Can Characteristics of Reciprocal Translocations Predict the Chance of Transferable Embryos in PGD Cycles?
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Subjects
2.2. Chromosomal Analysis and Pachytene Shape Statistics
| Variable | Categories/Definition | Example |
|---|---|---|
| Pachytene-diagram a | Translocation predisposed to the adjacent-1 or adjacent-2 type disjunction |  |
| Translocation predisposed to tertiary monosomy or trisomy (3:1 segregation) | 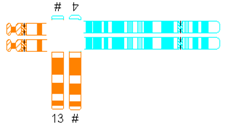 | |
| Translocation segregating by either adjacent-1 or 3:1 type |  | |
| Quadrivalent b | Fairly symmetric quadrivalent | 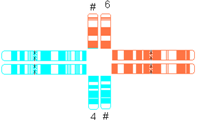 |
| One very small translocated segment | 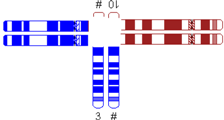 | |
| Both translocated segments very small |  | |
| One very small translocated segment and one very small nontranslocated segment | 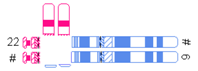 | |
| Location of breakpoints | Both in the centromeric region | |
| Both in the middle of the chromosomal arm | ||
| Both in the telomeric region | ||
| Other combination | ||
| Ratio of chromosomal length (cm) | Length of largest translocation chromosome divided by length of smallest translocation chromosome (A/B) | 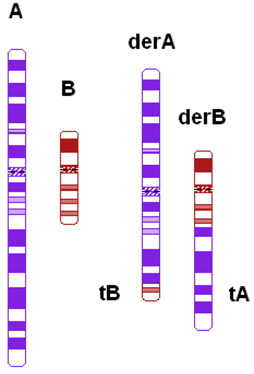 |
| Ratio of chromosomal length with correction (cm) | Abovementioned variable with correction for heterochromatine (in chromosome 1, 9, and 16) and acrosomes | |
| Ratio of the relative sizes of both translocated segments c | Ratio of the relative size of one translocated segment (tA/A) and the relative size of the other translocated segment (tB/B) in such a way that the largest relative size is divided by the smallest relative size (tA/A)/(tB/B) | |
| Total length of the translocated segments c | tA + tB |
2.3. IVF and PGD
2.4. Statistical Analysis
3. Results and Discussion
| Embryo Results per Couple | Reciprocal Translocations | Robertsonian Translocations |
|---|---|---|
| 85 Couples | 35 Couples | |
| Median (Interquartile Range)/Couple | Median (Interquartile Range)/Couple | |
| {Total Number of Embryos} | {Total Number of Embryos} | |
| Number of biopsied embryos | 16 (17) {1527} | 12 (8) {503} |
| Number of balanced embryos | 2 (2) {194} | 3 (4) {123} |
| Number of embryos with inconclusive results | 1 (3) {142} | 1 (2) {48} |
| Number of unbalanced embryos | 12 (14) {1169} | 7 (8) {325} |
| Number of excluded embryos a | {22} | {7} |
| Percentage of balanced embryos | 11.43 (12.45) | 22.22 (22.84) |
| Variable | N | Median % Balanced Embryos | p-Value a |
|---|---|---|---|
| Type of Translocation | |||
| Reciprocal | 85 | 11.43 | <0.001 |
| Robertsonian Symmetrical | 27 | 28.00 | ref |
| Robertsonian asymmetrical | 8 | 15.59 | 0.002 |
| Carrier | |||
| Female | 41 | 11.43 | ref |
| Male | 44 | 11.81 | 0.383 |
| Pachytene-Diagram | |||
| Adjacent-1 or 2 | 48 | 12.50 | ref |
| 3:1 | 14 | 12.50 | 0.534 |
| Adjacent-1 and 3:1 | 23 | 10.00 | 0.846 |
| Quadrivalent | |||
| Symmetrical | 23 | 11.11 | ref |
| 1 small translocated segment | 22 | 13.03 | 0.354 |
| Both translocated segments small | 30 | 13.39 | 0.753 |
| 1 small translocated segment en 1 small nontranslocated segment | 10 | 2.27 | 0.109 |
| Location of Breakpoints | |||
| Both in centromeric region | 11 | 12.50 | ref |
| Both in middle of arm | 3 | 7.69 | 0.35 |
| Both in telomeric region | 27 | 13.56 | 0.697 |
| Other combination | 44 | 11.11 | 0.316 |
| Ratio of chromosomal length (cm) | 85 | 11.43 | 0.776 |
| Ratio of chromosomal length with correction (cm) | 85 | 11.43 | 0.505 |
| Ratio of the relative sizes of both translocated segments | 85 | 11.43 | 0.048 |
| Total length of the translocated segments (Mb) | 85 | 11.43 | 0.561 |
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgements
Conflicts of interest
References
- Scriven, P.; Flinter, F.; Khalaf, Y.; Lashwood, A.; Mackie Ogilvie, C. Benefits and drawbacks of preimplantation genetic diagnosis (PGD) for reciprocal translocations: Lessons from a prospective cohort study. Eur. J. Hum. Genet. 2013, 21, 1035–1041. [Google Scholar] [CrossRef]
- Peng, H.; Chao, A.; Wang, T.; Chang, Y.; Chang, S. Prenatally diagnosed balanced chromosome rearrangements: Eight years’ experience. J. Reprod. Med. 2006, 51, 699–703. [Google Scholar]
- Hook, E.B.; Hamerton, J.L. The Frequency of Chromosome Abnormalities in Consecutive Newborn Studies-Differences between Studies-Results by Sex and by Severity of Phenotypic Involvement. In Population Genetics; Hook, E.B., Porter, L.H., Eds.; Academic Press: New York, NY, USA, 1977; pp. 66–79. [Google Scholar]
- Vozdova, M.; Oracova, E.; Kasikova, K.; Prinosilova, P.; Rybar, R.; Horinova, V.; Gaillyova, R.; Rubes, J. Balanced chromosomal translocations in men: Relationships among semen parameters, chromatin integrity, sperm meiotic segregation and aneuploidy. J. Assist. Reprod. Genet. 2013, 30, 391–405. [Google Scholar] [CrossRef]
- Munné, S.; Morrison, L.; Fung, J.; Marquez, C.; Weier, U.; Bahe, M.; Sable, D.; Grundfeld, L.; Schoolcraft, B.; Scott, R.; et al. Spontaneous abortions are reduced after preconception diagnosis of translocations. J. Assist. Reprod. Genet. 1998, 15, 290–296. [Google Scholar] [CrossRef]
- Munné, S.; Sandalinas, M.; Escudero, T.; Fung, J.; Gianaroli, L.; Cohen, J. Outcome of preimplantation genetic diagnosis of translocations. Fertil. Steril. 2000, 73, 1209–1218. [Google Scholar] [CrossRef]
- Otani, T.; Roche, M.; Mizuike, M.; Colls, P.; Escudero, T.; Munné, S. Preimplantation genetic diagnosis significantly improves the pregnancy outcome of translocation carriers with a history of recurrent miscarriage and unsuccessful pregnancies. Reprod. Biomed. Online 2006, 13, 869–874. [Google Scholar] [CrossRef]
- Franssen, M.T.M.; Musters, A.M.; van der Veen, F.; Repping, S.; Leschot, N.J.; Bossuyt, P.M.M.; Goddijn, M.; Korevaar, J.C. Reproductive outcome after PGD in couples with recurrent miscarriage carrying a structural chromosome abnormality: A systematic review. Hum. Reprod. Update 2011, 17, 467–475. [Google Scholar] [CrossRef]
- Ko, D.; Cho, J.; Park, S.; Kim, J.; Koong, M.; Song, I.; Kang, I.; Lim, C. Clinical outcomes of preimplantation genetic diagnosis (PGD) and analysis of meiotic segregation modes in reciprocal translocation carriers. Am. J. Med. Genet. 2010, 152, 1428–1433. [Google Scholar]
- Harper, J.C.; Wilton, L.; Traeger Synodinos, J.; Goossens, V.; Moutou, C.; SenGupta, S.B.; Pehlivan Budak, T.; Renwick, P.; de Rycke, M.; Geraedts, J.P.M.; et al. The ESHRE PGD Consortium: 10 Years of data collection. Hum. Reprod. Update 2012, 18, 234–247. [Google Scholar] [CrossRef]
- Sermon, K.; van-Steirteghem, A.; Liebaers, I. Preimplantation genetic diagnosis. Lancet 2004, 363, 1633–1641. [Google Scholar] [CrossRef]
- Scriven, P.N.; Handyside, A.H.; Ogilvie, C.M. Chromosome translocations: Segregation modes and strategies for preimplantation genetic diagnosis. Prenat. Diagn. 1998, 18, 1437–1449. [Google Scholar] [CrossRef]
- Gianaroli, L.; Magli, M.C.; Ferraretti, A.P.; Munné, S.; Balicchia, B.; Escudero, T.; Crippa, A. Possible interchromosomal effect in embryos generated by gametes from translocation carriers. Hum. Reprod. 2002, 17, 3201–3207. [Google Scholar]
- Anton, E.; Vidal, F.; Blanco, J. Reciprocal translocations: Tracing their meiotic behavior. Genet. Med. 2008, 10, 730–738. [Google Scholar]
- Jalbert, P.; Sele, B.; Jalbert, H. Reciprocal translocations: A way to predict the mode of imbalanced segregation by pachytene-diagram drawing. Hum. Genet. 1980, 55, 209–222. [Google Scholar] [CrossRef]
- Gardner, R.J.M.; Sutherland, G.R. Chromosome Abnormalities and Genetic Counseling; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Cohen, O.; Cans, C.; Mermet, M.A.; Demongeot, J.; Jalbert, P. Viability thresholds for partial trisomies and monosomies. A study of 1159 viable unbalanced reciprocal translocations. Hum. Genet. 1994, 93, 188–194. [Google Scholar]
- Daniel, A.; Hook, E.B.; Wulf, G. Risks of unbalanced progeny at amniocentesis to carriers of chromosome rearrangements: Data from United States and Canadian laboratories. Am. J. Med. Genet. 1989, 33, 14–53. [Google Scholar] [CrossRef]
- Midro, A.T.; Stengel Rutkowski, S.; Stene, J. Experiences with risk estimates for carriers of chromosomal reciprocal translocations. Clin. Genet. 1992, 41, 113–122. [Google Scholar]
- Mackie, O.C.; Scriven, P. Meiotic outcomes in reciprocal translocation carriers ascertained in 3-day human embryos. Eur. J. Hum. Genet. 2002, 10, 801–806. [Google Scholar] [CrossRef]
- Lim, C.; Cho, J.; Song, I.; Kang, I.; Yoon, Y.; Jun, J. Estimation of chromosomal imbalances in preimplantation embryos from preimplantation genetic diagnosis cycles of reciprocal translocations with or without acrocentric chromosomes. Fertil. Steril. 2008, 90, 2144–2151. [Google Scholar] [CrossRef]
- Lled, B.; Ortiz, J.; Morales, R.; Ten, J.; de la Fuente, P.E.; Garca-Ochoa, C.; Bernabeu, R. The paternal effect of chromosome translocation carriers observed from meiotic segregation in embryos. Hum. Reprod. 2010, 25, 1843–1848. [Google Scholar] [CrossRef]
- Kent, W.J.; Sugnet, C.; Furey, T.; Roskin, K.; Pringle, T.; Zahler, A.; Haussler, D. The human genome browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef]
- International Standing Committee on Human Cytogenetic Nomenclature (ISCN). ISCN (2005): An International System for Human Cytogenetic Nomenclature, Karger, S. Inc.: Basel, Switzerland, 2005.
- Harton, G.L.; Harper, J.C.; Coonen, E.; Pehlivan, T.; Vesela, K.; Wilton, L. ESHRE PGD consortium best practice guidelines for fluorescence in situ hybridization-based PGD. Hum. Reprod. 2011, 26, 25–32. [Google Scholar] [CrossRef]
- Coonen, E.; Hopman, A.H.; Geraedts, J.P.; Ramaekers, F.C. Application of in-situ hybridization techniques to study human preimplantation embryos: A review. Hum. Reprod. Update 1998, 4, 135–152. [Google Scholar] [CrossRef]
- Goossens, V.; Traeger-Synodinos, J.; Coonen, E.; de Rycke, M.; Moutou, C.; Pehlivan, T.; Derks-Smeets, I.A.; Harton, G. ESHRE PGD Consortium data collection XI: Cycles from January to December 2008 with pregnancy follow-up to October 2009. Hum. Reprod. 2012, 27, 1887–1911. [Google Scholar] [CrossRef]
- Keymolen, K.; van Berkel, K.; Vorsselmans, A.; Staessen, C.; Liebaers, I. Pregnancy outcome in carriers of Robertsonian translocations. Am. J. Med. Genet. 2011, 155, 2381–2385. [Google Scholar] [CrossRef]
- Ko, D.S.; Cho, J.W.; Lee, H.S.; Kim, J.Y.; Kang, I.S.; Yang, K.M.; Lim, C.K. Preimplantation genetic diagnosis outcomes and meiotic segregation analysis of Robertsonian translocation carriers. Fertil. Steril. 2013, 99, 1369–1376. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dul, E.; Van Echten-Arends, J.; Groen, H.; Kastrop, P.; Wissen, L.A.-v.; Engelen, J.; Land, J.; Coonen, E.; Van Ravenswaaij-Arts, C. Can Characteristics of Reciprocal Translocations Predict the Chance of Transferable Embryos in PGD Cycles? J. Clin. Med. 2014, 3, 348-358. https://doi.org/10.3390/jcm3020348
Dul E, Van Echten-Arends J, Groen H, Kastrop P, Wissen LA-v, Engelen J, Land J, Coonen E, Van Ravenswaaij-Arts C. Can Characteristics of Reciprocal Translocations Predict the Chance of Transferable Embryos in PGD Cycles? Journal of Clinical Medicine. 2014; 3(2):348-358. https://doi.org/10.3390/jcm3020348
Chicago/Turabian StyleDul, Elsbeth, Jannie Van Echten-Arends, Henk Groen, Peter Kastrop, Lucie Amory-van Wissen, John Engelen, Jolande Land, Edith Coonen, and Conny Van Ravenswaaij-Arts. 2014. "Can Characteristics of Reciprocal Translocations Predict the Chance of Transferable Embryos in PGD Cycles?" Journal of Clinical Medicine 3, no. 2: 348-358. https://doi.org/10.3390/jcm3020348




