castaing, D.; Smail, A.; Adam, R.; Cailliez, V.; Laurent, A.; Lemoine, A.; Bismuth, H. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann. Surg. 2000, 231, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Palavecino, M.; Chun, Y.S.; Madoff, D.C.; Zorzi, D.; Kishi, Y.; Kaseb, A.O.; Curley, S.A.; Abdalla, E.K.; Vauthey, J.N. Major hepatic resection for hepatocellular carcinoma with or without portal vein embolization: Perioperative outcome and survival. Surgery 2009, 145, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Castellano, E.; Downward, J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. Genes Cancer 2011, 2, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.D.; Corcoran, R.B.; Engelman, J.A. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010, 28, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Oklu, R.; Walker, T.G.; Wicky, S.; Hesketh, R. Angiogenesis and current antiangiogenic strategies for the treatment of cancer. J. Vasc. Interv. Radiol. 2010, 21, 1791–1805. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, W.; van den Esschert, J.W.; van Lienden, K.P.; van Gulik, T.M. Induction of tumor growth after preoperative portal vein embolization: Is it a real problem? Ann. Surg. Oncol. 2009, 16, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, N.; Tada, K.; Seki, M.; Ohta, H.; Azekura, K.; Ueno, M.; Ohta, K.; Yamaguchi, T.; Matsubara, T.; Takahashi, T.; et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology 2001, 34, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, L.T.; van Lienden, K.P.; Doets, A.; Busch, O.R.; Gouma, D.J.; van Gulik, T.M. Tumor progression after preoperative portal vein embolization. Ann. Surg. 2012, 256, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Van Lienden, K.P.; van den Esschert, J.W.; de Graaf, W.; Bipat, S.; Lameris, J.S.; van Gulik, T.M.; van Delden, O.M. Portal vein embolization before liver resection: A systematic review. Cardiovasc. Interv. Radiol. 2013, 36, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Melstrom, L.G.; Arnaoutakis, D.; Jarnagin, W.; Brown, K.; D’Angelica, M.; Covey, A.; DeMatteo, R.; Allen, P.; Kingham, T.P.; et al. Chemotherapy after portal vein embolization to protect against tumor growth during liver hypertrophy before hepatectomy. JAMA Surg. 2013, 148, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Beal, I.K.; Anthony, S.; Papadopoulou, A.; Hutchins, R.; Fusai, G.; Begent, R.; Davies, N.; Tibballs, J.; Davidson, B. Portal vein embolisation prior to hepatic resection for colorectal liver metastases and the effects of periprocedure chemotherapy. Br. J. Radiol. 2006, 79, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Covey, A.M.; Brown, K.T.; Jarnagin, W.R.; Brody, L.A.; Schwartz, L.; Tuorto, S.; Sofocleous, C.T.; D’Angelica, M.; Getrajdman, G.I.; DeMatteo, R.; et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann. Surg. 2008, 247, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Goere, D.; Farges, O.; Leporrier, J.; Sauvanet, A.; Vilgrain, V.; Belghiti, J. Chemotherapy does not impair hypertrophy of the left liver after right portal vein obstruction. J. Gastrointest. Surg. 2006, 10, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Madoff, D.C.; Hicks, M.E.; Abdalla, E.K.; Morris, J.S.; Vauthey, J.N. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: Safety and effectiveness—Study in 26 patients. Radiology 2003, 227, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Dias-Santagata, D.; Akhavanfard, S.; David, S.S.; Vernovsky, K.; Kuhlmann, G.; Boisvert, S.L.; Stubbs, H.; McDermott, U.; Settleman, J.; Kwak, E.L.; et al. Rapid targeted mutational analysis of human tumours: A clinical platform to guide personalized cancer medicine. EMBO Mol. Med. 2010, 2, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Nagino, M.; Nimura, Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: A review. World J. Surg. 2007, 31, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Fantini, M.C.; Wirtz, S.; Nikolaev, A.; Lehr, H.A.; Galle, P.R.; Rose-John, S.; Neurath, M.F. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle 2005, 4, 217–220. [Google Scholar] [CrossRef]
- Misale, S.; Yaeger, R.; Hobor, S.; Scala, E.; Janakiraman, M.; Liska, D.; Valtorta, E.; Schiavo, R.; Buscarino, M.; Siravegna, G.; et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012, 486, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Phipps, A.I.; Buchanan, D.D.; Makar, K.W.; Win, A.K.; Baron, J.A.; Lindor, N.M.; Potter, J.D.; Newcomb, P.A. KRAS-mutation status in relation to colorectal cancer survival: The joint impact of correlated tumour markers. Br. J. Cancer 2013, 108, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Stracquadanio, G.; Wang, X.; Wallace, M.D.; Grawenda, A.M.; Zhang, P.; Hewitt, J.; Zeron-Medina, J.; Castro-Giner, F.; Tomlinson, I.P.; Goding, C.R.; et al. The importance of p53 pathway genetics in inherited and somatic cancer genomes. Nat. Rev. Cancer 2016, 16, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.B.; Routhier, J.; Konkin, T.; Sabo, E.; Pricolo, V.E. Epidermal growth factor receptor, c-MET, beta-catenin, and p53 expression as prognostic indicators in stage II colon cancer: A tissue microarray study. Clin. Cancer Res. 2004, 10, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Deipolyi, A.R.; Iafrate, A.J.; Zhu, A.X.; Ergul, E.A.; Ganguli, S.; Oklu, R. High lung shunt fraction in colorectal liver tumors is associated with distant metastasis and decreased survival. J. Vasc. Interv. Radiol. 2014, 25, 1604–1608. [Google Scholar] [CrossRef] [PubMed]
- Sheth, R.A.; Hesketh, R.; Kong, D.S.; Wicky, S.; Oklu, R. Barriers to drug delivery in interventional oncology. J. Vasc. Interv. Radiol. 2013, 24, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
| Study Population | No Chemotherapy | Chemotherapy | p Value |
|---|---|---|---|
| Number of patients | 67 | 9 | |
| % Male | 61% | 56% | 0.745 |
| Age | 63 ± 1 year | 50 ± 3 year | <0.001 |
| Pre-PVE FLR | 35 ± 1% | 35 ± 3% | 0.960 |
| Tumor volume | 115 ± 31 cc | 105 ± 50 cc | 0.910 |
| Number of tumors | 3.2 ± 0.3 | 5.7 ± 1.4 | 0.122 |
| Etiology | 0.131 | ||
| HCC | 25% | 0 | |
| Cholangiocarcinoma | 15% | 0 | |
| Colorectal cancer | 54% | 89% | |
| Other metastasis | 6% | 11% |
| Age | Gender | Etiology | Chemotherapy Regimen |
|---|---|---|---|
| 66 | Male | Colorectal cancer | FOLFOX/bevacizumab |
| 44 | Female | Colorectal cancer | 5FU |
| 39 | Female | Colorectal cancer | FOLFIRINOX |
| 55 | Female | Colorectal cancer | FOLFOX |
| 40 | Male | Colorectal cancer | FOLFOX/bevacizumab, FOLFIRI/cetuximab |
| 50 | Male | Colorectal cancer | FOLFOX |
| 61 | Female | Colorectal cancer | FOLFOX |
| 51 | Male | Colorectal cancer | FOLFIRI, bevacizumab, 5-FU |
| 40 | Male | GIST | Sunitinib |
| Volume Changes after PVE | No Chemotherapy | Chemotherapy | p Value |
|---|---|---|---|
| % Change in tumor volume | 28% ± 13% | −24 ± 23% | 0.026 |
| % Daily change in tumor volume | 0.5% ± 0.2% | −0.3% ± 0.3% | 0.092 |
| % Change in FLR volume | 34% ± 4% | 28% ± 8% | 0.740 |
| % Daily change in FLR volume | 0.7% ± 0.1% | 0.4% ± 0.1% | 0.287 |
| % Cases that underwent hepatectomy | 79% | 88% | 0.489 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deipolyi, A.R.; Zhang, Y.S.; Khademhosseini, A.; Naidu, S.; Borad, M.; Sahin, B.; Mathur, A.K.; Oklu, R. Portal Vein Embolization: Impact of Chemotherapy and Genetic Mutations. J. Clin. Med. 2017, 6, 26. https://doi.org/10.3390/jcm6030026
Deipolyi AR, Zhang YS, Khademhosseini A, Naidu S, Borad M, Sahin B, Mathur AK, Oklu R. Portal Vein Embolization: Impact of Chemotherapy and Genetic Mutations. Journal of Clinical Medicine. 2017; 6(3):26. https://doi.org/10.3390/jcm6030026
Chicago/Turabian StyleDeipolyi, Amy R., Yu Shrike Zhang, Ali Khademhosseini, Sailendra Naidu, Mitesh Borad, Burcu Sahin, Amit K. Mathur, and Rahmi Oklu. 2017. "Portal Vein Embolization: Impact of Chemotherapy and Genetic Mutations" Journal of Clinical Medicine 6, no. 3: 26. https://doi.org/10.3390/jcm6030026
APA StyleDeipolyi, A. R., Zhang, Y. S., Khademhosseini, A., Naidu, S., Borad, M., Sahin, B., Mathur, A. K., & Oklu, R. (2017). Portal Vein Embolization: Impact of Chemotherapy and Genetic Mutations. Journal of Clinical Medicine, 6(3), 26. https://doi.org/10.3390/jcm6030026






