1. Introduction
Metabolic syndrome (MS) is a complex metabolic disorder influenced by genetic and environmental factors [
1]. In Western societies, the high increase of MS, including cardiovascular disease (CVD), seems to be due to changes in lifestyle (e.g., increased consumption of food high in refined sugar and decreased physical activities). CVD represents a major cause of premature death in Western countries [
2]. Therefore, there is growing interest in identifying novel therapeutic approaches including a particular focus on nutrition and dietary interventions.
Cardiac energy metabolic shifts occur as a normal response to diverse physiological and dietary conditions and as a component of the pathophysiological processes that accompany heart disease. It is well established that insulin and fatty acids (FAs) are important modulators of cardiac substrate utilization [
3]. In the heart, there is a fine-tuning of high rates of myocellular FAs uptake and of mitochondrial fatty acid oxidation. When the rate of FA delivery to the heart increases (e.g., diabetes, high fat feeding) it may cause a mismatch between FA uptake and oxidation leading to excessive intracellular storage of the bio-active lipid intermediates within the cardiomyocytes that could subsequently lead to cardiac dysfunction [
4,
5]. Accordingly, several studies have shown that lipids accumulate in the heart of diabetic animals [
6,
7]. Myocardial FA uptake is largely regulated by the membrane fatty acid transporter (FAT/CD36) [
8]. Chiu
et al. [
9] showed that a myocardial lipid accretion due to an increase of fat uptake leads to myocyte apoptosis and cardiomyopathy. An enhanced long-chain acyl CoA (LC ACoA) uptake and channeling into triglycerides was observed in the heart of obese Zucker rats [
10]. The accumulation of triglyceride is likely toxic to the myocardium and has been linked with insulin resistance (IR) and cardiac dysfunction [
6,
11]. Besides, it is generally acknowledged that dietary factors, among them FAs, up-regulated the transcription of genes encoding for proteins involved in cardiac FA transport and metabolism, most likely through the activation of peroxisome proliferator activated receptor α (PPARα) (e.g., expression of muscle-type carnitine palmitoyltransferase 1 (M-CPT1) [
12,
13].
On the other hand, high sucrose, high fructose and/or high fat diets have been used to induce metabolic and physiological alterations in rodents, mimicking several aspects of the MS in humans such as dyslipidemia, IR and adiposity [
14]. Furthermore, we have previously demonstrated that the cardiac muscle of rats chronically fed a sucrose-rich diet (SRD) showed a significant increase of lipid storage accompanied by a significant reduction of basal and insulin stimulated glucose uptake and metabolism (isolated perfusion according to Langendorff’s recirculating mode), as well as in the activities of key enzymes involved in glucose metabolism [
15,
16].
Epidemiological data show that a high intake of
n-3 polyunsaturated fatty acids (
n-3 PUFAs) from fish is associated with a lower incidence of heart failure and cardio protective function [
17]. Moreover, different epidemiological and clinical studies have suggested that a high concentration of dietary α-linolenic acid 18:3
n-3, (ALA) is associated with a decreased risk of CVD [
18,
19]. Recent studies carried out in rats by Folino
et al. [
20] showed that ALA protects against cardiac injury and remodeling induced by beta-adrenergic over stimulation, and that a protective role is played by β
2 adrenergic receptors which mediate the activation of the Src kinase-phosphatidylinositol-3-kinase protective pathway. The seeds of
Salvia hispanica L, commonly known as chia seeds, contain the richest botanical oil source of ALA and high amounts of fiber and minerals. Poudyal
et al. [
21,
22] have recently shown that the administration of chia oil improved heart left ventricular dimensions, contractility, volume and stiffness as well as hypertension, glucose tolerance and insulin sensitivity in rats fed a high fat-high fructose diet. In this line, recent studies of our group have demonstrated that the administration of chia seeds as a dietary source of fat in rats fed an SRD reversed dyslipidemia and IR, improved adipose tissue dysfunction and glucose and lipid metabolism in the skeletal muscle [
23,
24,
25]. However, the effect of chia seeds on myocardial substrate utilization has been only partially investigated in this experimental model [
21,
22].
