Clinical Evaluation of Corneal Endothelial Parameters following Laser Refractive Surgery in Myopic Eyes: A Review
Abstract
:1. Introduction
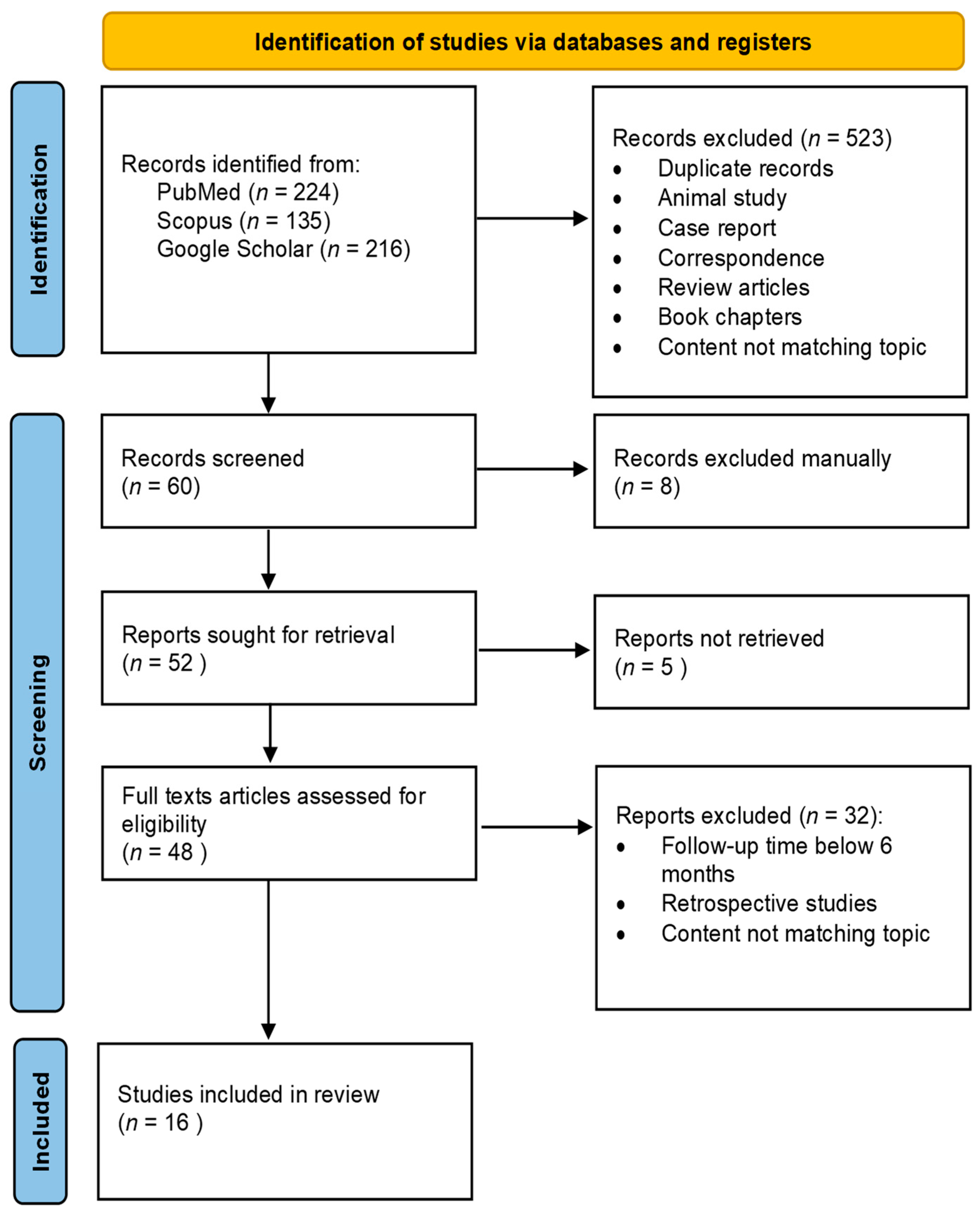
2. Materials and Methods
3. Results
3.1. Surface Ablation
| Study | Study Size | Age (Mean, SD, Range) (Years) | Correction Method | Excimer Laser Firing Frequency (Hz) | Exam Method | Follow-Up Time (Mean, SD, Range) (Months) | Ablation Depth (Mean, SD, Range) (µm) | SE (Mean, SD) (Diopters) | ECD (Mean, SD) | CV (Mean, SD) | HEX (Mean, SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Eyes | Pre-Op | Pre-Op | Post-Op | Pre-Op | Post-Op | Pre-Op | Post-Op | |||||||
| Amano S. et al., 1993 [39] | 20 | 26 | 33, N/S, (22–57) | PRK | N/S | SM | 12, N/S, N/S | 38, N/S, (19–50) | −5.80, N/S, (−2.75–−10.50) | 3221 ± 216 | 3177 ± 185 | 0.24 ± 0.09 | 0.22 ± 0.05 | N/S | N/S |
| Carones F. et al., 1994 [38] | 61 | 76 | 30, N/S, (20–49) | PRK | 10 | SM | 12, N/S, N/S | 72, N/S, (24–113) | −6.60, N/S, (−1.75–−13.50) | 2657 ± 298 | 2656 ± 289 | 30.27 ± 5.99 | 26.35 ± 5.29 * | 63.8 ± 9.9 | 67.1 ± 9.1 * |
| Pallikaris I.G. et al., 1994 [28] | 20 | 10 | N/S, N/S, (22–46) | PRK | 20 | SM | 12, N/S, N/S | N/S | −13.65 ± 1.21 (−10.75–−23.12) | 2800 ± 218 | 2690 ± 239 * | N/S | N/S | N/S | N/S |
| Mardelli P.G. et al., 1995 [45] | 32 | 35 | 39, N/S, (22–57) | PRK | 5 | SM | 38, N/S, (12–54) | N/S | N/S | 2950 ± 329 | 2907 ± 337 | 0.29 ± 0.01 | 0.28 ± 0.05 * | 65.8 ± 5.6 | 64.2 ± 5.6 * |
| Trocmé S.D. et al., 1996 [37] | 14 | 28 | N/S | PRK | 10 | SM | 12, N/S, N/S | N/S | N/S, N/S, (−2.00–−6.00) | A: 2889 ± 283 | 2988 ± 292 | A: 0.30 ± 0.05 | 0.28 ± 0.05 * | A: 58.6 ± 10.9 | 64.1 ± 11.0 * |
| B: 3086 ± 320 | 2966 ± 332 | B: 0.31 ± 0.05 | 0.27 ± 0.05 * | B: 57.1 ± 9.2 | 63.5 ± 9.7 * | ||||||||||
| C: 3184 ± 385 | 2963 ± 296 * | C: 0.32 ± 0.06 | 0.27 ± 0.04 * | C: 60.1 ± 8.6 | 63.7 ± 7.6 | ||||||||||
| Spadea L. et al., 1996 [40] | 50 | 50 | 34 ± 10 (20–60) | PRK | 10 | SM | 11 ± 6 (6–34) | 67 ± 20 (31–111) | −7.80 ± 3.70 (−2.50–−17.00) | 2674 ± 398 | 2578 ± 402 | N/S | N/S | N/S | N/S |
| Pallikaris I.G. et al., 1996 [41] | 42 | 42 | 29 ± 6, N/S, N/S | PRK | N/S | SM | 6, N/S, N/S | N/S | −4.13 ± 1.24 (−1.62–−6.12) | 3115 ± 322 | 3220 ± 333 | N/S | N/S | N/S | N/S |
| Cennamo G. et al., 1997 [42] | 18 | 36 | 30, N/S, (19–57) | PRK | 20 | SM | 12, N/S, N/S | 91 ± 12(62–116) | −10.30 ± 1.40, N/S | 2818 ± 337 | 2894 ± 301 | 79.00 ± 2.30 | 81.00 ± 5.00 | N/S | N/S |
| Frueh B.E. et al., 1998 [43] | 12 | 18 | 35 ± 12, N/S | PRK | 13 | IVCM | 17 ± 8 (12–24) | (35–100) | −5,30 ± 2.10, N/S | 2678 ± 311 | 2658 ± 256 | N/S | N/S | N/S | N/S |
| Pallikaris I.G. et al., 1999 [44] | 102 | 102 | 31 ± 7 (19–52) | PRK | 32 | SM | 12, N/S, N/S | N/S | −4.22 ± 1.22 (−1.50–−6.25) | 2593 ± 377 | 2678 ± 358 | N/S | N/S | N/S | N/S |
| Diakonis V.F. et al., 2007 [32] | 15 | 15 | N/S | PRK MMC(+) | 400 | IVCM | 12, N/S, N/S | 50 ± 12, N/S | −3.32 ± 0.48 (−2.00–−4.75) | 2757 ± 117 | 2721 ± 113 | N/S | N/S | N/S | N/S |
| 15 | N/S | Epi-LASIK | 53 ± 10, N/S | −3.54 ± 0.65 (−2.75–−5.00) | 2769 ± 158 | 2760 ± 102 | N/S | N/S | N/S | N/S | |||||
| Zhao L. et al., 2008 [22] | 89 | 174 | 28 ± 6 (18–45) | LASEK MMC(+) | 120 | SM | 6, N/S, N/S | 134 ± 35 (52–224) | −7.68 ± 2.74 (−1.95–−14.95) | 2755 ± 373 | 2770 ± 399 | 31.45 ± 8.26 | 32.55 ± 9.07 | 66.0 ± 25.8 | 70.7 ± 24.3 |
| Nassiri N. et al., 2008 [35] | 48 | 76 | 29 ± 7 (18–47) | PRK MMC(+) | N/S | SM | 6, N/S, N/S | 81 ± 18, N/S | −4.19 ± 1.27 (N/S) | 2740 ± 361 | 2682 ± 385 * | N/S | N/S | N/S | N/S |
| 33 | 86 | PRK MMC(−) | 43 ± 12, N/S | −2.14 ± 0.81 (N/S) | 2744 ± 333 | 2752 ± 316 * | N/S | N/S | N/S | N/S | |||||
| Patel S. et al., 2009 [29] | 16 | 9 | 39 ± 6 (31–44) | PRK | N/S | SM | 108, N/S, N/S | 31 ± 19, N/S | −3.50 ± 1.70 (−1.25–−5.75) | 2641 ± 340 | 2559 ± 423 | 0.36 ± 0.03 | 0.32 ± 0.04 * | 51.0 ± 5.0 | 58.0 ± 5.0 * |
| Amoozadeh J. et al., 2009 [30] | 12 | 24 | 28 ± 6 (20–35) | PRK | N/S | IVCM | 6, N/S, N/S | 62 ± 15 (38–69) | −2.85 ± 0.99 (−1.00–−4.00) | 2983 ± 293 | 3025 ± 404 | N/S | N/S | 51.3 ± 11.0 | 53.3 ± 10.2 |
| Sia et al., 2014 [31] | 167 | 334 | 34 ± 8 (21–57) | PRK MMC(+) | N/S | SM | 12, N/S, N/S | 83 ±16 (56–129) | −5.99 ± 1.40 (−3.88–−9.38) | 2914 ± 352 | 2859 ± 298 | N/S | N/S | N/S | N/S |
| LASEK | 83 ± 16 (53–114) | 2787 ± 329 | 2860 ±407 | N/S | N/S | N/S | N/S | ||||||||
| PRK | 84 ± 16 (56–120) | 2870 ±375 | 2828 ±345 | N/S | N/S | N/S | N/S | ||||||||
| Gharaee H. et al., 2018 [36] | 48 | 96 | 27 ± 5 (18–34) | PRK MMC(+) | 100 | SM | 6, N/S, N/S | N/S | −3.57 ± 0.18 (−0.50–−6.75) | 2800 ± 37 | 2764 ± 38 | 0.37 ± 0.00 | 0.40 ± 0.10 * | 39.7 ± 1.3 | 36.9 ± 1.3 |
| Adib-Moghaddam S. et al., 2018 [23] | 71 | 142 | 28 ± 6 (22–45) | Trans-PRK MMC(+) | 500 | SM | 12, N/S, N/S | 122 ± 22, N/S | −3.20 ± 1.23, N/S | 2864 ± 295 | N/S | 30.64 ± 4.97 | 33.12 ± 4.72 * | 56.4 ± 10.8 | 56.4 ± 10.8 |
| Trans-PRK MMC(−) | 123 ± 21, N/S | −3.28 ± 1.22, N/S | 2830 ± 302 | N/S | 29.68 ± 5.28 | 30.23 ± 5.16 * | 58.8 ± 9.6 | 58.8 ± 9.61 | |||||||
3.2. LASIK and FemtoLASIK
3.3. Femtosecond Lenticule Extraction
3.4. Comparative Studies
4. Discussion
4.1. The Impact of the Excimer and/or Femtosecond Lasers
4.2. The Role of Attempted Correction (ES, AD) and Residual Stromal Thickness (RST)
4.3. The Effect of Contact Lenses (CL)
4.4. The Role of Mitomycin (MMC)
4.5. Laser Refractive Surgery in Corneal Dystrophies
4.6. Study Limitations
- Heterogeneity in Refractive Surgical Procedures and Equipment: The impact on corneal endothelial cell density may differ depending on the specific refractive surgery techniques, the lasers used, and the range of parameters involved.
- Diversity in Study Populations: Variability in patient demographics, including the ethnic origin, age, sex, and pre-existing ocular conditions, may occur across studies, potentially influencing endothelial cell counts.
- Inconsistencies in Measurement Techniques: Differences in the methods used for corneal endothelial cell counting, such as variations in microscopy techniques (specular vs. in vivo confocal) or imaging devices, may introduce measurement bias and affect the reliability of pooled results.
- Limited Standardization of Reporting: Lack of standardized reporting guidelines for corneal endothelial cell counting after refractive surgery (different techniques) may lead to inconsistent data presentation and hinder the comparability of studies.
- Patient Selection Bias: Studies may not adequately account for patient selection biases, such as excluding individuals with certain risk factors or comorbidities, potentially limiting the generalizability of the findings.
- Small Study Effects: Small studies with limited sample sizes may show larger effects than larger studies, leading to potential bias when pooling the results.
5. Conclusions
- The corneal endothelium is not directly affected by the LRS. However, the energy of the laser, pressure changes, and surgical manipulations during the procedure may lead to minor and transient endothelial cell loss. This loss is usually well within the cornea’s regenerative capacity.
- While short-term studies often show minimal (if any) impact on the corneal endothelium, the long-term effects of laser vision correction are still being studied. Most research indicates that the endothelium remains stable over time for the vast majority of patients. Nevertheless, individual factors and variations in LRS methods may play a role.
- Patient selection is critical to ensuring safety. Individuals with pre-existing corneal conditions or endothelial issues (Fuchs’ dystrophy) may not be good candidates for laser vision correction.
- In general, most studies indicate that PRK, LASEK, LASIK, FemtoLASIK, and SMILE procedures do not significantly affect the corneal endothelium. However, some studies revealed that laser refractive treatment might cause endothelial cell density and morphological changes. Future studies are needed to further elucidate the possible effect of excimer and femtosecond laser procedures on the corneal endothelium.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ang, M.; Gatinel, D.; Reinstein, D.Z.; Mertens, E.; Alió Del Barrio, J.L.; Alió, J.L. Refractive surgery beyond 2020. Eye 2021, 35, 362–382. [Google Scholar] [CrossRef]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Jones, C. Refractive Surgery. Market Report. MarketScope 2020, 2020, 1–283. [Google Scholar]
- Sandoval, H.P.; Donnenfeld, E.D.; Kohnen, T.; Lindstrom, R.L.; Potvin, R.; Tremblay, D.M.; Solomon, K.D. Modern laser in situ keratomileusis outcomes. J. Cataract. Refract. Surg. 2016, 42, 1224–1234. [Google Scholar] [CrossRef]
- Kim, T.I.; Alió Del Barrio, J.L.; Wilkins, M.; Cochener, B.; Ang, M. Refractive surgery. Lancet 2019, 393, 2085–2098. [Google Scholar] [CrossRef]
- Bourne, W.M.; Nelson, L.R.; Hodge, D.O. Central corneal endothelial cell changes over a ten-year period. Investig. Ophthalmol. Vis. Sci. 1997, 38, 779–782. [Google Scholar]
- Gambato, C.; Longhin, E.; Catania, A.G.; Lazzarini, D.; Parrozzani, R.; Midena, E. Aging and corneal layers, an in vivo corneal confocal microscopy study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.; Tok Çevik, M.; Görkem Çevik, S.; Duman, R.; Perente, İ. Corneal endothelial cell density in healthy Caucasian population. Saudi J. Ophthalmol. 2016, 30, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S. Corneal endothelial cell dysfunction, etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 251584141881580. [Google Scholar] [CrossRef] [PubMed]
- McCarey, B.E.; Edelhauser, H.F.; Lynn, M.J. Review of Corneal Endothelial Specular Microscopy for FDA Clinical Trials of Refractive Procedures, Surgical Devices, and New Intraocular Drugs and Solutions. Cornea 2008, 27, 1–16. [Google Scholar] [CrossRef]
- Hara, M.; Morishige, N.; Chikama, T.; Nishida, T. Comparison of Confocal Biomicroscopy and Noncontact Specular Microscopy for Evaluation of the Corneal Endothelium. Cornea 2003, 22, 512–515. [Google Scholar] [CrossRef]
- Seiler, T. Current evaluation of myopia correction with the excimer laser. Ophthalmologe 1995, 92, 379–384. [Google Scholar] [PubMed]
- Seiler, T.; McDonnell, P.J. Excimer laser photorefractive keratectomy. Surv. Ophthalmol. 1995, 40, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, T.; Kastis, G.A.; Suárez, C.; Bor, Z.; Bron, W.E. Time-resolved observations of shock waves and cavitation bubbles generated by femtosecond laser pulses in corneal tissue and water. Lasers Surg. Med. 1996, 19, 23–31. [Google Scholar] [CrossRef]
- Lubatschowski, H.; Maatz, G.; Heisterkamp, A.; Hetzel, U.; Drommer, W.; Welling, H.; Ertmer, H. Application of ultrashort laser pulses for intrastromal refractive surgery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Netto, M.V.; Mohan, R.R.; Medeiros, F.W.; Dupps, W.J., Jr.; Sinha, S.; Krueger, R.R.; Stapleton, W.M.; Rayborn, M.; Suto, S.; Wilson, S.E. Femtosecond Laser and Microkeratome Corneal Flaps, Comparison of Stromal Wound Healing and Inflammation. J. Refract. Surg. 2007, 23, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Randleman, J.B.; Woodward, M.; Lynn, M.J.; Stulting, R.D. Risk Assessment for Ectasia after Corneal Refractive Surgery. Ophthalmology 2008, 115, 37–50.e4. [Google Scholar] [CrossRef]
- Song, J.S.; Kim, J.H.; Yang, M.; Sul, D.; Kim, H.M. Mitomycin-C Concentration in Cornea and Aqueous Humor and Apoptosis in the Stroma After Topical Mitomycin-C Application. Cornea 2007, 26, 461–467. [Google Scholar] [CrossRef]
- Vroman, D.T.; Solomon, K.D.; Holzer, M.P.; Peng, Q.; Apple, D.J.; Bowie, E.M. Endothelial decompensation after laser in situ keratomileusis. J. Cataract. Refract. Surg. 2002, 28, 2045–2049. [Google Scholar] [CrossRef]
- Dastjerdi, M.H.; Sugar, A. Corneal decompensation after laser in situ keratomileusis in fuchs’ endothelial dystrophy. Cornea 2003, 22, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Q.; Wei, R.L.; Ma, X.Y.; Zhu, H. Effect of intraoperative mitomycin-C on healthy corneal endothelium after laser-assisted subepithelial keratectomy. J. Cataract. Refract. Surg. 2008, 34, 1715–1719. [Google Scholar] [CrossRef]
- Adib-Moghaddam, S.; Soleyman-Jahi, S.; Tefagh, G.; Tofighi, S.; Grentzelos, M.A.; Kymionis, G.D. Comparison of Single-Step Transepithelial Photorefractive Keratectomy with or Without Mitomycin C in Mild to Moderate Myopia. J. Refract. Surg. 2018, 34, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Simaroj, P.; Kosalprapai, K.; Chuckpaiwong, V. Effect of laser in situ keratomileusis on the corneal endothelium. J. Refract. Surg. 2003, 19 (Suppl. S2), S237–S240. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.; Albarrán-Diego, C.; Sakla, H.F.; Ferrer-Blasco, T.; Javaloy, J. Effects of LASIK on corneal endothelium using the 15-kHz IntraLase femtosecond laser. J. Refract. Surg. 2011, 27, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Igarashi, A.; Ishii, R.; Sato, N.; Nishimoto, H.; Shimizu, K. Early clinical outcomes, including efficacy and endothelial cell loss, of refractive lenticule extraction using a 500 kHz femtosecond laser to correct myopia. J. Cataract. Refract. Surg. 2012, 38, 1996–2002. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Xie, S.; Wu, D.; Wu, W.; Xu, L. Short-term and long-term effects of small incision lenticule extraction (SMILE) on corneal endothelial cells. Cont. Lens Anterior Eye 2015, 38, 334–338. [Google Scholar] [CrossRef]
- Pallikaris, I.G.; Siganos, D.S. Excimer laser in situ keratomileusis and photorefractive keratectomy for correction of high myopia. J. Refract. Corneal Surg. 1994, 10, 498–510. [Google Scholar] [CrossRef]
- Patel, S.V. Corneal Endothelial Cell Loss 9 Years After Excimer Laser Keratorefractive Surgery. Arch. Ophthalmol. 2009, 127, 1423. [Google Scholar] [CrossRef]
- Amoozadeh, J.; Aliakbari, S.; Behesht-Nejad, A.H.; Seyedian, M.A.; Rezvan, B.; Hashemi, H. Confocal microscopy of corneal stroma and endothelium after LASIK and PRK. J. Refract. Surg. 2009, 25 (Suppl. S10), S963–S967. [Google Scholar] [CrossRef]
- Sia, R.K.; Ryan, D.S.; Edwards, J.D.; Stutzman, R.D.; Bower, K.S. The U.S. Army Surface Ablation Study, comparison of PRK, MMC-PRK, and LASEK in moderate to high myopia. J. Refract. Surg. 2014, 30, 256–264. [Google Scholar] [CrossRef]
- Diakonis, V.F.; Pallikaris, A.; Kymionis, G.D.; Markomanolakis, M.M. Alterations in endothelial cell density after photorefractive keratectomy with adjuvant mitomycin. Am. J. Ophthalmol. 2007, 144, 99–103. [Google Scholar] [CrossRef]
- Klingler, K.N.; McLaren, J.W.; Bourne, W.M.; Patel, S.V. Corneal endothelial cell changes 5 years after laser in situ keratomileusis, Femtosecond laser versus mechanical microkeratome. J. Cataract. Refract. Surg. 2012, 38, 2125–2130. [Google Scholar] [CrossRef]
- Shaaban, Y.M.; Badran, T.A.F. Comparison Between the Effect of Femtosecond Laser in situ Keratomileusis (FS-LASIK) and Femtosecond Small Incision Lenticule Extraction (FS-SMILE) on the Corneal Endothelium. Clin. Ophthalmol. 2020, 14, 2543–2550. [Google Scholar] [CrossRef]
- Nassiri, N.; Farahangiz, S.; Rahnavardi, M.; Rahmani, L.; Nassiri, N. Corneal endothelial cell injury induced by mitomycin-C in photorefractive keratectomy, Nonrandomized controlled trial. J. Cataract. Refract. Surg. 2008, 34, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Gharaee, H.; Zarei-Ghanavati, S.; Alizadeh, R.; Abrishami, M. Endothelial cell changes after photorefractive keratectomy with graded usage of mitomycin C. Int. Ophthalmol. 2018, 38, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Trocmé, S.D.; Mack, K.A.; Gill, K.S.; Gold, D.H.; Milstein, B.A.; Bourne, W.M. Central and peripheral endothelial cell changes after excimer laser photorefractive keratectomy for myopia. Arch. Ophthalmol. 1996, 114, 925–928. [Google Scholar] [CrossRef]
- Carones, F.; Brancato, R.; Venturi, E.; Morico, A. The corneal endothelium after myopic excimer laser photorefractive keratectomy. Arch. Ophthalmol. 1994, 112, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.; Shimizu, K. Corneal endothelial changes after excimer laser photorefractive keratectomy. Am. J. Ophthalmol. 1993, 116, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Spadea, L.; Dragani, T.; Blasi, M.A.; Mastrofini, M.C.; Balestrazzi, E. Specular microscopy of the corneal endothelium after excimer laser photorefractive keratectomy. J. Cataract. Refract. Surg. 1996, 22, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Pallikaris, l.; McDonald, M.B.; Siganos, D.; Klonos, G.; Detorakis, S.; Frey, R.; Downes, R.; Gauthier, C.A. Tracker-Assisted Photorefractive Keratectomy for Myopia of -1 to -6 Diopters. J. Refract. Surg. 1996, 12, 240–247. [Google Scholar] [CrossRef]
- Cennamo, G.; Rosa, N.; Del Prete, A.; Breve, M.A.; Sebastiani, A. The corneal endothelium 12 months after photorefractive keratectomy in high myopia. Acta Ophthalmol. Scand. 1997, 75, 128–130. [Google Scholar] [CrossRef]
- Frueh, B.E.; Cadez, R.; Böhnke, M. In vivo confocal microscopy after photorefractive keratectomy in humans. A prospective, long-term study. Arch. Ophthalmol. 1998, 116, 1425–1431. [Google Scholar] [CrossRef]
- Pallikaris, I.G.; Koufala, K.I.; Siganos, D.S.; Papadaki, T.G.; Katsanevaki, V.J.; Tourtsan, V.; McDonald, M.B. Photorefractive keratectomy with a small spot laser and tracker. J. Refract. Surg. 1999, 15, 137–144. [Google Scholar]
- Mardelli, P.G.; Piebenga, L.W.; Matta, C.S.; Hyde, L.L.; Gira, J. Corneal endothelial status 12 to 55 months after excimer laser photorefractive keratectomy. Ophthalmology 1995, 102, 544–549, discussion 548–549. [Google Scholar] [CrossRef]
- Marshall, J.; Trokel, S.; Rothery, S.; Schubert, H. An Ultrastructural Study of Corneal Incisions Induced by an Excimer Laser at 193 nm. Ophthalmology 1985, 92, 749–758. [Google Scholar] [CrossRef]
- Hanna, K.D. Corneal Stromal Wound Healing in Rabbits After 193-nm Excimer Laser Surface Ablation. Arch. Ophthalmol. 1989, 107, 895. [Google Scholar] [CrossRef]
- Woodward, M.A.; Edelhauser, H.F. Corneal endothelium after refractive surgery. J. Cataract. Refract. Surg. 2011, 37, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.; Vanathi, M. Specular microscopy in clinical practice. Indian J. Ophthalmol. 2021, 69, 517. [Google Scholar] [CrossRef] [PubMed]
- Benetz, B.A.; Gal, R.L.; Ruedy, K.J.; Rice, C.; Beck, R.W.; Kalajian, A.D.; Lass, J.H.; Cornea Donor Study Group. Specular Microscopy Ancillary Study Methods for Donor Endothelial Cell Density Determination of Cornea Donor Study Images. Curr. Eye Res. 2006, 31, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kitzmann, A.S.; Winter, E.J.; Nau, C.B.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. Comparison of corneal endothelial cell images from a noncontact specular microscope and a scanning confocal microscope. Cornea 2005, 24, 980–984. [Google Scholar] [CrossRef]
- Imre, L.; Nagymihály, A. Reliability and reproducibility of corneal endothelial image analysis by in vivo confocal microscopy. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 356–360. [Google Scholar] [CrossRef]
- Collins, M.J.; Carr, J.D.; Stulting, R.D.; Azar, R.G.; Waring, G.O.; Smith, R.E.; Thompson, K.P.; Edelhauser, H.F. Effects of laser in situ keratomileusis (LASIK) on the corneal endothelium 3 years postoperatively. Am. J. Ophthalmol. 2001, 131, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kymionis, G.D.; Kankariya, V.P.; Plaka, A.D.; Reinstein, D.Z. Femtosecond laser technology in corneal refractive surgery, a review. J. Refract. Surg. 2012, 28, 912–920. [Google Scholar] [CrossRef]
- Pidro, A.; Biscevic, A.; Pjano, M.; Mravicic, I.; Bejdic, N.; Bohac, M. Excimer Lasers in Refractive Surgery. Acta Inform. Med. 2019, 27, 278. [Google Scholar] [CrossRef]
- Montés-Micó, R.; Rodríguez-Galietero, A.; Alió, J.L. Femtosecond Laser versus Mechanical Keratome LASIK for Myopia. Ophthalmology 2007, 114, 62–68. [Google Scholar] [CrossRef]
- Edelhauser, H.F. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea 2000, 19, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Sorenson, A.L.; Krishnasamy, S.; Carlson, A.N.; Edelhauser, H.F. Acute Corneal Endothelial Changes After Laser In Situ Keratomileusis. Cornea 2001, 20, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Helena, M.C.; Baerveldt, F.; Kim, W.J.; Wilson, S.E. Keratocyte apoptosis after corneal surgery. Invest. Ophthalmol. Vis. Sci. 1998, 39, 276–283. [Google Scholar]
- Özbilen, K.T.; Altinkurt, E.; Ceylan, N.A.; Bilgin, G.S.; Gözüm, N. Effect of Myopic Femtosecond Laser-Assisted LASIK on Anterior Chamber Inflammation (Flare Values) and Corneal Endothelium, A Prospective before and after Study. J. Ophthalmol. 2021, 2021, 2395028. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Chen, Y.T.; Yeh, S.I.; Yau, C.W. Errors of residual stromal thickness estimation in LASIK. Ophthal. Surg. Lasers Imag. 2008, 39, 107–113. [Google Scholar] [CrossRef]
- Moshirfar, M.; Somani, S.N.; Patel, B.C. Small Incision Lenticule Extraction. 2023 Jun 12. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mohammad-Rabei, H.; Moravej, R.; Almasi-Nasrabadi, M.; Rezazadeh, P.; Manafi, N.; Noorizadeh, F. Effect of mitomycin-C on corneal endothelial cell parameters after refractive surface ablation procedures. Med. Hypothesis Discov. Innov. Ophthalmol. 2022, 10, 156–164. [Google Scholar] [CrossRef]
- Pérez-Santonja, J.J. Evaluation of Endothelial Cell Changes 1 Year After Excimer Laser In Situ Keratomileusis. Arch. Ophthalmol. 1997, 115, 841. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Lin, J.; Bair, P.; Chen, W.; Tsai, Y.; Tseng, S. Effects of Laser In Situ Keratomileusis on the Corneal Endothelium. Kaohsiung J. Med. Sci. 2005, 21, 272–276. [Google Scholar] [CrossRef]
- Wiffen, S.J.; Hodge, D.O.; Bourne, W.M. The Effect of Contact Lens Wear on the Central and Peripheral Corneal Endothelium. Cornea 2000, 19, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Khodadoust, A.A.; Hirst, L.W. Diurnal Variation in Corneal Endothelial Morphologyp. Ophthalmology 1984, 91, 1125–1128. [Google Scholar] [CrossRef]
- Carones, F.; Vigo, L.; Scandola, E.; Vacchini, L. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy. J. Cataract. Refract. Surg. 2002, 28, 2088–2095. [Google Scholar] [CrossRef]
- Torres, R.M.; Merayo-Lloves, J.; Daya, S.M.; Blanco-Mezquita, J.T.; Espinosa, M.; Nozal, M.J.; Beral, J.B.; Bernal, J. Presence of mitomycin-C in the anterior chamber after photorefractive keratectomy. J. Cataract. Refract. Surg. 2006, 32, 67–71. [Google Scholar] [CrossRef] [PubMed]
- De Benito-Llopis, L.; Teus, M.A.; Ortega, M. Effect of mitomycin-C on the corneal endothelium during excimer laser surface ablation. J. Cataract. Refract. Surg. 2007, 33, 1009–1013. [Google Scholar] [CrossRef]
- Al-Mohaimeed, M.M. Effect of Prophylactic Mitomycin C on Corneal Endothelium Following Transepithelial Photorefractive Keratectomy in Myopic Patients. Clin. Ophthalmol. 2022, 16, 2813–2822. [Google Scholar] [CrossRef]
- Majmudar, P.A.; Schallhorn, S.C.; Cason, J.B.; Donaldson, K.E.; Kymionis, G.D.; Shtein, R.M.; Verity, S.M.; Frajo, A.A. Mitomycin-C in Corneal Surface Excimer Laser Ablation Techniques. Ophthalmology 2015, 122, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Rocha-de-Lossada, C.; Rachwani-Anil, R.; Colmenero-Reina, E.; Borroni, D.; Sánchez-González, J.M. Laser refractive surgery in corneal dystrophies. J. Cataract. Refract. Surg. 2021, 47, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Feiz, V.; Feilmeier, M.R.; Kang, P.C. Laser in situ keratomileusis in patients with corneal guttata and family history of Fuchs’ endothelial dystrophy. J. Cataract. Refract. Surg. 2005, 31, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Barsam, C.A.; Tanner, M.C. Laser in situ keratomileusis in patients with posterior polymorphous dystrophy. Cornea 2005, 24, 230–232. [Google Scholar] [CrossRef]
- Bower, K.S.; Trudo, E.W.; Ryan, D.S.; Sia, R.K.; Mines, M.J.; Stutzman, R.D.; Wrobleski, K.J. Photorefractive keratectomy in posterior polymorphous dystrophy with vesicular and band subtypes. J. Cataract. Refract. Surg. 2011, 37, 1101–1108. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.H.; Koo, H.J. Small-incision lenticule extraction in posterior polymorphic corneal dystrophy. J. Cataract. Refract. Surg. 2016, 42, 795–797. [Google Scholar] [CrossRef]
| Study | Study Size | Age (Mean, SD, Range) (Years) | Correction Method | Excimer Laser Firing Frequency (Hz) | Femtosecond Laser Firing Frequency (kHz) | Exam Method | Follow-Up Time (Mean, SD, Range) (Months) | Ablation Depth (Mean, SD, Range) (µm) | SE (Mean, SD) (Diopters) | ECD (Mean, SD) | CV (Mean, SD) | HEX (Mean, SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Eyes | Pre-Op | Pre-Op | Post-Op | Pre-Op | Post-Op | Pre-Op | Post-Op | ||||||||
| Pallikaris I.G. et al., 1994 [28] | 20 | 10 | N/S, N/S, (31–58) | LASIK | 20 | Microkeratome | SM | 12, N/S, N/S | N/S | −11.00 ± 0.86 (−8.00–−16.00) | 2744 ± 217 | 2644 ± 235 * | N/S | N/S | N/S | N/S |
| Simaroj P. et al., 2003 [24] | 105 | 180 | 32 ± 9 (16–55) | LASIK | 30 | Microkeratome | SM | 12, N/S, N/S | N/S | N/S, N/S, (−0.75–−15.00) | 2547 ± 282 | 2508 ± 290 | 58.33 ± 11.50% | 58.61 ± 9.89% | N/S | N/S |
| Patel S. et al., 2009 [29] | 16 | 20 | 34 ± 8 (23–47) | LASIK | N/S | Microkeratome | SM | 108, N/S, N/S | 66 ± 34, N/S | −6.20 ± 1.40 (−4.00–−9.25) | 2925 ± 303 | 2741 ± 308 * | 0.33 ± 0.05 | 0.33 ± 0.03 | 55.0 ± 9.0 | 56.0 ± 5.0 |
| Amoozadeh J. et al., 2009 [30] | 4 | 8 | 28 ± 7 (19–36) | LASIK | N/S | Microkeratome | IVCM | 6 N/S, N/S | 61 ± 17 (36–71) | −2.94 ± 0.96 (−2.00–−4.25) | 3022 ± 224 | 3030 ± 186 | N/S | N/S | 52.2 ± 11.4 | 53.0 ± 7.6 |
| Muñoz G. et al., 2011 [25] | 62 | 62 | 32 ± 7 (21–42) | FemtoLASIK CL(−) | N/S | 15 | SM | 12 N/S, N/S | 45 ± 30 (12–123) | N/S, N/S, (−0.75–−9.00) | 3605 ± 219 | 3564 ± 232 | N/S | N/S | 35.1 ± 11.7 | 34.4 ± 11.2 |
| 76 | 76 | FemtoLASIK CL(+) | N/S | 15 | 48 ± 23 (19–113) | N/S, N/S, (−0.75–−9.00) | 3401 ± 292 | 3587 ± 262 * | N/S | N/S | 31.0 ± 5.1 | 32.5 ± 4.0 | ||||
| Klingler K. et al., 2012 [33] | 18 | 17 | 38 ± 10 (22–54) | FemtoLASIK | N/S | 15 | IVCM | 60, N/S, N/S | N/S | N/S | 2758 ± 406 | 2829 ± 317 | 32.00 ± 6.00 | 31.00 ± 4.00 | 59.0 ± 8.0 | 57.0 ± 7.0 |
| 17 | LASIK | N/S | Microkeratome | 2773 ± 399 | 2853 ± 355 | 32.00 ± 6.00 | 32.00 ± 3.00 | 59.0 ± 6.0 | 57.0 ± 6.0 | |||||||
| Shaaban Y.M. et al., 2020 [34] | 20 | 40 | 29 ± 5 (19–37) | FemtoLASIK | N/S | Microkeratome | SM | 6, N/S, N/S | N/S | −4.06 ± 1.94, N/S | 2947 ± 151 | 2901 ± 150 * | 29.48 ± 2.87 | 31.75 ± 3.27 * | 60.1 ± 2.9 | 57.1 ± 3.2 * |
| Study | Study Size | Age (Mean, SD, Range) (Years) | