Using Electric Stimulation of the Spinal Muscles and Electromyography during Motor Tasks for Evaluation of the Role in Development and Progression of Adolescent Idiopathic Scoliosis
Abstract
:1. Introduction
1.1. Electromyography (EMG)
1.2. Transcutanous Electric Stimulation
1.3. The Current Standard Practices for AIS Prediction and Treatment
1.4. The Role of Muscle Imbalance in the Development and Progression of AIS
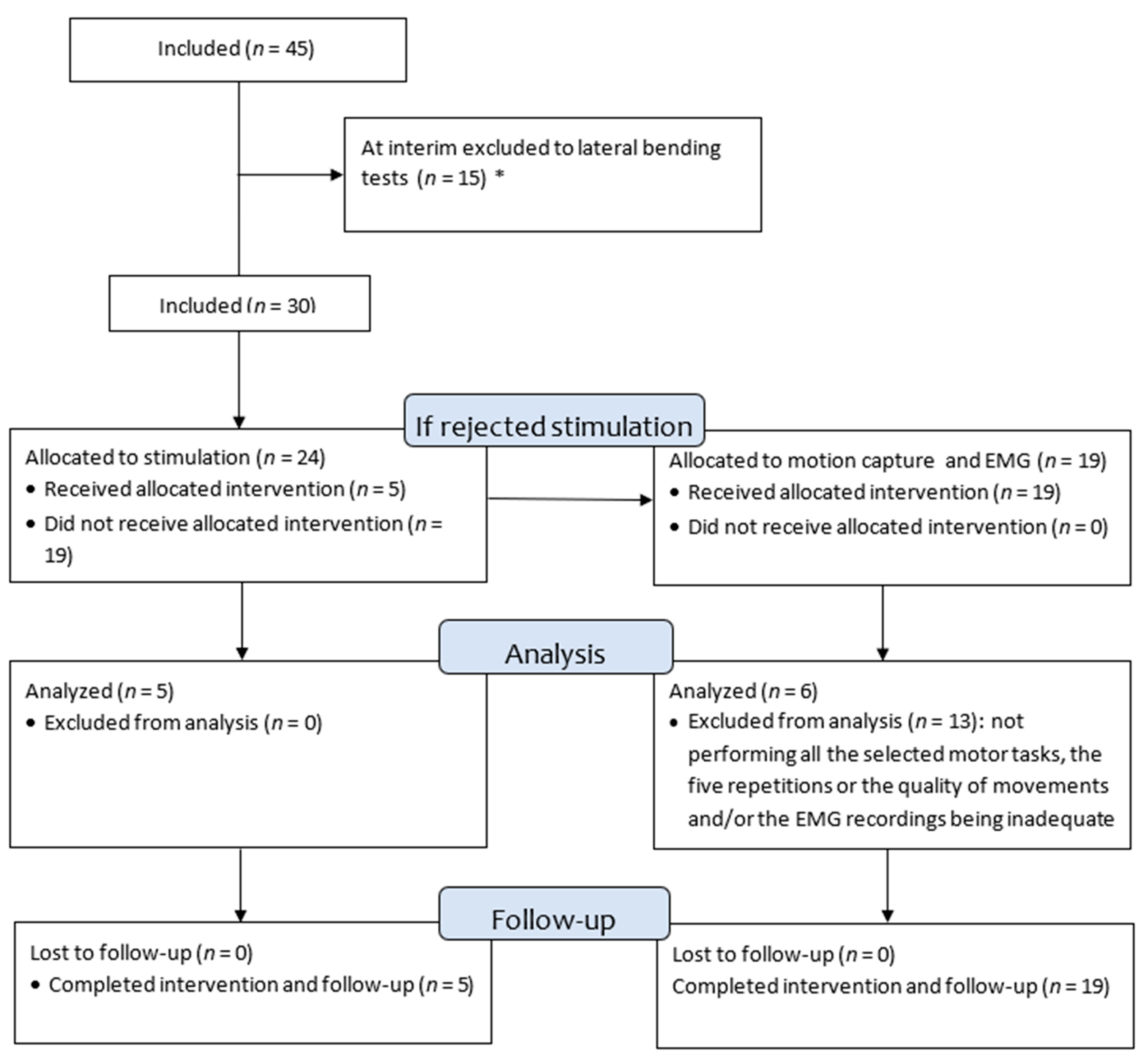
2. Material and Methods
2.1. Design of the Study
2.2. Radiological Evaluation
2.3. Muscle Activity Evaluated by EMG in Various Motor Tasks
2.3.1. Motion Capture
2.3.2. EMG
2.4. Electric Stimulation of the Spinal Muscles
2.5. Data Analysis
3. Results
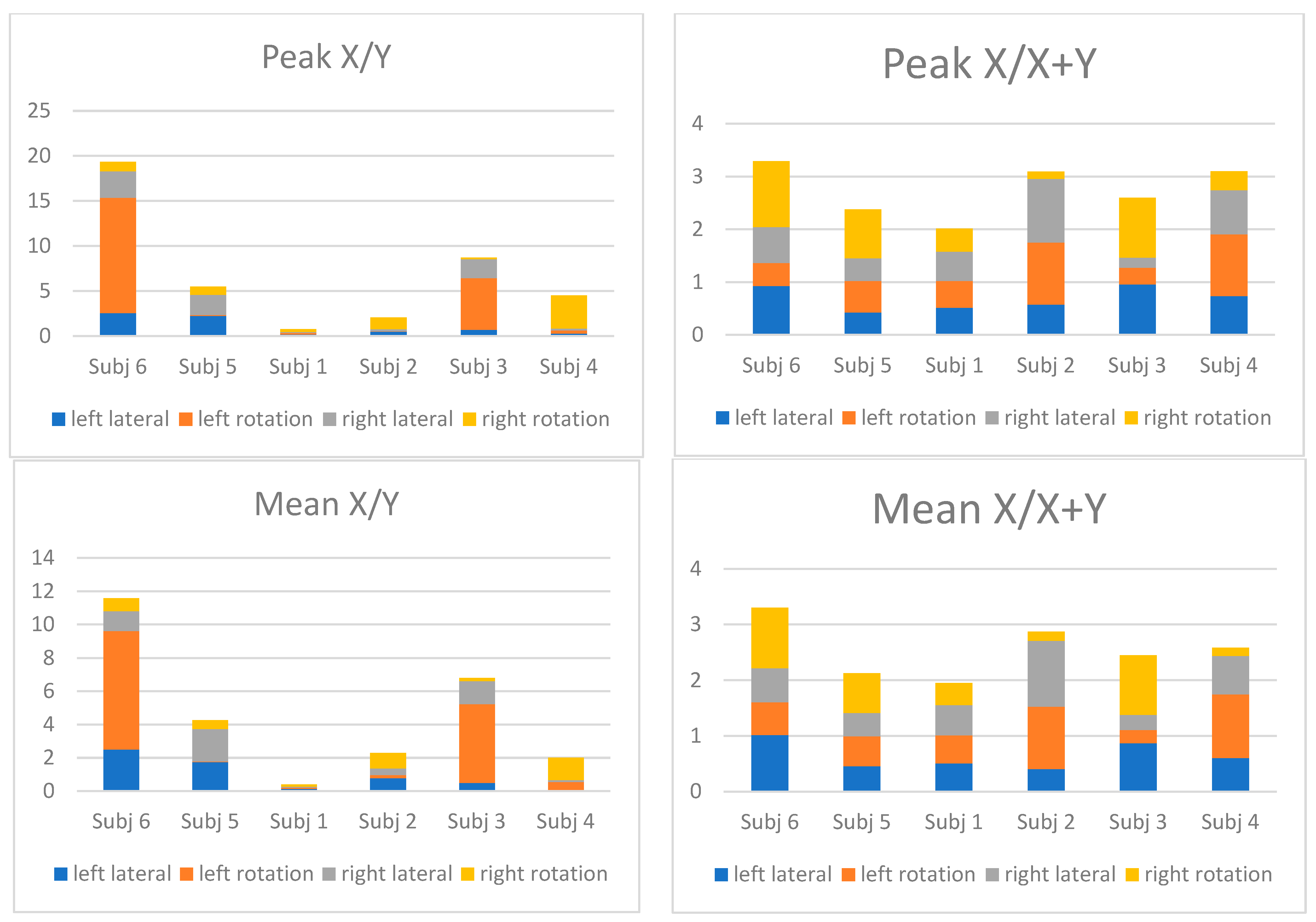
3.1. EMG Ratio
3.2. Electric Stimulation
4. Discussion
4.1. EMG
4.2. Electric Stimulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cobb, J.R. Outline for the Study of Scoliosis. Instr. Course Lect. AAOS 1948, 5, 261–275. [Google Scholar]
- Grivas, T.B.; Vasiliadis, E.; Mouzakis, V.; Mihas, C.; Koufopoulos, G. Association between Adolescent Idiopathic Scoliosis Prevalence and Age at Menarche in Different Geographic Latitudes. Scoliosis 2006, 1, 9. [Google Scholar] [CrossRef]
- Zhang, J.; Cheuk, K.-Y.; Xu, L.; Wang, Y.; Feng, Z.; Sit, T.; Cheng, K.-L.; Nepotchatykh, E.; Lam, T.-P.; Liu, Z.; et al. A Validated Composite Model to Predict Risk of Curve Progression in Adolescent Idiopathic Scoliosis. EClinicalMedicine 2020, 18, 100236. [Google Scholar] [CrossRef]
- Wong, L.P.K.; Cheung, P.W.H.; Cheung, J.P.Y. Curve Type, Flexibility, Correction, and Rotation Are Predictors of Curve Progression in Patients with Adolescent Idiopathic Scoliosis Undergoing Conservative Treatment: A Systematic Review. Bone Jt. J. 2022, 104-B, 424–432. [Google Scholar] [CrossRef]
- Ragborg, L.C.; Dragsted, C.; Ohrt-Nissen, S.; Andersen, T.; Gehrchen, M.; Dahl, B. Health-Related Quality of Life in Patients 40 Years after Diagnosis of an Idiopathic Scoliosis. Bone Jt. J. 2023, 105-B, 166–171. [Google Scholar] [CrossRef]
- Watanabe, K.; Ohashi, M.; Hirano, T.; Katsumi, K.; Mizouchi, T.; Tashi, H.; Minato, K.; Hasegawa, K.; Endo, N. Health-Related Quality of Life in Nonoperated Patients With Adolescent Idiopathic Scoliosis in the Middle Years: A Mean 25-Year Follow-up Study. Spine 2020, 45, E83–E89. [Google Scholar] [CrossRef]
- Tang, N.L.S.; Yeung, H.-Y.; Hung, V.W.Y.; Di Liao, C.; Lam, T.-P.; Yeung, H.-M.; Lee, K.-M.; Ng, B.K.-W.; Cheng, J.C.-Y. Genetic Epidemiology and Heritability of AIS: A Study of 415 Chinese Female Patients. J. Orthop. Res. 2012, 30, 1464–1469. [Google Scholar] [CrossRef]
- Marya, S.; Tambe, A.D.; Millner, P.A.; Tsirikos, A.I. Adolescent Idiopathic Scoliosis: A Review of Aetiological Theories of a Multifactorial Disease. Bone Jt. J. 2022, 104-B, 915–921. [Google Scholar] [CrossRef]
- Cheng, J.C.; Castelein, R.M.; Chu, W.C.; Danielsson, A.J.; Dobbs, M.B.; Grivas, T.B.; Gurnett, C.A.; Luk, K.D.; Moreau, A.; Newton, P.O.; et al. Adolescent Idiopathic Scoliosis. Nat. Rev. Dis. Primers 2015, 1, 15030. [Google Scholar] [CrossRef]
- Wong, C. Mechanism of Right Thoracic Adolescent Idiopathic Scoliosis at Risk for Progression; a Unifying Pathway of Development by Normal Growth and Imbalance. Scoliosis 2015, 10, 2. [Google Scholar] [CrossRef]
- Kouwenhoven, J.-W.M.; Smit, T.H.; van der Veen, A.J.; Kingma, I.; van Dieën, J.H.; Castelein, R.M. Effects of Dorsal versus Ventral Shear Loads on the Rotational Stability of the Thoracic Spine: A Biomechanical Porcine and Human Cadaveric Study. Spine 2007, 32, 2545–2550. [Google Scholar] [CrossRef]
- Janssen, M.M.A.; Kouwenhoven, J.-W.M.; Schlösser, T.P.C.; Viergever, M.A.; Bartels, L.W.; Castelein, R.M.; Vincken, K.L. Analysis of Preexistent Vertebral Rotation in the Normal Infantile, Juvenile, and Adolescent Spine. Spine 2011, 36, E486–E491. [Google Scholar] [CrossRef]
- Castelein, R.M.; Pasha, S.; Cheng, J.C.; Dubousset, J. Idiopathic Scoliosis as a Rotatory Decompensation of the Spine. J. Bone Miner. Res. 2020, 35, 1850–1857. [Google Scholar] [CrossRef]
- Modi, H.N.; Suh, S.-W.; Yang, J.-H.; Hong, J.-Y.; Venkatesh, K.; Muzaffar, N. Spontaneous Regression of Curve in Immature Idiopathic Scoliosis—Does Spinal Column Play a Role to Balance? An Observation with Literature Review. J. Orthop. Surg. Res. 2010, 5, 80. [Google Scholar] [CrossRef]
- Soucacos, P.N.; Zacharis, K.; Gelalis, J.; Soultanis, K.; Kalos, N.; Beris, A.; Xenakis, T.; Johnson, E.O. Assessment of Curve Progression in Idiopathic Scoliosis. Eur. Spine J. 1998, 7, 270–277. [Google Scholar] [CrossRef]
- Riddle, H.F.; Roaf, R. Muscle Imbalance in the Causation of Scoliosis. Lancet 1955, 268, 1245–1247. [Google Scholar] [CrossRef]
- Kouwenhoven, J.-W.M.; Castelein, R.M. The Pathogenesis of Adolescent Idiopathic Scoliosis: Review of the Literature. Spine 2008, 33, 2898–2908. [Google Scholar] [CrossRef]
- Machida, M.; Saito, M.; Dubousset, J.; Yamada, T.; Kimura, J.; Shibasaki, K. Pathological Mechanism of Idiopathic Scoliosis: Experimental Scoliosis in Pinealectomized Rats. Eur. Spine J. 2005, 14, 843–848. [Google Scholar] [CrossRef]
