Horizon 2020 in Diabetic Kidney Disease: The Clinical Trial Pipeline for Add-On Therapies on Top of Renin Angiotensin System Blockade
Abstract
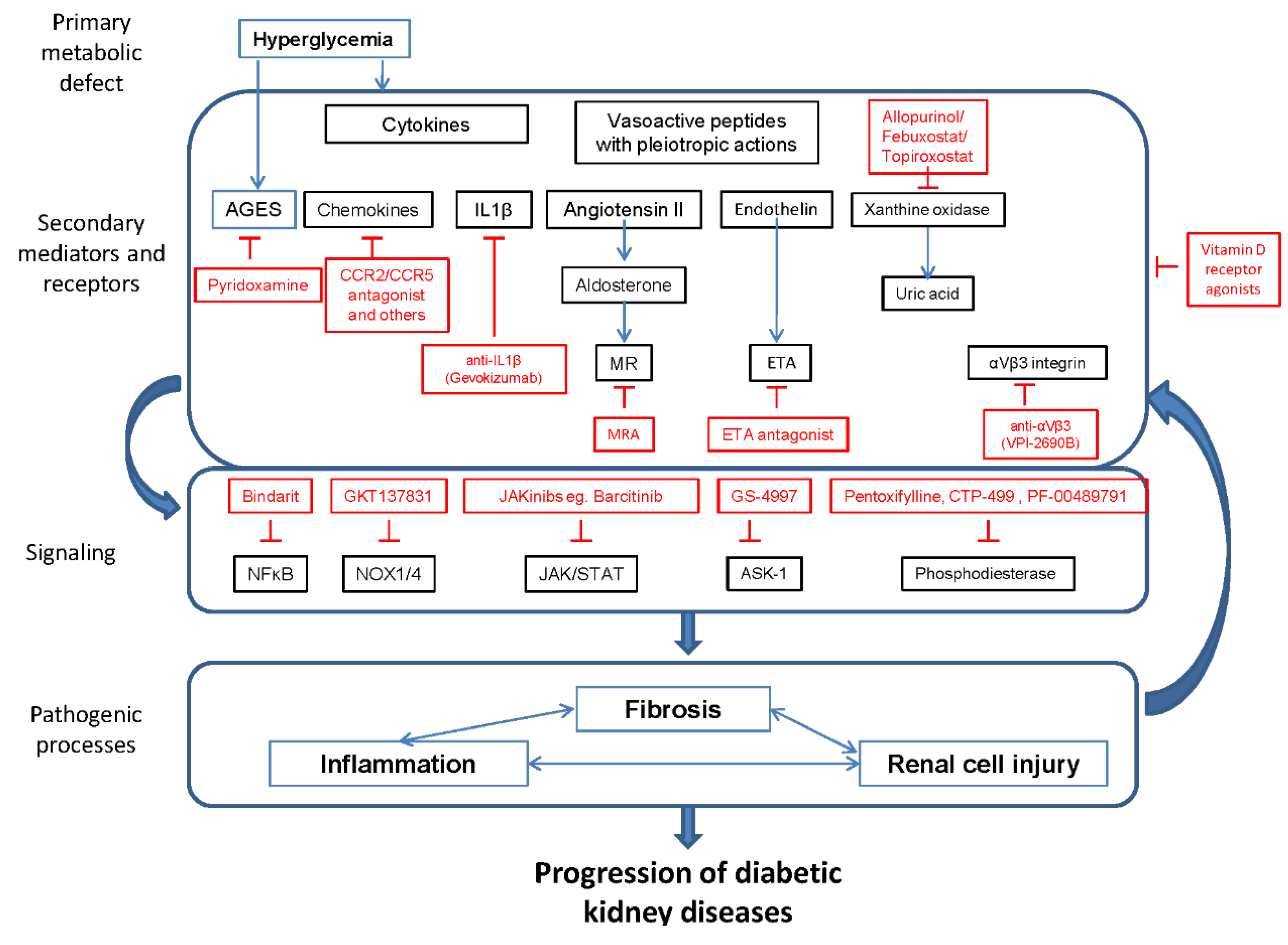
:1. Introduction
| Target | Drug | ClinicalTrials.gov Identifier | Phase | Enrollment (Expected) | Status | Completed Date (or Expectation) |
|---|---|---|---|---|---|---|
| Vitamin D receptor agonist | Paricalcitol | VITAL NCT00421733 | II | 281 | Completed | June 2009 |
| PROCEED NCT01393808 | II | 112 | Ongoing | June 2015 | ||
| Cholecalciferol | NCT00552409 | II and III | 22 | Completed | June 2010 | |
| Calcitriol | NCT01673204 | IV | 276 | Ongoing | April 2014 | |
| Endothelin receptor antagonists | Avosentan | NCT00120328 | III | 2364 | Terminated * | February 2007 |
| Atrasentan | NCT00920764 | II | 92 | Completed | May 2010 | |
| RADAR NCT01356849 | II | 149 | Completed | August 2012 | ||
| SONAR NCT 01858532 | III | 4148 | Ongoing | February 2017 | ||
| Mineralocorticoid receptor blockers | Spironolactone | NCT00317954 | IV | 48 | Completed | July 2005 |
| PRIORITY NCT02040441 | II and III | 3500 | Ongoing | December 2017 | ||
| Eplerenone | NCT00315016 | II | 30 | Completed | July 2011 | |
| MT-3995 | NCT01756703 | II | 67 | Completed | August 2014 | |
| BAY 94-8862 (Finerenone) | NCT01874431 | II | 821 | Completed | August 2014 | |
| Cs3150 | NCT02345057 | II | 325 | Ongoing | July 2016 | |
| Xanthine oxidase Inhibitor | Topiroxostat | NCT02327754 | II | 60 | Ongoing | December 2016 |
| Allopurinol | PERL NCT02017171 | III | 480 | Ongoing | December 2018 | |
| Phosphodiesterase inhibitor | Pentoxifylline | NCT00663949 | II and III | 70 | Completed | January 2008 |
| PF0489791 | NCT01200394 | II | 256 | Completed | August 2013 | |
| CTP499 | NCT01487109 | II | 170 | Completed | January 2015 | |
| Serotonin receptor antagonists | Sarpogrelate | SONATA NCT01869881 | IV | 166 | Ongoing | December 2014 |
| Nuclear factor erythroid 2-related factor 2 (Nrf2) activators | Bardoxolone RTA-402 | NCT00811889 | II | 227 | Completed | December 2010 |
| BEACON NCT00664027 | III | 2185 | Terminated ** | October 2012 | ||
| NCT02316821 | II | 72 | Ongoing | December 2017 | ||
| Chemokine inhibitors | PF-04634817 | NCT01712061 | II | 226 | Completed | September 2014 |
| CCX 140-B | NCT01447147 | II | 332 | Completed | December 2014 | |
| BMS-813160 | NCT01752985 | II | 120 | Ongoing | December 2015 | |
| NFκB inhibitors | Bindarit | NCT01109212 | II | 100 | Completed | December 2008 |
| Jakinibs | Baricitinib | NCT01683409 | II | 129 | Completed | November 2014 |
| Antioxidants | N-Acetylcysteine | NCT00556465 | II and III | 69 | Completed | June 2007 |
| NCT00915200 | II | 225 | Ongoing | March 2015 | ||
| NOX-E36 | NCT01547897 | II | 76 | Completed | December 2013 | |
| Probucol | NCT01726816 | II | 126 | Completed | September 2014 | |
| Glutathione | NCT01265563 | II | 110 | Ongoing | February 2015 | |
| GKT137831 | NCT02010242 | II | 200 | Completed | March 2015 | |
| Colchicine | NCT02035891 | I, II, III and IV | 160 | Ongoing | June 2018 | |
| Galectin-3 antagonist | GCS-100 | NCT02312050 | II | 375 | Ongoing | Sep 2016 |
| Integrin blocker | VPI-2690B | NCT02251067 | II | 300 | Ongoing | August 2017 |
| Apoptosis signal-regulating kinase 1 | GS-4997 | NCT02177786 | II | 300 | Ongoing | August 2016 |
| Antifibrotic therapies | Pirfenidone | NCT00063583 | I and II | 77 | Completed | March 2009 |
| LY2382770 Anti-TGF-β1 mAb | NCT01113801 | II | 400 | Completed *** | July 2014 | |
| RAGE inhibitor | TTP488 | NCT00287183 | II | 110 | Completed | August 2009 |
| Glycosaminoglycans | Sulodexide | NCT00130208 | III | 1000 | Completed | February 2008 |
| NCT00130312 | IV | 1248 | Terminated **** | March 2008 | ||
| Soften NCT01316068 | IV | 80 | Ongoing | August 2012 | ||
| Metalloproteinase inhibitor | XL784 | NCT00312780 | II | 125 | Completed | December 2007 |
| Inhibitors of epidermal growth factor ligands | TGF-α/epiregulin inhibitor LY3016859 | NCT01774981 | I and II | 64 | Ongoing | September 2015 |
| ACTH receptor | ACTH | ACTH-NRDN NCT01028287 | IV | 15 | Completed | July 2011 |
| NCT01601236 | II | 40 | Ongoing | January 2015 | ||
| DPP-4 Inhibitor | Linagliptin | NCT02376075 | III | 43 | Completed | September 2014 |
| RENALIS NCT02106104 | IV | 48 | Ongoing | May 2016 | ||
| PKCβ inhibition | LY333531 (Ruboxistaurin) | NCT00044148 | II | Not Provided | Completed | Not Provided |
| Inhibitors of AGE formation | Pyridoxamine | NCT00734253 | II | 317 | Completed | August 2010 |
| PIONEER NCT02156843 | III | 600 | Ongoing | December 2017 |
| Drug | Comparator | Months | N | Study Population | Primary Endpoint | Results |
|---|---|---|---|---|---|---|
| Paricalcitol | Placebo vs.(low and high dose of drug) | 6 | 281 | T2DM, on RAS blockade. GFR: 15–90. UACR: 100–3000 | UACR | Low dose group: −14%, High dose group: −20, Placebo group: −3%. Drug versus placebo −15% (p = 0.071) |
| Atrasentan | Placebo (vs. 3 doses of the drug) | 2 | 89 | T2DM, on RAS blockade. GFR: >20 UACR: 100–3000 | UACR | Reduction by 35%–42% versus 11% for placebo (p < 0.005) |
| Placebo (vs. 2 doses of the drug) | 3 | 211 | T2DM, on RAS blockade. GFR: 30–75 UACR: 300–3500 | UACR | Reduction by 35%–38% | |
| BAY 94-8862 (Finerenone) | Placebo (vs. 7 doses of the drug) | 3 | 821 | T2DM, on RAS blockade. GFR: 30–90 UACR: 30–300 and 300–3000 | UACR | Dose-dependently reduced UACR. Mean ratio of UACR in the two highest doses vs. placebo was 0.62 and 0.67 (p < 0.0001 either) |
| PF0489791 | Placebo | 3 | 256 | T2DM, on RAS blockade. GFR: 30–90 UACR: >300 | UACR | Significant reduction in UACR (15.7%) compared to placebo |
| CTP499 | Placebo | 12 | 177 | T2DM, on RAS blockade. GFR: 23–89. UACR: 200–5000 if male 300–5000 if female | UACR after 24 weeks | Failed to meet the primary endpoint. Serum creatinine after 48 weeks lower (mean increase in CTP499; 0.13 mg/dL versus Placebo: 0.21 mg/dL, p = 0.057) |
| Bardoxolone RTA-402 | Placebo (vs. 3 doses of the drug) | 12 | 227 | T2DM, on RAS blockade. GFR: 20–45 | GFR at 24 weeks | Significant increases in GFR, as compared with placebo (low dose group: +8. Medium dose: +11. High dose: +10 (p < 0.001). |
| CCX 140-B | Placebo | 13 | 332 | T2DM, on RAS blockade. GFR: >25 UACR: 100–3000 | UACR | Decreased albuminuria by 24% and after an initial reduction in eGFR, decreased the slope of eGFR loss |
| Pirfenidone | Placebo (vs. 2 doses of the drug) | 12 | 77 | DMT1 and T2DM, not specifically on RAS blockade. GFR: 20–75 | GFR after 1 year | Mean GFR increased in pirfenidone +3.3 whereas decreased in placebo −2.2 (p = 0.026) |
| LY2382770 Anti-TGF-β1 mAb | Placebo | 12 | 416 | DMT1 and T2DM, on RAS blockade. GFR: PCR > or equal 800 | Serum creatinine | Terminate: futility |
| Pyridoxamine | Placebo (vs. 2 doses of the drug) | 12 | 317 | T2DM, on RAS blockade. sCr 1.3–3.3 female or 1.5–3.5 male. PCR > 1200 | Serum creatinine | Failed to meet primary endpoint. Subgroup analysis: in the lowest tertile of baseline sCr, Pyridorin associated with a lower average change in serum creatinine concentration at 52 weeks (drug 1: −0.28 drug 2: 0.07 placebo: 0.14 (p = 0.05) |
2. Current Therapy for Diabetic Kidney Disease
3. Ongoing Clinical Trials
3.1. Optimizing Already Tested Approaches or Drugs
3.1.1. Vitamin D Receptor Activators
3.1.2. Endothelin Receptor Antagonists
3.1.3. Mineralocorticoid Receptor Antagonists
3.1.4. Xanthine Oxidase Inhibitors
3.1.5. Phosphodiesterase Inhibitors
3.1.6. Serotonin Receptor Antagonists
3.2. Novel Therapeutic Approaches
3.2.1. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Activators
3.2.2. Chemokine Inhibitors
3.2.3. Anti-IL-1β Antibodies
3.2.4. NFκB Inhibitors
3.2.5. Jakinibs
3.2.6. Antioxidants
3.2.7. Galectin-3 Antagonist
3.2.8. Integrin Blocker
3.2.9. Apoptosis Signal-Regulating Kinase 1 (ASK1, Mitogen-Activated Protein Kinase Kinase Kinase 5, MAP3K5) Inhibitors
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- United States Renal Data System (USRDS) 2014 Annual Data Report. Available online: http://www.usrds.org/2014/download/V2_Ch_01_ESRD_Incidence_Prevalence_14.pdf (accessed on 29 March 2015).
- de Boer, I.H.; Rue, T.C.; Hall, Y.N.; Heagerty, P.J.; Weiss, N.S.; Himmelfarb, J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011, 305, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A. Translational nephrology: What translational research is and a bird’s-eye view on translational research in nephrology. Clin. Kidney J. 2015, 8, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, B.; Ortiz, A.; Gomez-Guerrero, C.; Egido, J. Therapeutic approaches to diabetic nephropathy—Beyond the RAS. Nat. Rev. Nephrol. 2014, 10, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Available online: www.clinicaltrials.gov (accessed on 2 April 2015).
- de Boer, I.H.; Rue, T.C.; Cleary, P.A.; Lachin, J.M.; Molitch, M.E.; Steffes, M.W.; Sun, W.; Zinman, B.; Brunzell, J.D.; White, N.H.; et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: An analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch. Intern. Med. 2011, 171, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Steffes, M.; Sun, W.; Rutledge, B.; Cleary, P.; de Boer, I.H.; Zinman, B.; Lachin, J. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010, 33, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.J.; Nguyen, Q.D.; Curhan, G.; Hsu, C.Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003, 289, 3273–3277. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, E.I.; Jerums, G.; Skene, A.; Crammer, P.; Power, D.; Cheong, K.Y.; Panagiotopoulos, S.; McNeil, K.; Baker, S.T.; Fioretto, P.; et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care 2013, 36, 3620–3626. [Google Scholar] [CrossRef] [PubMed]
- Justo, P.; Sanz, A.B.; Egido, J.; Ortiz, A. 3,4-Dideoxyglucosone-3-ene induces apoptosis in renal tubular epithelial cells. Diabetes 2005, 54, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Nino, M.D.; Sanz, A.B.; Lorz, C.; Gnirke, A.; Rastaldi, M.P.; Nair, V.; Egido, J.; Ruiz-Ortega, M.; Kretzler, M.; Ortiz, A. BASP1 promotes apoptosis in diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Ito, S.; Izzo, J.L., Jr.; Januszewicz, A.; Katayama, S.; Menne, J.; Mimran, A.; Rabelink, T.J.; Ritz, E.; Ruilope, L.M.; et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N. Engl. J. Med. 2011, 364, 907–917. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014, 37, S14–S80. [Google Scholar]
- Fried, L.F.; Emanuele, N.; Zhang, J.H.; Brophy, M.; Conner, T.A.; Duckworth, W.; Leehey, D.J.; McCullough, P.A.; O’Connor, T.; Palevsky, P.M.; et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 2013, 369, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.; Schmieder, R.E.; McQueen, M.; Dyal, L.; Schumacher, H.; Pogue, J.; Wang, X.; Maggioni, A.; Budaj, A.; Chaithiraphan, S.; et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 2008, 372, 547–553. [Google Scholar] [CrossRef]
- Gentile, G.; Remuzzi, G.; Ruggenenti, P. Dual renin-angiotensin system blockade for nephroprotection: Still under scrutiny. Nephron 2015, 129, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Esteras, R.; Perez-Gomez, M.V.; Rodriguez-Osorio, L.; Ortiz, A.; Fernandez-Fernandez, B. Combination use of medicines from two classes of RAS blocking agents: Risk of hyperkalaemia, hypotension, and impaired renal function. Ther. Adva. Drug Saf. (accepted).
- Raval, A.D.; Thakker, D.; Rangoonwala, A.N.; Gor, D.; Walia, R. Vitamin B and its derivatives for diabetic kidney disease. Cochrane Database Syst. Rev. 2015, 1. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: http://www.clinicaltrials.gov (accessed on 29 March 2015).
- Lewis, E.J.; Greene, T.; Spitalewiz, S.; Blumenthal, S.; Berl, T.; Hunsicker, L.G.; Pohl, M.A.; Rohde, R.D.; Raz, I.; Yerushalmy, Y.; et al. Pyridorin in type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 2012, 23, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gonzalez, J.F.; Muros, M.; Mora-Fernandez, C.; Herrera, H.; Meneses, B.; Garcia, J. Pentoxifylline for renoprotection in diabetic nephropathy: The PREDIAN study. Rationale and basal results. J. Diabetes Complicat. 2011, 25, 314–319. [Google Scholar] [CrossRef] [PubMed]
- de Zeeuw, D.; Agarwal, R.; Amdahl, M.; Audhya, P.; Coyne, D.; Garimella, T.; Parving, H.H.; Pritchett, Y.; Remuzzi, G.; Ritz, E.; et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 2010, 376, 1543–1551. [Google Scholar] [CrossRef]
- Kohan, D.E.; Pritchett, Y.; Molitch, M.; Wen, S.; Garimella, T.; Audhya, P.; Andress, D.L. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am. Soc. Nephrol. 2011, 22, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Lewis, J.B.; Greene, T.; Hunsicker, L.G.; Berl, T.; Pohl, M.A.; de Zeeuw, D.; Heerspink, H.L.; Rohde, R.D.; Atkins, R.C.; et al. Sulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: A randomized controlled trial. Am. J. Kidney Dis. 2011, 58, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Tumlin, J.A.; Galphin, C.M.; Rovin, B.H. Advanced diabetic nephropathy with nephrotic range proteinuria: A pilot study of the long-term efficacy of subcutaneous ACTH gel on proteinuria, progression of CKD, and urinary levels of VEGF and MCP-1. J. Diabetes Res. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Bakris, G.L.; Toto, R.D.; McGill, J.B.; Hu, K.; Anderson, P.W. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 2005, 28, 2686–2690. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O.; et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014, 311, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gomez, M.V.; Ortiz-Arduan, A.; Lorenzo-Sellares, V. Vitamin D and proteinuria: A critical review of molecular bases and clinical experience. Nefrologia 2013, 33, 716–726. [Google Scholar] [PubMed]
- Sanchez-Nino, M.D.; Bozic, M.; Cordoba-Lanus, E.; Valcheva, P.; Gracia, O.; Ibarz, M.; Fernandez, E.; Navarro-Gonzalez, J.F.; Ortiz, A.; Valdivielso, J.M. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2012, 302, F647–F657. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Nino, M.D.; Sanz, A.B.; Carrasco, S.; Saleem, M.A.; Mathieson, P.W.; Valdivielso, J.M.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Globotriaosylsphingosine actions on human glomerular podocytes: Implications for Fabry nephropathy. Nephrol. Dial. Transplant. 2011, 26, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, P.; Patel, N.A.; Peterson, C.; Bills, J.E.; Bekele, D.M.; Bunaye, Z.; Light, R.P.; Agarwal, R. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: A randomized double-blind pilot trial. Hypertension 2008, 52, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Chittineni, H.; Packman, M.; Dutka, P.; Ali, N.; Durie, N. Oral paricalcitol in the treatment of patients with CKD and proteinuria: A randomized trial. Am. J. Kidney Dis. 2009, 54, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Rojas-Rivera, J.; Polanco, N.; Morales, E.; Morales, J.M.; Egido, J.; Amado, A.; Praga, M. Effects of oral paricalcitol on secondary hyperparathyroidism and proteinuria of kidney transplant patients. Transplantation 2013, 95, e49–e52. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Sanchez-Nino, M.D.; Rojas, J.; Egido, J. Paricalcitol for reduction of albuminuria in diabetes. Lancet 2011, 377, 635–636. [Google Scholar] [CrossRef]
- Gomez-Garre, D.; Largo, R.; Liu, X.H.; Gutierrez, S.; Lopez-Armada, M.J.; Palacios, I.; Egido, J. An orally active ETA/ETB receptor antagonist ameliorates proteinuria and glomerular lesions in rats with proliferative nephritis. Kidney Int. 1996, 50, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Garre, D.; Largo, R.; Tejera, N.; Fortes, J.; Manzarbeitia, F.; Egido, J. Activation of NF-kappaB in tubular epithelial cells of rats with intense proteinuria: Role of angiotensin II and endothelin-1. Hypertension 2001, 37, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vita, J.; Ruiz-Ortega, M.; Ruperez, M.; Esteban, V.; Sanchez-Lopez, E.; Plaza, J.J.; Egido, J. Endothelin-1, via ETA receptor and independently of transforming growth factor-beta, increases the connective tissue growth factor in vascular smooth muscle cells. Circ. Res. 2005, 97, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.; Green, D.; Jamerson, K.; Ruilope, L.M.; Kuranoff, S.J.; Littke, T.; Viberti, G.; ASCEND Study Group. Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 527–535. [Google Scholar] [CrossRef] [PubMed]
- de Zeeuw, D.; Coll, B.; Andress, D.; Brennan, J.J.; Tang, H.; Houser, M.; Correa-Rotter, R.; Kohan, D.; Lambers Heerspink, H.J.; Makino, H.; et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Brem, A.S.; Morris, D.J.; Gong, R. Aldosterone-induced fibrosis in the kidney: Questions and controversies. Am. J. Kidney Dis. 2011, 58, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Ruilope, L.M.; Agarwal, R.; Chan, J.C.; Cooper, M.E.; Gansevoort, R.T.; Haller, H.; Remuzzi, G.; Rossing, P.; Schmieder, R.E.; Nowack, C.; et al. Rationale, design, and baseline characteristics of ARTS-DN: A randomized study to assess the safety and efficacy of finerenone in patients with type 2 diabetes mellitus and a clinical diagnosis of diabetic nephropathy. Am. J. Nephrol. 2014, 40, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Rossing, K.; Schjoedt, K.J.; Smidt, U.M.; Boomsma, F.; Parving, H.H. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: A randomized, double-masked, cross-over study. Diabetes Care 2005, 28, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Siwy, J.; Schanstra, J.P.; Argiles, A.; Bakker, S.J.; Beige, J.; Boucek, P.; Brand, K.; Delles, C.; Duranton, F.; Fernandez-Fernandez, B.; et al. Multicentre pective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diaprosbetic nephropathy. Nephrol. Dial. Transplant. 2014, 29, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- European Union Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention Of early diabetic nephRopathy In TYpe 2 diabetic patients with normoalbuminuria (PRIORITY). Available online: www.eu-priority.org/ (accessed on 29 March 2015).
- Epstein, M. Mineralocorticoid receptor antagonists: Part of an emerging treatment paradigm for chronic kidney disease. Lancet Diabetes Endocrinol. 2014, 2, 925–927. [Google Scholar] [CrossRef]
- Bakris, G.; Nowack, C.; Ruilope, L.M. Results of ARTS-DN: A Randomized Study to Assess the Safety and Efficacy of Finerenone in Patients with Type 2 Diabetes and Diabetic Nephropahty; World Congress of Nephrology: Cape Town, South Africa, 2015. [Google Scholar]
- Ficociello, L.H.; Rosolowsky, E.T.; Niewczas, M.A.; Maselli, N.J.; Weinberg, J.M.; Aschengrau, A.; Eckfeldt, J.H.; Stanton, R.C.; Galecki, A.T.; Doria, A.; et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: Results of a 6-year follow-up. Diabetes Care 2010, 33, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincon, A.; Arroyo, D.; Luno, J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. 2010, 5, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Maahs, D.M.; Caramori, L.; Cherney, D.Z.; Galecki, A.T.; Gao, C.; Jalal, D.; Perkins, B.A.; Pop-Busui, R.; Rossing, P.; Mauer, M.; et al. Uric acid lowering to prevent kidney function loss in diabetes: The preventing early renal function loss (PERL) allopurinol study. Curr. Diab. Rep. 2013, 13, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Kimura, K.; Itoh, S.; Inaba, M.; Uchida, S.; Tomino, Y.; Makino, H.; Matsuo, S.; Yamamoto, T.; Ohno, I.; et al. The effect of febuxostat to prevent a further reduction in renal function of patients with hyperuricemia who have never had gout and are complicated by chronic kidney disease stage 3: Study protocol for a multicenter randomized controlled study. Trials 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Omidvar, B.; Beladi-Mousavi, S.S.; Lak, E.; Vaziri, S. The effect of pentoxifylline on reduction of proteinuria among patients with type 2 diabetes under blockade of angiotensin system: A double blind and randomized clinical trial. Nefrologia 2012, 32, 790–796. [Google Scholar] [PubMed]
- Navarro-Gonzalez, J.F.; Mora-Fernandez, C.; Muros de Fuentes, F.M.; Chahin, J.; Mendez, M.L.; Gallego, E.; Macia, M.; del Castillo, C.N.; Rivero, A.; Getino, M.A.; et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: The PREDIAN trial. J. Am. Soc. Nephrol. 2015, 26, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Bridson, G.; Ke, J.; Wu, L.; Erol, H.; Graham, P.; Lin, C.H.; Braman, V.; Zhao, H.; Liu, J.F.; et al. Quantitative analyses of CTP-499 and five major metabolites by core-structure analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 963, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Diamond, S.A.; Pergola, S.E.; Shipley, J.E.; Wu, L.; Sabounjian, L.A.; Graham, P.B. Effect of CTP-499 on renal function in patients with type 2 diabetes and kidney disease. Am. J. Kidney Dis. 2014, 63, A1–A120. [Google Scholar]
- Kidney Week. Selective inhibition of phosphodiesterase type 5 reduces macroalbuminuira in subjects with type 2 diabetes, and overt nephropathy. In Proceedings of the Kidney Week 2014: American Society of Nephrology Annual Meeting, Philadelphia, PA, USA, 11–16 November 2014.
- Park, S.Y.; Rhee, S.Y.; Oh, S.; Kwon, H.S.; Cha, B.Y.; Lee, H.J.; Lee, H.C.; Kim, Y.S. Evaluation of the effectiveness of sarpogrelate on the surrogate markers for macrovascular complications in patients with type 2 diabetes. Endocr. J. 2012, 59, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Kasho, M.; Sakai, M.; Sasahara, T.; Anami, Y.; Matsumura, T.; Takemura, T.; Matsuda, H.; Kobori, S.; Shichiri, M. Serotonin enhances the production of type IV collagen by human mesangial cells. Kidney Int. 1998, 54, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Satoh, M.; Namikoshi, T.; Haruna, Y.; Fujimoto, S.; Arakawa, S.; Komai, N.; Tomita, N.; Sasaki, T.; Kashihara, N. Blockade of serotonin 2A receptor improves glomerular endothelial function in rats with streptozotocin-induced diabetic nephropathy. Clin. Exp. Nephrol. 2008, 12, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kanai, H.; Hiromura, K.; Kuroiwa, T.; Maezawa, A.; Yano, S.; Naruse, T. Role of serotonin in nephrotoxic serum nephritis in WKY rats. J. Lab. Clin. Med. 1997, 129, 557–566. [Google Scholar] [CrossRef]
- Hamasaki, Y.; Doi, K.; Maeda-Mamiya, R.; Ogasawara, E.; Katagiri, D.; Tanaka, T.; Yamamoto, T.; Sugaya, T.; Nangaku, M.; Noiri, E. A 5-hydroxytryptamine receptor antagonist, sarpogrelate, reduces renal tubulointerstitial fibrosis by suppressing PAI-1. Am. J. Physiol. Renal. Physiol. 2013, 305, F1796–F1803. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- de Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Corna, D.; Nava, V.; Locatelli, M.; Abbate, M.; Gaspari, F.; Carrara, F.; Sangalli, F.; Remuzzi, G.; Benigni, A. Analogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am. J. Physiol. Renal. Physiol. 2013, 304, F808–F819. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Miller, D.H.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.; Kita, M.; Yang, M.; Raghupathi, K.; Novas, M.; Sweetser, M.T.; et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 2012, 367, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Moreno, S.; Rubio-Navarro, A.; Sastre, C.; Blanco-Colio, L.M.; Gomez-Guerrero, C.; Ortiz, A.; Egido, J. Targeting chemokines in proteinuria-induced renal disease. Expert Opin. Ther. Targets 2012, 16, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, S.G.; Ryu, M.; Kulkarni, O.P.; Schmid, H.; Lichtnekert, J.; Gruner, S.; Green, L.; Mattei, P.; Hartmann, G.; Anders, H.J. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 2011, 80, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.; Miao, Z.; Dairaghi, D.J.; Krasinski, A.; Wang, Y.; Zhao, B.N.; Baumgart, T.; Ertl, L.S.; Pennell, A.; Seitz, L.; et al. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am. J. Physiol. Renal. Physiol. 2013, 305, F1288–F1297. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.J.; Miao, Z.; Zhao, B.N.; Ertl, L.S.; Wang, Y.; Krasinski, A.; Walters, M.J.; Powers, J.P.; Dairaghi, D.J.; Baumgart, T.; et al. Experimental evidence for the use of CCR2 antagonists in the treatment of type 2 diabetes. Metabolism 2013, 62, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- ChemoCentryx. Available online: http://ir.chemocentryx.com/releasedetail.cfm?ReleaseID=887402 (accessed on 29 March 2015).
- Blech, M.; Peter, D.; Fischer, P.; Bauer, M.M.; Hafner, M.; Zeeb, M.; Nar, H. One target-two different binding modes: Structural insights into gevokizumab and canakinumab interactions to interleukin-1beta. J. Mol. Biol. 2013, 425, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Issafras, H.; Corbin, J.A.; Goldfine, I.D.; Roell, M.K. Detailed mechanistic analysis of gevokizumab, an allosteric anti-IL-1beta antibody with differential receptor-modulating properties. J. Pharmacol. Exp. Ther. 2014, 348, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.M. Antibodies to watch in 2015. MAbs 2015, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- European Union Clinical Trials Register. Available online: www.clinicaltrialsregister.eu (accessed on 29 March 2015).
- Sanz, A.B.; Sanchez-Nino, M.D.; Ramos, A.M.; Moreno, J.A.; Santamaria, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. NF-kappaB in renal inflammation. J. Am. Soc. Nephrol. 2010, 21, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Mora, E.; Guglielmotti, A.; Biondi, G.; Sassone-Corsi, P. Bindarit: An anti-inflammatory small molecule that modulates the NFkappaB pathway. Cell Cycle 2012, 11, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Ble, A.; Mosca, M.; Di Loreto, G.; Guglielmotti, A.; Biondi, G.; Bombardieri, S.; Remuzzi, G.; Ruggenenti, P. Antiproteinuric effect of chemokine C-C motif ligand 2 inhibition in subjects with acute proliferative lupus nephritis. Am. J. Nephrol. 2011, 34, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P. Effects of MCP-1 inhibition by bindarit therapy in type 2 diabetes subjects with micro- or macro-albuminuria. J. Am. Soc. Nephrol. 2010, 21 (Suppl. 1), 44A. [Google Scholar]
- Berthier, C.C.; Zhang, H.; Schin, M.; Henger, A.; Nelson, R.G.; Yee, B.; Boucherot, A.; Neusser, M.A.; Cohen, C.D.; Carter-Su, C.; et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 2009, 58, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Sanchez, R.; Berzal, S.; Sanchez-Nino, M.D.; Neria, F.; Goncalves, S.; Calabia, O.; Tejedor, A.; Calzada, M.J.; Caramelo, C.; Deudero, J.J.; et al. AG490 promotes HIF-1alpha accumulation by inhibiting its hydroxylation. Curr. Med. Chem. 2012, 19, 4014–4023. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Suzuki, N.; van Ypersele de Strihou, S.C. Diabetic nephropathy: Are there new and potentially promising therapies targeting oxygen biology? Kidney Int. 2013, 84, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Banes, A.K.; Shaw, S.; Jenkins, J.; Redd, H.; Amiri, F.; Pollock, D.M.; Marrero, M.B. Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. Am. J. Physiol. Renal Physiol. 2004, 286, F653–F659. [Google Scholar] [CrossRef] [PubMed]
- Taira, M.; Inaba, M.; Takada, K.; Baba, S.; Fukui, J.; Ueda, Y.; Kwon, A.H.; Hisha, H.; Kamiyama, Y.; Ikehara, S. Treatment of streptozotocin-induced diabetes mellitus in rats by transplantation of islet cells from two major histocompatibility complex disparate rats in combination with intra bone marrow injection of allogeneic bone marrow cells. Transplantation 2005, 79, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Munoz, G.; Lopez-Parra, V.; Lopez-Franco, O.; Fernandez-Vizarra, P.; Mallavia, B.; Flores, C.; Sanz, A.; Blanco, J.; Mezzano, S.; Ortiz, A.; et al. Suppressors of cytokine signaling abrogate diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Genovese, M.; Keystone, E.; Schlichting, D.; Beattie, S.; Macias, W. Baricitinib, an oraljanus kinase inhibitor, in the treatment of rheumatoid arthritis: Safety and efficacy in an open-label, long-term extension study. Ann. Rheum. Dis. 2014, 73. [Google Scholar] [CrossRef]
- Dang, Z.; MacKinnon, A.; Marson, L.P.; Sethi, T. Tubular atrophy and interstitial fibrosis after renal transplantation is dependent on galectin-3. Transplantation 2012, 93, 477–484. [Google Scholar] [CrossRef] [PubMed]
- de Boer, R.A.; Lok, D.J.; Jaarsma, T.; van der Meer, P.; Voors, A.A.; Hillege, H.L.; van Veldhuisen, D.J. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2011, 43, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Bertocchi, A.P.; Campanhole, G.; Wang, P.H.; Goncalves, G.M.; Damiao, M.J.; Cenedeze, M.A.; Beraldo, F.C.; de Paula, A.T.; Dos Reis, M.A.; Mazzali, M.; et al. A Role for galectin-3 in renal tissue damage triggered by ischemia and reperfusion injury. Transpl. Int. 2008, 21, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.T.; Hughes, J.; Sethi, T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 2008, 172, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Karpf, R.J. Effects of emotions on altruism and social inference in retarded adolescents. Psychol. Rep. 1977, 41, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Kobayashi, S.; Hemmi, N.; Ikee, R.; Hyodo, N.; Saigusa, T.; Namikoshi, T.; Yamada, M.; Suzuki, S.; Miura, S. Galectin-3-positive cell infiltration in human diabetic nephropathy. Nephrol. Dial. Transplant. 2004, 19, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Delgado, G.; Wanner, C.; Blouin, K.; Pilz, S.; Tomaschitz, A.; Kleber, M.E.; Dressel, A.; Willmes, C.; Krane, V.; et al. Galectin-3, renal function, and clinical outcomes: Results from the LURIC and 4D Studies. J. Am. Soc. Nephrol. 2015. [Google Scholar] [CrossRef]
- Iacobini, C.; Oddi, G.; Menini, S.; Amadio, L.; Ricci, C.; Di Pippo, C.; Sorcini, M.; Pricci, F.; Pugliese, F.; Pugliese, G. Development of age-dependent glomerular lesions in galectin-3/AGE-receptor-3 knockout mice. Am. J. Physiol. Renal Physiol. 2005, 289, F611–F621. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Pricci, F.; Iacobini, C.; Leto, G.; Amadio, L.; Barsotti, P.; Frigeri, L.; Hsu, D.K.; Vlassara, H.; Liu, F.T.; et al. Accelerated diabetic glomerulopathy in galectin-3/AGE receptor 3 knockout mice. FASEB J. 2001, 15, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; Sonnenberg, A. Cell-matrix adhesion of podocytes in physiology and disease. Nat. Rev. Nephrol 2013, 9, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Gingras, D.; Bendayan, M. Alterations of vitronectin and its receptor alpha(v) integrin in the rat renal glomerular wall during diabetes. Am. J. Kidney Dis. 2001, 38, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.H.; Pedigo, C.E.; Guzman, J.; Correa-Medina, M.; Wei, C.; Villarreal, R.; Mitrofanova, A.; Leclercq, F.; Faul, C.; Li, J.; et al. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J. Am. Soc. Nephrol. 2015, 26, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Maile, L.A.; Busby, W.H.; Gollahon, K.A.; Flowers, W.; Garbacik, N.; Garbacik, S.; Stewart, K.; Nichols, T.; Bellinger, D.; Patel, A.; et al. Blocking ligand occupancy of the αVβ3 integrin inhibits the development of nephropathy in diabetic pigs. Endocrinology 2014, 155, 4665–4675. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Naguro, I.; Runchel, C.; Ichijo, H. The roles of ASK family proteins in stress responses and diseases. Cell Commun. Signal. 2009, 7. [Google Scholar] [CrossRef] [PubMed]
- Kawarazaki, Y.; Ichijo, H.; Naguro, I. Apoptosis signal-regulating kinase 1 as a therapeutic target. Expert Opin. Ther. Targets 2014, 18, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, Y.; Gu, H.; Reece, E.A.; Fang, S.; Gabbay-Benziv, R.; Aberdeen, G.; Yang, P. Ask1 gene deletion blocks maternal diabetes-induced endoplasmic reticulum stress in the developing embryo by disrupting the unfolded protein response signalosome. Diabetes 2015, 64, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, T.; Fukuo, K.; Yasuda, O.; Hotta, M.; Miyazaki, J.; Takemura, Y.; Kawamoto, H.; Ichijo, H.; Ogihara, T. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes 2006, 55, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Takeda, K.; Kadowaki, H.; Ueda, I.; Namba, Y.; Ouchi, Y.; Nishitoh, H.; Ichijo, H. Involvement of ASK1-p38 pathway in the pathogenesis of diabetes triggered by pancreatic β cell exhaustion. Biochim. Biophys. Acta 2013, 1830, 3656–3663. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Gomez, M.V.; Sanchez-Niño, M.D.; Sanz, A.B.; Martín-Cleary, C.; Ruiz-Ortega, M.; Egido, J.; Navarro-González, J.F.; Ortiz, A.; Fernandez-Fernandez, B. Horizon 2020 in Diabetic Kidney Disease: The Clinical Trial Pipeline for Add-On Therapies on Top of Renin Angiotensin System Blockade. J. Clin. Med. 2015, 4, 1325-1347. https://doi.org/10.3390/jcm4061325
Perez-Gomez MV, Sanchez-Niño MD, Sanz AB, Martín-Cleary C, Ruiz-Ortega M, Egido J, Navarro-González JF, Ortiz A, Fernandez-Fernandez B. Horizon 2020 in Diabetic Kidney Disease: The Clinical Trial Pipeline for Add-On Therapies on Top of Renin Angiotensin System Blockade. Journal of Clinical Medicine. 2015; 4(6):1325-1347. https://doi.org/10.3390/jcm4061325
Chicago/Turabian StylePerez-Gomez, Maria Vanessa, Maria Dolores Sanchez-Niño, Ana Belen Sanz, Catalina Martín-Cleary, Marta Ruiz-Ortega, Jesus Egido, Juan F. Navarro-González, Alberto Ortiz, and Beatriz Fernandez-Fernandez. 2015. "Horizon 2020 in Diabetic Kidney Disease: The Clinical Trial Pipeline for Add-On Therapies on Top of Renin Angiotensin System Blockade" Journal of Clinical Medicine 4, no. 6: 1325-1347. https://doi.org/10.3390/jcm4061325