Thus, the aims of the present study were the following: (i) to further explore the mechanisms underlying the impaired lipid metabolism in the heart muscle of dyslipidemic insulin-resistant rats fed an SRD; (ii) to investigate if chia seeds as a dietary intervention could improve or even revert cardiac lipotoxicity. To achieve these goals: (a) we analyzed the protein mass levels of FAT/CD36 both at basal conditions and under insulin stimulation and the mitochondrial oxidation of LC ACoA by the activity and protein mass levels of the enzyme M-CPT1; (b) since the effect of FAs or FA derivatives in cardiac myocytes are considered to be PPARα mediated, we measured the protein mass level of this receptor; (c) we evaluated the protein mass levels of uncoupling protein 2 (UCP2), which plays a major role in the mitochondrial FAs flux. Additionally, the activities of the pyruvate dehydrogenase complex (PDHc) and lipid storage were assessed. The study was conducted in rats fed an SRD during 6 months, during which permanent dyslipidemia, IR, abnormal glucose homeostasis and visceral adiposity were present before the source of dietary fat, corn oil (CO), was replaced by an isocaloric amount of chia seeds for the last three months of the experimental period in half of the animals.
4. Discussion
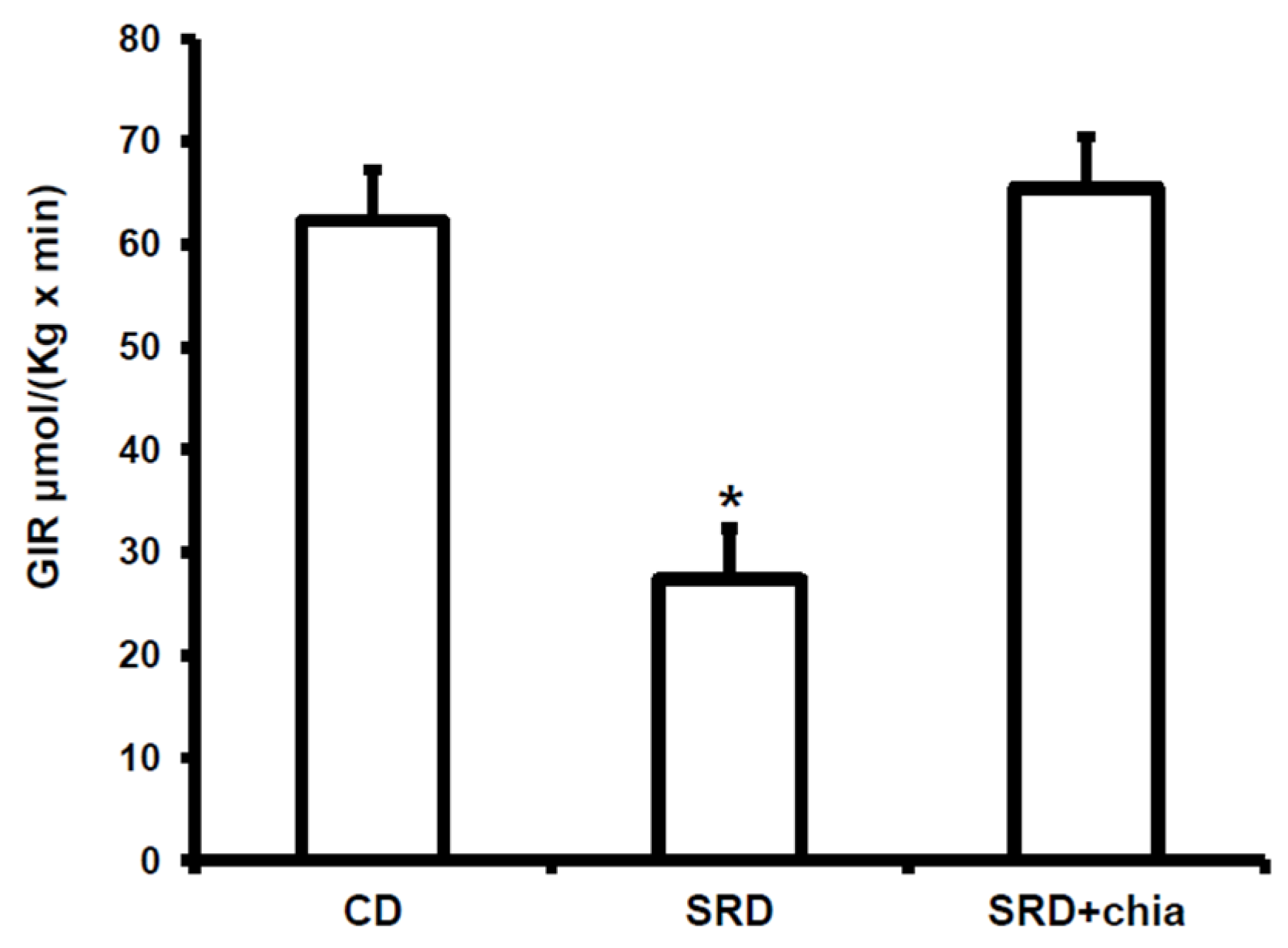
The present study provides new information on the mechanisms involved in heart muscle lipotoxicity in dyslipemic insulin resistant rats fed an SRD and explores the possible beneficial effects of dietary chia supplementation on reversed or improved pre-exiting impaired cardiac lipid metabolism. Disruption of the sensitive balance between FAs and glucose in the heart and increased intramyocellular fat contents and fatty acid metabolites are likely to play a pivotal role in the development of insulin resistance, cardiac lipotoxicity and heart dysfunction [
36]. FAT/CD36 plays a pivotal role in governing myocardial FAs uptake [
8]. In the present work, the increased intracellular LC ACoA in the heart of SRD-fed rats is accompanied by a significant increase of protein mass level of FAT/CD36 in the plasma membrane, suggesting that the enhanced amount of FAT/CD36 on sarcolemma elicits an increased rate of FAs uptake. In this regard, an increased availability of plasma free fatty acids and triglyceride levels is recorded in the SRD-fed group. Moreover, despite a significant increase of both the M-CPT1 activity and its protein mass level, triglyceride accumulates in the heart of this dietary group. It is possible that the increased flux of FAs to the heart exceeds the mitochondrial oxidative capacity leading to an increase of FAs storage into the triglyceride pool. The dynamic equilibrium between triglyceride stores and their metabolites cause accumulation of DAG and ceramide during prolonged long-chain fatty acid (LCFA) influx. Although in the present study we did not measure the level of ceramide, our previous results [
16] and the present data show an increase of DAG concentration in the heart of rats fed an SRD. Both metabolites are implicated in counteracting insulin signaling, reducing insulin responsiveness and altering its ability to regulate substrate handling [
4]. In this regard, the present data show that insulin stimulated the cell surface recruitment of FAT/CD36 in the heart of CD-fed rats. However, insulin was unable to further recruit FAT/CD36 to the sarcolemma in the heart of SRD-fed rats that was completely insensitive to the stimulus of the hormone. Similarly, in cardiac myocytes from obese Zucker rats, Coort
et al. [
10] reported that insulin failed to alter the sub cellular localization of FAT/CD36 and the rate of LCFA uptake and triglyceride esterification. Besides, in cardiamyocytes of Wistar rats in which a high-fat diet induced cardiac contractile dysfunction, Ouwens
et al. [
5] demonstrated that a permanent presence of FAT/CD36 in the sarcolemma membrane resulted in the enhancement of LCFA uptake and myocardial triglyceride accumulation.
PPARα and its co-activator PPARγ co-activator 1 alpha (PGC-1α) play an important role in the transcriptional regulation of cardiac energy metabolism, and the effect of FAs in cardiac myocytes is considered to be PPARα mediated [
37]. Several lines of evidence suggested that LCFAs that induce the gene expression of M-CPT1 and other enzymes in the cellular fatty acid utilization pathway are namely mediated by PPARα transcriptional control [
13]. LCFAs: linoleic acid 18:2
n-6, ALA and docosahexaenoic acid 22:6,
n-3 (DHA) among them, and a variety of related compounds serve as PPARα ligands [
38]. In this context, the present study shows a significant increase of the relative abundance of the protein mass level of PPARα and the mitochondrial M-CPT1 activity in the heart of SRD-fed rats. These results suggest that a chronic high exposure to Fas, which enhances their uptake, induces the activation of PPARα protein expression that, in turn, encodes the proteins responsible for FAs oxidation, M-CPT1 among others. Since an increase of intramyocardial lipids is observed in the SRD heart, it is possible that a disruption of the balance between lipid oxidation-storage occurs in the heart muscle of this dyslipemic insulin-resistant model. In this vein, Buchanam
et al. [
39] have documented an increased PPARα and PGC-1α expression in murine insulin-resistant hearts. A high fat diet also activates PPARα in the heart and stimulates expression of key proteins involved in fatty acid oxidation [
40].
It is well known that dietary
n-3 PUFAs, mainly eicosapentaenoic acid 20:5,
n-3 (EPA) and DHA, improve cardiac function [
17]. ALA could be a valuable source of
n-3 long-chain FAs via elongase/desaturase activities. Dietary ALA exerts a protective effect on the CVD [
18]. In this regard, and confirming previous results, the present work demonstrates a reversion of dyslipidemia, abnormal glucose homeostasis and whole body peripheral insulin insensitivity when dietary chia seed replaced CO in the SRD-fed rats. The reverse of dyslipidemia by chia seed led to a significant reduction of lipid storage in the heart of SRD-fed rats, reaching values similar to those observed in the heart of CD-fed rats. Moreover, at basal conditions (beginning of the clamp study) a decrease of FAT/CD36 protein mass level suggests that a different milieu (decreased plasma lipids levels) prevented the robust relocations of fatty acid translocase to the sarcolemma, which was otherwise seen in the cardiac muscle of SRD-fed rats and, therefore, reduced the influx of FAs. Furthermore, the heart of dietary chia-fed rats was sensitive to the stimulus of insulin. As in the CD-fed group, the hormone significantly induced the translocation of FAT/CD36 to the plasma membrane. Recently, in isolated rat cardiomyocytes incubated under insulin resistance evoking conditions, Franekova
et al. [
41] demonstrated that the inclusion of EPA and DHA to the medium prevented the persistent translocation of CD36 to the sarcolemma and protected the metabolic and functional properties of the cardiomyocytes. In this regard, we previously demonstrated that the administration of dietary fish oil to SRD-fed rats was able to reverse heart muscle lipotoxicity and benefit key enzyme activities involved in the glucose metabolism [
16]. At present, we are unaware of other studies concerning the effect of the long-term consumption of dietary chia and/or ALA on lipid metabolism in the cardiac muscle of SRD-fed rats. However, the reversion of the impaired glucose oxidation, as well as the accretion of triglyceride and fatty acid derivatives, the normalization of the enhanced sarcolemmal FAT/CD36 and the significantly reduced mitochondrial oxidative flux suggests that dietary chia seeds could improve the altered balance of heart fuel utilization. Interestingly, compared with CD-fed rats the protein mass level of the nuclear receptor PPARα and the activity of its target enzyme M-CPT1 were still higher in the heart of the SRD + chia group. Our results do not provide data on the mechanisms underlying the effect of chia seed on this nuclear receptor but it was shown that ALA and DHA, among others, are natural occurring ligands of PPARα in the heart [
38]. In this regard, it has been demonstrated by our group [
23] and others [
21,
42] that chia seeds change the plasma fatty acid profile increasing ALA, EPA, docosapentaenoic acid 22:5,
n-3 (DPA) and DHA levels as well as the
n-3/
n-6 FAs ratio in rats fed an SRD, a control diet, or a high fat, high fructose diet. Thus, we do not discard the possibility that the different plasma FA profiles to which the heart was exposed could contribute to this finding.
On the other hand, an increase of FAs could induce UCP2 expression through PPARα activation in adult rat cardiomyocytes [
43]. Besides, an increase of UCP2 and PPARα was recorded in the heart of ob/ob mice [
39]. However, under the present experimental protocol, our results showed that the mitochondrial protein mass levels of UCP2 were similar in the three experimental groups. Besides, we are unaware of any other studies that evaluated the potential role of UCP2 in altering myocardial substrate in the heart muscle of SRD rats, and the effect of either chia seeds or oil upon UCP2. Further studies will be needed to evaluate this matter.
Chia seeds were able to decrease the systolic blood pressure that developed in the SRD-fed rats. In this regard, Poudyal
et al. [
21], in rats fed a high fructose-high fat diet, recorded that chia seed normalized systolic blood pressure increasing DPA and DHA contents in FAs of the heart, and Rousseau
et al. [
44] showed a decrease in blood pressure and increased DHA and EPA in cardiac phospholipids in rats fed a high fructose diet supplemented with either DHA or EPA. Interestingly, we observed an increase of DHA and ALA in the FA phospholipids of cardiac membrane in the SRD + chia group (data not shown). Moreover, Vuksan
et al. [
45] showed that a long-term supplementation with Salba (
S. hispanica L) attenuates systolic blood pressure and emerging cardiovascular risk factors, safely beyond conventional therapy, while maintaining good glycemic and lipid control in well-controlled type-2 diabetic patients.