- Jiang, J.; Meng, Y.; Jin, X.; Zhang, C.; Zhao, J.; Wang, C.; Gao, R.; Zhou, X. Volumetric and Fatty Infiltration Imbalance of Deep Paravertebral Muscles in Adolescent Idiopathic Scoliosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 2089–2095. [Google Scholar] [CrossRef]
- Zoabli, G.; Mathieu, P.A.; Aubin, C.-E. Back Muscles Biometry in Adolescent Idiopathic Scoliosis. Spine J. 2007, 7, 338–344. [Google Scholar] [CrossRef]
- Cheung, J.; Veldhuizen, A.G.; Halberts, J.P.K.; Sluiter, W.J.; Van Horn, J.R. Geometric and Electromyographic Assessments in the Evaluation of Curve Progression in Idiopathic Scoliosis. Spine 2006, 31, 322–329. [Google Scholar] [CrossRef]
- Smidt, G.L.; Van Meter, S.E.; Hartman, M.D.; Messaros, S.E.; Rubsam, D.L.; Anderson Welk, K. Spine Configuration and Range of Motion in Normals and Scoliotics. Clin. Biomech. 1994, 9, 303–309. [Google Scholar] [CrossRef]
- Smidt, G.L.; Blanpied, P.R.; White, R.W. Exploration of Mechanical and Electromyographic Responses of Trunk Muscles to High-Intensity Resistive Exercise. Spine 1989, 14, 815–830. [Google Scholar] [CrossRef] [PubMed]
- McIntire, K.L.; Asher, M.A.; Burton, D.C.; Liu, W. Trunk Rotational Strength Asymmetry in Adolescents with Idiopathic Scoliosis: An Observational Study. Scoliosis 2007, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Starcević-Klasan, G.; Cvijanović, O.; Peharec, S.; Zulle, M.; Arbanas, J.; Ivancić Jokić, N.; Bakarcić, D.; Malnar-Dragojević, D.; Bobinac, D. Anthropometric Parameters as Predictors for Iliopsoas Muscle Strength in Healthy Girls and in Girls with Adolescent Idiopathic Scoliosis. Coll. Antropol. 2008, 32, 461–466. [Google Scholar]
- Kennelly, K.P.; Stokes, M.J. Pattern of Asymmetry of Paraspinal Muscle Size in Adolescent Idiopathic Scoliosis Examined by Real-Time Ultrasound Imaging. A Preliminary Study. Spine 1993, 18, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Meier, M.; Grob, D.; Müntener, M. Paraspinal Muscle Fibre Type Alterations Associated with Scoliosis: An Old Problem Revisited with New Evidence. Eur. Spine J. 1998, 7, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.T.T.; Claus, A.; Izatt, M.T.; Pivonka, P.; Tucker, K. Is Spinal Neuromuscular Function Asymmetrical in Adolescents with Idiopathic Scoliosis Compared to Those without Scoliosis?: A Narrative Review of Surface EMG Studies. J. Electromyogr. Kinesiol. 2022, 63, 102640. [Google Scholar] [CrossRef]
- Grivas, T.B.; Burwell, R.G.; Kechagias, V.; Mazioti, C.; Fountas, A.; Kolovou, D.; Christodoulou, E. Idiopathic and Normal Lateral Lumbar Curves: Muscle Effects Interpreted by 12th Rib Length Asymmetry with Pathomechanic Implications for Lumbar Idiopathic Scoliosis. Scoliosis Spinal Disord. 2016, 11, 35. [Google Scholar] [CrossRef]
- Weinstein, S.L.; Dolan, L.A.; Cheng, J.C.Y.; Danielsson, A.; Morcuende, J.A. Adolescent Idiopathic Scoliosis. Lancet 2008, 371, 1527–1537. [Google Scholar] [CrossRef]
- Kim, H.; Lee, C.-K.; Yeom, J.S.; Lee, J.H.; Cho, J.H.; Shin, S.I.; Lee, H.-J.; Chang, B.-S. Asymmetry of the Cross-Sectional Area of Paravertebral and Psoas Muscle in Patients with Degenerative Scoliosis. Eur. Spine J. 2013, 22, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Gosvig, K.; Sonne-Holm, S. The Role of the Paravertebral Muscles in Adolescent Idiopathic Scoliosis Evaluated by Temporary Paralysis. Scoliosis Spinal Disord. 2017, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.T.; Rabczuk, T. Scoliosis Conservative Treatment: A Review of Literature. J. Craniovertebral Junction Spine 2018, 9, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, I.M.; van Dam, F.; Zarzycki, D.; Rymarczyk, A.; Sebastianowicz, P. Short-Duration Electrostimulation in the Treatment of Idiopathic Scoliosis. Ortop. Traumatol. Rehabil. 2004, 6, 82–89. [Google Scholar] [PubMed]
- Axelgaard, J. Transcutaneous Electrical Muscle Stimulation for the Treatment of Progressive Spinal Curvature Deformities. Int. Rehabil. Med. 1984, 6, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Axelgaard, J.; Brown, J.C. Lateral Electrical Surface Stimulation for the Treatment of Progressive Idiopathic Scoliosis. Spine 1983, 8, 242–260. [Google Scholar] [CrossRef] [PubMed]
- Dutro, C.L.; Keene, K.J. Electrical Muscle Stimulation in the Treatment of Progressive Adolescent Idiopathic Scoliosis: A Literature Review. J. Manip. Physiol. Ther. 1985, 8, 257–260. [Google Scholar]
- Bertrand, S.L.; Drvaric, D.M.; Lange, N.; Lucas, P.R.; Deutsch, S.D.; Herndon, J.H.; Roberts, J.M. Electrical Stimulation for Idiopathic Scoliosis. Clin. Orthop. Relat. Res. 1992, 276, 176–181. [Google Scholar] [CrossRef]
- Baek, S.O.; Ahn, S.H.; Jones, R.; Cho, H.K.; Jung, G.S.; Cho, Y.W.; Tak, H.J. Activations of Deep Lumbar Stabilizing Muscles by Transcutaneous Neuromuscular Electrical Stimulation of Lumbar Paraspinal Regions. Ann. Rehabil. Med. 2014, 38, 506–513. [Google Scholar] [CrossRef]
- Baek, S.O.; Cho, H.K.; Kim, S.Y.; Jones, R.; Cho, Y.W.; Ahn, S.H. Changes in Deep Lumbar Stabilizing Muscle Thickness by Transcutaneous Neuromuscular Electrical Stimulation in Patients with Low Back Pain. J. Back Musculoskelet. Rehabil. 2016, 30, 121–127. [Google Scholar] [CrossRef]
- Baek, S.O.; Cho, H.K.; Jung, G.S.; Son, S.M.; Cho, Y.W.; Ahn, S.H. Verification of an Optimized Stimulation Point on the Abdominal Wall for Transcutaneous Neuromuscular Electrical Stimulation for Activation of Deep Lumbar Stabilizing Muscles. Spine J. 2014, 14, 2178–2183. [Google Scholar] [CrossRef]
- Wong, C.; Adriansen, J.; Jeppsen, J.; Balslev-Clausen, A. Intervariability in Radiographic Parameters and General Evaluation of a Low-Dose Fluoroscopic Technique in Patients with Idiopathic Scoliosis. Acta Radiol. Open 2021, 10, 20584601211043256. [Google Scholar] [CrossRef]
- Negrini, S.; Donzelli, S.; Aulisa, A.G.; Czaprowski, D.; Schreiber, S.; de Mauroy, J.C.; Diers, H.; Grivas, T.B.; Knott, P.; Kotwicki, T.; et al. 2016 SOSORT Guidelines: Orthopaedic and Rehabilitation Treatment of Idiopathic Scoliosis during Growth. Scoliosis Spinal Disord. 2018, 13, 3. [Google Scholar] [CrossRef]
- Farahpour, N.; Younesian, H.; Bahrpeyma, F. Electromyographic Activity of Erector Spinae and External Oblique Muscles during Trunk Lateral Bending and Axial Rotation in Patients with Adolescent Idiopathic Scoliosis and Healthy Subjects. Clin. Biomech. 2015, 30, 411–417. [Google Scholar] [CrossRef]
- Bruyneel, A.-V.; Chavet, P.; Bollini, G.; Allard, P.; Berton, E.; Mesure, S. Dynamical Asymmetries in Idiopathic Scoliosis during Forward and Lateral Initiation Step. Eur. Spine J. 2009, 18, 188–195. [Google Scholar] [CrossRef]
- Hellig, T.; Rick, V.; Mertens, A.; Nitsch, V.; Brandl, C. Investigation of Observational Methods Assessing Workload of Static Working Postures Based on Surface Electromyography. Work 2019, 62, 185–195. [Google Scholar] [CrossRef] [PubMed]
- CefarCompex_Practical_Guide_DK. Available online: https://www.djoglobal.eu/media/storage.djoglobal.eu/en_US/Documents/Marketing_documents/Rehab_Theta_Physio_User_Manual___Practical_Guide_-_Archive_(4526420-4526435_Rev_H_2019).pdf (accessed on 14 February 2024).
- Cheung, J.; Veldhuizen, A.G.; Halbertsma, J.P.K.; Maurits, N.M.; Sluiter, W.J.; Cool, J.C.; Van Horn, J.R. The Relation between Electromyography and Growth Velocity of the Spine in the Evaluation of Curve Progression in Idiopathic Scoliosis. Spine 2004, 29, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.-C.; Hong, C.-Z.; Lai, C.-L.; Tan, S.-H. Postural Control Strategies Related to Anticipatory Perturbation and Quick Perturbation in Adolescent Idiopathic Scoliosis. Spine 2011, 36, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.; Arsenault, A.B.; Larivière, C.; DeSerres, S.J.; Rivard, C.-H. Assessment of the Paraspinal Muscles of Subjects Presenting an Idiopathic Scoliosis: An EMG Pilot Study. BMC Musculoskelet. Disord. 2005, 6, 14. [Google Scholar] [CrossRef]
- de Oliveira, A.S.; Gianini, P.E.S.; Camarini, P.M.F.; Bevilaqua-Grossi, D. Electromyographic Analysis of Paravertebral Muscles in Patients with Idiopathic Scoliosis. Spine 2011, 36, E334–E339. [Google Scholar] [CrossRef]
- Cheung, M.-C.; Yip, J.; Lai, J.S.K. Biofeedback Posture Training for Adolescents with Mild Scoliosis. BioMed Res. Int. 2022, 2022, 5918698. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ko, J.Y.; Jang, J.Y.; Lee, S.; Beom, J.; Ryu, J.S. Asymmetrical Activation and Asymmetrical Weakness as Two Different Mechanisms of Adolescent Idiopathic Scoliosis. Sci. Rep. 2021, 11, 17582. [Google Scholar] [CrossRef]
- Sung, P.S.; Park, M.S. Lumbar Spine Coordination during Axial Trunk Rotation in Adolescents with and without Right Thoracic Idiopathic Scoliosis. Hum. Mov. Sci. 2020, 73, 102680. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.Y.H.; Ma, A.C.F.; Cheung, T.S.K.; Chan, J.C.L.; Kwok, R.W.Y.; Fu, A.C.L.; Tsang, S.M.H. Effect of Muscle Fatigue of the Thoracic Erector Spinae on Neuromuscular Control When Performing the Upper Extremity Functional Tasks in People with Adolescent Idiopathic Scoliosis. PLoS ONE 2023, 18, e0281001. [Google Scholar] [CrossRef]
- Lau, R.W.-L.; Kwan, R.L.-C.; Cheng, J.C.-Y.; Hui, S.S.-C.; Lam, T.-P. Patients with Adolescent Idiopathic Scoliosis Have Higher Metabolic Cost during High-Intensity Interval Training. Int. J. Environ. Res. Public Health 2023, 20, 2155. [Google Scholar] [CrossRef]
- Bruggi, M.; Lisi, C.; Rodigari, A.; Nava, M.; Carlisi, E.; Toffola, E.D. Monitoring Iliopsoas Muscle Contraction in Idiopathic Lumbar Scoliosis Patients. G. Ital. Med. Lav. Ergon. 2014, 36, 186–191. [Google Scholar] [PubMed]
- Wilczyński, J. Relationship between Muscle Tone of the Erector Spinae and the Concave and Convex Sides of Spinal Curvature in Low-Grade Scoliosis among Children. Children 2021, 8, 1168. [Google Scholar] [CrossRef]
- DeVries, H.A. “Efficiency of Electrical Activity” as a Physiological Measure of the Functional State of Muscle Tissue. Am. J. Phys. Med. 1968, 47, 10–22. [Google Scholar]
- Lam, G.C.; Hill, D.L.; Le, L.H.; Raso, J.V.; Lou, E.H. Vertebral Rotation Measurement: A Summary and Comparison of Common Radiographic and CT Methods. Scoliosis 2008, 3, 16. [Google Scholar] [CrossRef]
- Stokes, I.A.F. Analysis and Simulation of Progressive Adolescent Scoliosis by Biomechanical Growth Modulation. Eur. Spine J. 2007, 16, 1621–1628. [Google Scholar] [CrossRef]
- Kowalski, I.M.; Szarek, J.; Zarzycki, D.; Rymarczyk, A. Experimental Scoliosis in the Course of Unilateral Surface Electrostimulation of the Paravertebral Muscles in Rabbits: Effects According to Stimulation Period. Eur. Spine J. 2001, 10, 490–494. [Google Scholar] [CrossRef]
- Jou, T. Studies of Experimental Scoliosis Produced by Electrical Stimulation With Special Reference to the Histochemical Properties of the Muscle. J. Nippon. Med. Sch. 1990, 57, 416–426. [Google Scholar] [CrossRef]
- Wojtkiewicz, J.; Kowalski, I.M.; Kmiec, Z.; Crayton, R.; Babinska, I.; Bladowski, M.; Szarek, J.; Kiebzak, W.; Majewski, M.; Barczewska, M.; et al. The Effect of Lateral Electrical Surface Stimulation (LESS) on Motor End-Plates in an Animal Model of Experimental Scoliosis. J. Physiol. Pharmacol. 2012, 63, 285–291. [Google Scholar]
- Kowalski, I.M.; Szarek, J.; Babińska, I.; Wojtkiewicz, J.; Andrzejewska, A.; Lipińska, J.; Majewski, M. Ultrastructural Features of Supraspinal Muscles in Rabbits after Long-Term Transcutaneous Lateral Electrical Surface Stimulation (LESS). Folia Histochem. Cytobiol. 2005, 43, 243–247. [Google Scholar]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Rockenfeller, R.; Günther, M.; Stutzig, N.; Haeufle, D.F.B.; Siebert, T.; Schmitt, S.; Leichsenring, K.; Böl, M.; Götz, T. Exhaustion of Skeletal Muscle Fibers Within Seconds: Incorporating Phosphate Kinetics Into a Hill-Type Model. Front. Physiol. 2020, 11, 306. [Google Scholar] [CrossRef]
- Schwartzmann, J.R.; Miles, M. Experimental production of scoliosis in rats and mice. J. Bone Jt. Surg. Am. Vol. 1945, 27, 59–69. [Google Scholar]
- Roaf, R. Rotation Movements of the Spine with Special Reference to Scoliosis. J. Bone Jt. Surg. Br. Vol. 1958, 40-B, 312–332. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Fusco, C.; Minozzi, S.; Atanasio, S.; Zaina, F.; Romano, M. Exercises Reduce the Progression Rate of Adolescent Idiopathic Scoliosis: Results of a Comprehensive Systematic Review of the Literature. Disabil. Rehabil. 2008, 30, 772–785. [Google Scholar] [CrossRef] [PubMed]
- Seleviciene, V.; Cesnaviciute, A.; Strukcinskiene, B.; Marcinowicz, L.; Strazdiene, N.; Genowska, A. Physiotherapeutic Scoliosis-Specific Exercise Methodologies Used for Conservative Treatment of Adolescent Idiopathic Scoliosis, and Their Effectiveness: An Extended Literature Review of Current Research and Practice. Int. J. Environ. Res. Public Health 2022, 19, 9240. [Google Scholar] [CrossRef]


| Subject | Age at Examination | Sex | Curve Type | Cobb Angle | History | MRI | Stimulation Amplitude | Electrode Placement | Brace History and Physiotheraphy |
| 1 | 14.7 | F | sinistrokonvex TL S | 22.3 | Stationary | 55 | unilateral concave | Soft brace and PT | |
| 2 | 16.4 | M | dextrokonvex TL > straight | 11.0 | Regression | 63 × 2 | bilateral symmetrical | No brace and no PT | |
| 3 | 25.7 | M | dexkonvex TL S | 11.6 | Stationary | Arcolysis L5/Spina bifida | 94 | unilateral concave | No brace and PT |
| 4 | 13.7 | M | sin. convex T > straight | 12.5 | Regression | 62 × 2 | bilateral symmetrical | No brace and no PT | |
| 5 | 16.5 | M | dextrokonvex TL S | 13.3 | Stationary | 94 | lower convex | No brace and PT * | |
| Subject | Age at Examination | Sex | Curve Type | Cobb Angle | History | MRI | Medical History | Surgical AIS | Brace History and Physiotheraphy |
| 1 | 14.9 | F | Low dextrokonvex T | 18 > 56 > 0 | Progression | Synrinx | CP | Operated shorthly after | Soft brace and PT |
| 2 | 14.7 | F | Sinkonvex TL | 19 > 26 > 21 | Regression | none | TDC | None | Soft brace and PT |
| 3 | 17.8 | F | Low dextrokonvex T | 26 > 19 > 17 | Regression | ia | TDC | None | No brace and PT * |
| 4 | 12 | F | Dextro konvex T | 13 > 11 > 9 | Regression | none | TDC | None | No brace and no PT |
| 5 | 12.8 | F | Dextrokonvex TL | 11 | Stationary | TDC | None | No brace and PT | |
| 6 | 14.3 | F | Dextrokonvex TL | 15 > 15 > 0 | straight | CP | None | Soft brace and PT |
| du | ds | αu | αs | *du | *ds | *αu | *αs | |
| Subject 1 | 137.2 | 136.4 | 26.5 | 28.8 | ||||
| Subject 2 | 150.9 | 128.9 | 1.3 | 4 | ||||
| Subject 3 | 88.3 | 87.4 | 5.7 | 6.3 | ||||
| Subject 4 | 73.8 | 70.7 | 2.8 | 1.4 | ||||
| Subject 5 | 138.7 | 106.9 | 10.9 | 11.7 | 112.4 | 105.3 | 18.8 | 21.5 |
| CA | Rdis | Rang | Run | Rn | ||||
| <6° | 0.066 | −0.894 | 0.414 | 13.55 | ||||
| ~10° | 0.23 | −0.073 | 0.025 | 0.32 | ||||
| >20 ° | 0.006 | −0.087 | 2.875 | 14.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.; Shayestehpour, H.; Koutras, C.; Dahl, B.; Otaduy, M.A.; Rasmussen, J.; Bencke, J. Using Electric Stimulation of the Spinal Muscles and Electromyography during Motor Tasks for Evaluation of the Role in Development and Progression of Adolescent Idiopathic Scoliosis. J. Clin. Med. 2024, 13, 1758. https://doi.org/10.3390/jcm13061758
Wong C, Shayestehpour H, Koutras C, Dahl B, Otaduy MA, Rasmussen J, Bencke J. Using Electric Stimulation of the Spinal Muscles and Electromyography during Motor Tasks for Evaluation of the Role in Development and Progression of Adolescent Idiopathic Scoliosis. Journal of Clinical Medicine. 2024; 13(6):1758. https://doi.org/10.3390/jcm13061758
Chicago/Turabian StyleWong, Christian, Hamed Shayestehpour, Christos Koutras, Benny Dahl, Miguel A. Otaduy, John Rasmussen, and Jesper Bencke. 2024. "Using Electric Stimulation of the Spinal Muscles and Electromyography during Motor Tasks for Evaluation of the Role in Development and Progression of Adolescent Idiopathic Scoliosis" Journal of Clinical Medicine 13, no. 6: 1758. https://doi.org/10.3390/jcm13061758







