Long-Term Weight Change after Initiating Second-Generation Antidepressants
Abstract
:1. Introduction
2. Experimental Section
2.1. Setting
2.2. Study Population
2.3. Data Collection
2.4. Antidepressant Drug Treatment
2.5. Weight Data
2.6. Covariates
2.7. Statistical Analysis
3. Results
3.1. Population Description
3.2. Intent-to-Treat Analysis Results
3.3. Per Protocol Analysis Results
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General. The Surgeon General's Call to Action to Prevent and Decrease Overweight and Obesity; U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General: Rockville, MD, USA, 2001.
- Arterburn, D. Obesity. Clin. Evid. 2004, 11, 762–776. [Google Scholar] [PubMed]
- Arterburn, D.E.; McDonell, M.B.; Hedrick, S.C.; Diehr, P.; Fihn, S.D. Association of body weight with condition-specific quality of life in male veterans. Am. J. Med. 2004, 117, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.E. Long-term prognosis of depression in primary care. Bull. World Health Organ. 2000, 78, 439–445. [Google Scholar] [PubMed]
- Simon, G.E. Social and economic burden of mood disorders. Biol. Psychiatry 2003, 54, 208–215. [Google Scholar] [CrossRef]
- Simon, G.E.; Chisholm, D.; Treglia, M.; Bushnell, D. Course of depression, health services costs, and work productivity in an international primary care study. Gen. Hosp. Psychiatry 2002, 24, 328–335. [Google Scholar] [CrossRef]
- Simon, G.E.; Von Korff, M.; Lin, E. Clinical and functional outcomes of depression treatment in patients with and without chronic medical illness. Psychol. Med. 2005, 35, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010. JAMA 2012, 307, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Shim, R.S.; Baltrus, P.; Ye, J.; Rust, G. Prevalence, treatment, and control of depressive symptoms in the united states: Results from the national health and nutrition examination survey (nhanes), 2005–2008. J. Am. Board Fam. Med. 2011, 24, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Linde, J.A.; Jeffery, R.W.; Finch, E.A.; Simon, G.E.; Ludman, E.J.; Operskalski, B.H.; Ichikawa, L.; Rohde, P. Relation of body mass index to depression and weighing frequency in overweight women. Prev. Med. 2007, 45, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.E.; Von Korff, M.; Saunders, K.; Miglioretti, D.L.; Crane, P.K.; van Belle, G.; Kessler, R.C. Association between obesity and psychiatric disorders in the us adult population. Arch. Gen. Psychiatry 2006, 63, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.; Pietrobelli, A.; Fontaine, K.R.; Sirey, J.A.; Faith, M.S. Depressive mood and obesity in us adults: Comparison and moderation by sex, age, and race. Int. J. Obes. (Lond.) 2006, 30, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of overweight and obesity in the united states, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Kuczmarski, R.J.; Johnson, C.L. Overweight and obesity in the united states: Prevalence and trends, 1960–1994. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Paulose-Ram, R.; Safran, M.A.; Jonas, B.S.; Gu, Q.; Orwig, D. Trends in psychotropic medication use among U.S. Adults. Pharmacoepidemiol. Drug Saf. 2007, 16, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Keith, S.W.; Redden, D.T.; Katzmarzyk, P.T.; Boggiano, M.M.; Hanlon, E.C.; Benca, R.M.; Ruden, D.; Pietrobelli, A.; Barger, J.L.; Fontaine, K.R.; et al. Putative contributors to the secular increase in obesity: Exploring the roads less traveled. Int. J. Obes. (Lond.) 2006, 30, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Ricca, V.; Mannucci, E.; Di Bernardo, M.; Rizzello, S.M.; Cabras, P.L.; Rotella, C.M. Sertraline enhances the effects of cognitive-behavioral treatment on weight reduction of obese patients. J. Endocrinol. Investig. 1996, 19, 727–733. [Google Scholar] [CrossRef]
- Serretti, A.; Mandelli, L. Antidepressants and body weight: A comprehensive review and meta-analysis. J. Clin. Psychiatry 2010, 71, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Aronne, L.J.; Segal, K.R. Weight gain in the treatment of mood disorders. J. Clin. Psychiatry 2003, 64 (Suppl. 8), 22–29. [Google Scholar] [PubMed]
- Berkowitz, R.I.; Fabricatore, A.N. Obesity, psychiatric status, and psychiatric medications. Psychiatr. Clin. North Am. 2005, 28, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Franco, K. Managing weight gain as a side effect of antidepressant therapy. Cleve Clin. J. Med. 2003, 70. [Google Scholar] [CrossRef]
- Kachur, S.G.; Hannan, C.L.; Ward, K.E. Antidepressant-induced weight gain. Med. Health R. I. 2005, 88, 359–361. [Google Scholar] [PubMed]
- Schwartz, T.L.; Nihalani, N.; Jindal, S.; Virk, S.; Jones, N. Psychiatric medication-induced obesity: A review. Obes. Rev. 2004, 5, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, S.R.; Castro, V.M.; Clements, C.C.; Rosenfield, H.R.; Murphy, S.N.; Fava, M.; Weilburg, J.B.; Erb, J.L.; Churchill, S.E.; Kohane, I.S.; et al. An electronic health records study of long-term weight gain following antidepressant use. JAMA Psychiatry 2014, 71, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Snow, V.; Denberg, T.D.; Forciea, M.A.; Owens, D.K.; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Using second-generation antidepressants to treat depressive disorders: A clinical practice guideline from the american college of physicians. Ann. Intern. Med. 2008, 149, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; Gaynes, B.N.; Hansen, R.A.; Thieda, P.; DeVeaugh-Geiss, A.; Krebs, E.E.; Moore, C.G.; Morgan, L.; Lohr, K.N. Comparative benefits and harms of second-generation antidepressants: Background paper for the american college of physicians. Ann. Intern. Med. 2008, 149, 734–750. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Geddes, J.R.; Higgins, J.P.; Churchill, R.; Watanabe, N.; Nakagawa, A.; Omori, I.M.; McGuire, H.; et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: A multiple-treatments meta-analysis. Lancet 2009, 373, 746–758. [Google Scholar] [CrossRef]
- Aubin, H.J.; Farley, A.; Lycett, D.; Lahmek, P.; Aveyard, P. Weight gain in smokers after quitting cigarettes: Meta-analysis. BMJ 2012, 345, e4439. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.D.R.; Stergachis, A. Group health cooperative. In Pharmacoepidemiology, 4th ed.; BL, S., Ed.; John Wiley and Sons: West Sussez, UK, 2005; pp. 223–239. [Google Scholar]
- Boudreau, D.M.; Daling, J.R.; Malone, K.E.; Gardner, J.S.; Blough, D.K.; Heckbert, S.R. A validation study of patient interview data and pharmacy records for antihypertensive, statin, and antidepressant medication use among older women. Am. J. Epidemiol. 2004, 159, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Buist, D.S.; LaCroix, A.Z.; Brenneman, S.K.; Abbott, T., 3rd. A population-based osteoporosis screening program: Who does not participate, and what are the consequences? J. Am. Geriatr. Soc. 2004, 52, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, D. Bariatric surgery. BMJ 2008, 337, a755. [Google Scholar] [CrossRef] [PubMed]
- Seaman, S.R.; White, I.R. Review of inverse probability weighting for dealing with missing data. Stat. Methods Med. Res. 2013, 22, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.M.; Hernan, M.A.; Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000, 11, 550–560. [Google Scholar] [CrossRef]
- Cole, S.R.; Hernan, M.A. Constructing inverse probability weights for marginal structural models. Am. J. Epidemiol. 2008, 168, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Follmann, D.; Proschan, M.; Leifer, E. Multiple outputation: Inference for complex clustered data by averaging analyses from independent data. Biometrics 2003, 59, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Kaplan, R.A.; Gadde, K.M.; Wadden, T.A.; Allison, D.B.; Brewer, E.R.; Leadbetter, R.A.; Richard, N.; Haight, B.; Jamerson, B.D.; et al. Bupropion SR vs. placebo for weight loss in obese patients with depressive symptoms. Obes. Res. 2002, 10, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M.; Pradko, J.F.; Haight, B.R.; Modell, J.G.; Rockett, C.B.; Learned-Coughlin, S. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim. Care Companion J. Clin. Psychiatry 2004, 6, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, D.M.; Arterburn, D.; Bogart, A.; Haneuse, S.; Theis, M.K.; Westbrook, E.; Simon, G. Influence of body mass index on the choice of therapy for depression and follow-up care. Obesity (Silver Spring) 2013, 21, E303–E313. [Google Scholar] [CrossRef] [PubMed]
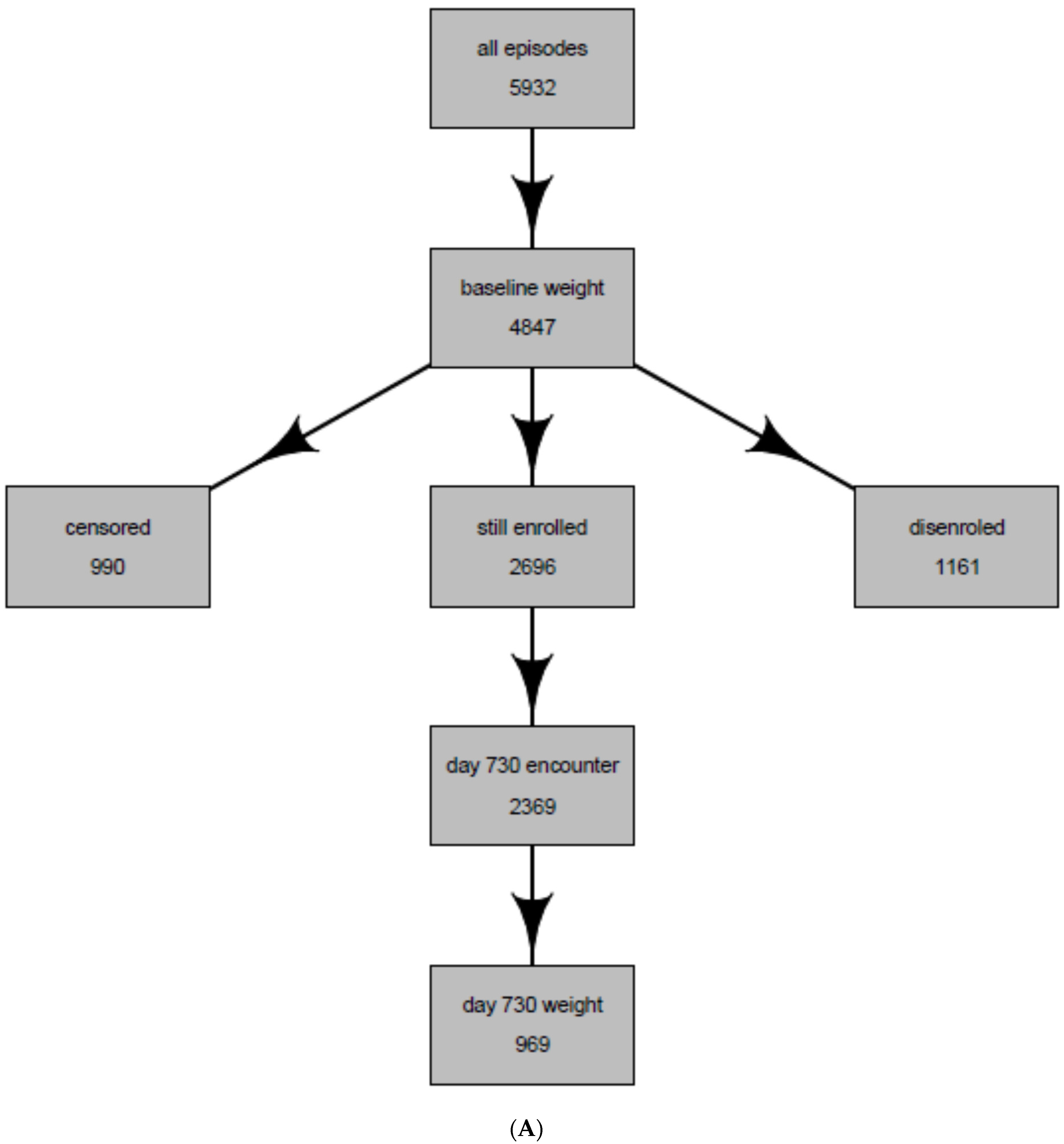
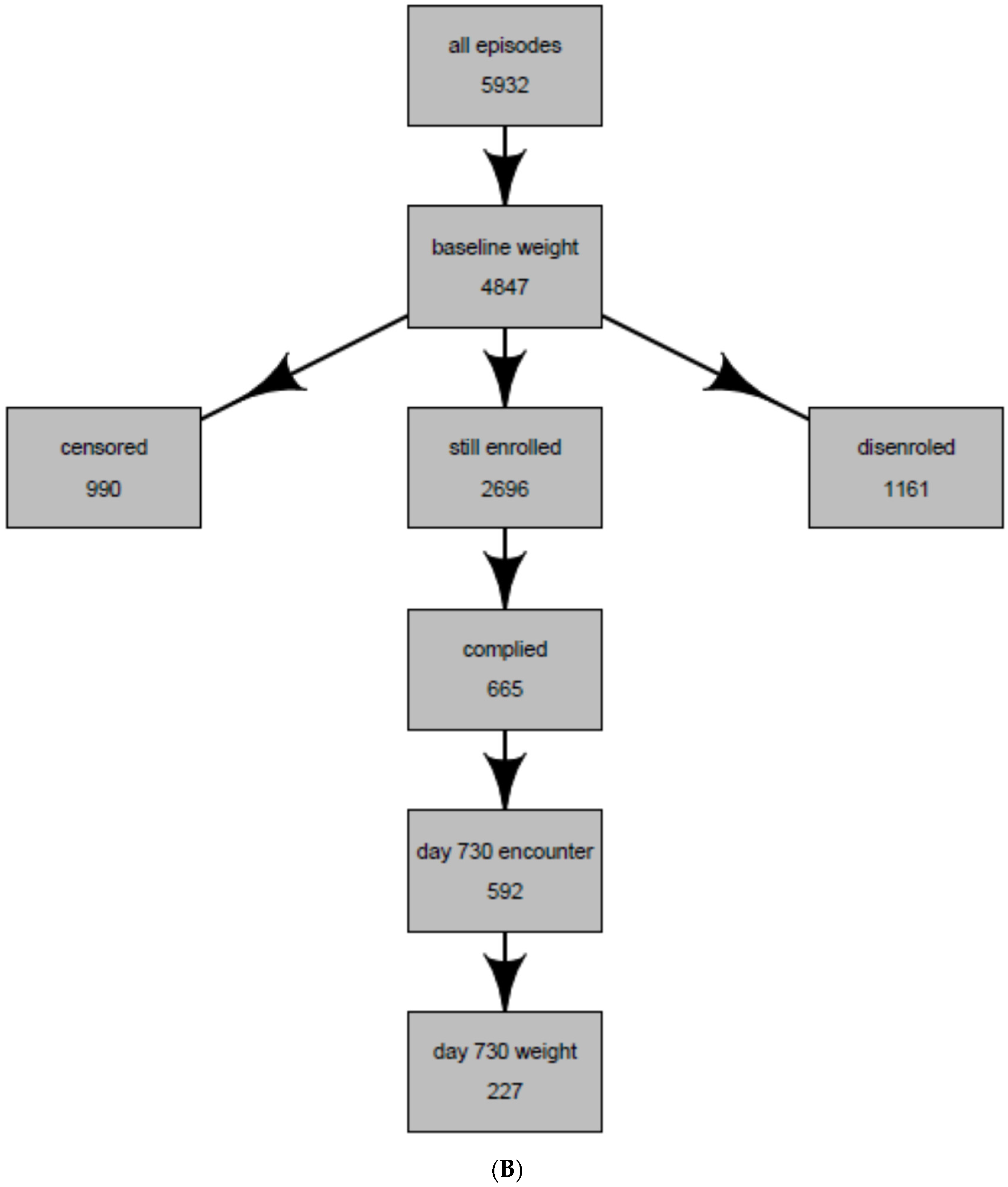
| Entire Population of Antidepressant Users * | Population Whose Weight Was Observed at 2 years (Intent-to-Treat Population) | Population Whose weight Was Observed and Were Continuously Treated with the Same Antidepressant at 2 years (Per-Protocol Population) | |
|---|---|---|---|
| Total | 5932 | 969 | 227 |
| Female, N (%) | 4018 (67.7%) | 651 (67.2%) | 158 (69.6%) |
| Age, N (%) | |||
| 18–29 years | 1114 (18.8%) | 119 (12.3%) | 17 (7.5%) |
| 30–49 years | 2691 (45.4%) | 438 (45.2%) | 96 (42.3%) |
| 50–65 years | 2127 (35.9%) | 412 (42.5%) | 114 (50.2%) |
| Weight, N (%) | |||
| 88–149 lbs. | 1176 (19.8%) | 200 (20.6%) | 42 (18.5%) |
| 150–179 lbs. | 1111 (18.7%) | 226 (23.3%) | 57 (25.1%) |
| 180–219 lbs. | 724 (12.2%) | 148 (15.3%) | 32 (14.1%) |
| 220–500 lbs. | 1188 (20.0%) | 268 (27.7%) | 69 (30.4%) |
| No baseline weight | 1085 (18.3%) | 0 (0%) | 0 (0%) |
| Body mass index, N (%) | |||
| Underweight: <18.5 | 41 (0.7%) | 9 (0.9%) | 1 (0.4%) |
| Normal: 18.5–24.9 | 1217 (20.5%) | 216 (22.3%) | 46 (20.3%) |
| Overweight: 25–29.9 | 1324 (22.3%) | 279 (28.8%) | 66 (29.1%) |
| Obese: ≥ 30.0 | 1957 (33.0%) | 441 (45.5%) | 110 (48.5%) |
| No baseline BMI | 1393 (23.5%) | 24 (2.5%) | 4 (1.8%) |
| Antidepressants, N (%) | |||
| Fluoxetine | 2842 (47.9%) | 506 (52.2%) | 127 (55.9%) |
| Bupropion | 877 (14.8%) | 129 (13.3%) | 25 (11%) |
| Citalopram | 1137 (19.2%) | 173 (17.9%) | 39 (17.2%) |
| Duloxetine | 37 (0.6%) | 8 (0.8%) | 0 (0.0%) |
| Mirtazapine | 36 (0.6%) | 5 (0.5%) | 1 (0.4%) |
| Paroxetine | 245 (4.1%) | 34 (3.5%) | 9 (4%) |
| Sertraline | 367 (6.2%) | 47 (4.9%) | 18 (7.9%) |
| Trazodone | 281 (4.7%) | 54 (5.6%) | 6 (2.6%) |
| Venlafaxine | 110 (1.9%) | 13 (1.3%) | 2 (0.9%) |
| Concurrent psychotherapy N (%) | 680 (11.5%) | 100 (10.3%) | 21 (9.3%) |
| Comorbid conditions, N (%) | |||
| Anxiety disorder | 1527 (25.7%) | 247 (25.5%) | 49 (21.6%) |
| Bipolar disorder | 78 (1.3%) | 9 (0.9%) | 4 (1.8%) |
| Schizophrenia | 1 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Schizoaffective disorder | 1 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Sleep disturbance | 381 (6.4%) | 80 (8.3%) | 18 (7.9%) |
| Smoker | 1923 (32.4%) | 282 (29.1%) | 49 (21.6%) |
| Unweighted Estimates | Weighted Estimates | |||||
|---|---|---|---|---|---|---|
| Estimate | p-Value | 95% CI | Estimate | p-Value | 95% CI | |
| Bupropion-non smoker | −7.6 | <0.01 | (−11.5, −3.7) | −7.1 | <0.01 | (−11.3, −2.8) |
| Bupropion-smoker | 1.0 | 0.65 | (−3.2, 5.2) | 2.2 | 0.33 | (−2.3, 6.8) |
| Citalopram | 0.3 | 0.82 | (−2.3, 2.9) | 1.2 | 0.40 | (−1.6, 4.1) |
| Duloxetine | −0.6 | 0.91 | (−11.4, 10.1) | −1.0 | 0.88 | (−13.5, 11.5) |
| Mirtazapine | 12.7 | 0.08 | (−1.5, 27.0) | 11.6 | 0.12 | (−2.8, 26.0) |
| Paroxetine | −0.5 | 0.84 | (−5.7, 4.7) | 0.8 | 0.78 | (−5.0, 6.6) |
| Sertraline | 3.3 | 0.15 | (−1.2, 7.9) | 5.9 | 0.02 | (0.8, 10.9) |
| Trazodone | 0.4 | 0.84 | (−3.9, 4.8) | 0.8 | 0.75 | (−3.9, 5.5) |
| Venlafaxine | −6.7 | 0.14 | (−15.5, 2.1) | −2.0 | 0.67 | (−11.3, 7.3) |
| Baseline Weight (lbs) | Baseline BMI (kg/m2) | Change in Weight at 2 years (lbs) | Change in BMI at 2 years (kg/m2) | |
|---|---|---|---|---|
| Fluoxetine: non-smoker | 191.4 | 30.6 | 4.6 | 0.7 |
| Fluoxetine: smoker | 186.2 | 29.5 | 6.7 | 1.1 |
| Bupropion: non-smoker | 199.1 | 31.5 | −2.4 | −0.4 |
| Bupropion: smoker | 194.0 | 30.2 | 6.9 | 1.1 |
| Citalopram | 186.6 | 29.8 | 5.9 | 0.9 |
| Duloxetine | 194.5 | 31.1 | 3.6 | 0.6 |
| Mirtazapine | 151.9 | 24.2 | 16.2 | 2.6 |
| Paroxetine | 189.9 | 30.1 | 5.5 | 0.9 |
| Sertraline | 187.5 | 30.0 | 10.5 | 1.7 |
| Trazodone | 188.1 | 29.8 | 5.4 | 0.9 |
| Venlafaxine | 183.6 | 29.1 | 2.6 | 0.4 |
| Unweighted Analysis | Weighted Analysis | |||||
|---|---|---|---|---|---|---|
| Estimate | p-value | 95% CI | Estimate | p-value | 95% CI | |
| Bupropion: non smoker | −7.6 | 0.049 | (−15.2, 0.0) | −8.4 | 0.041 | (−16.5, −0.3) |
| Bupropion: smoker | 13.3 | 0.016 | (2.5, 24.1) | 14.2 | 0.001 | (3.4, 24.9) |
| Citalopram | 1.9 | 0.49 | (−3.5, 7.2) | 2.9 | 0.32 | (−2.9, 8.7) |
| Paroxetine | 0.9 | 0.86 | (−9.2, 11.0) | 0.3 | 0.96 | (−10.5, 11.1) |
| Sertraline | 2.3 | 0.53 | (−5.0, 9.6) | 5.5 | 0.17 | (−2.4, 13.4) |
| Trazodone | 0.9 | 0.88 | (−11.4,13.3) | −0.1 | 0.99 | (−13.2, 13.0) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arterburn, D.; Sofer, T.; Boudreau, D.M.; Bogart, A.; Westbrook, E.O.; Theis, M.K.; Simon, G.; Haneuse, S. Long-Term Weight Change after Initiating Second-Generation Antidepressants. J. Clin. Med. 2016, 5, 48. https://doi.org/10.3390/jcm5040048
Arterburn D, Sofer T, Boudreau DM, Bogart A, Westbrook EO, Theis MK, Simon G, Haneuse S. Long-Term Weight Change after Initiating Second-Generation Antidepressants. Journal of Clinical Medicine. 2016; 5(4):48. https://doi.org/10.3390/jcm5040048
Chicago/Turabian StyleArterburn, David, Tamar Sofer, Denise M. Boudreau, Andy Bogart, Emily O. Westbrook, Mary Kay Theis, Greg Simon, and Sebastien Haneuse. 2016. "Long-Term Weight Change after Initiating Second-Generation Antidepressants" Journal of Clinical Medicine 5, no. 4: 48. https://doi.org/10.3390/jcm5040048






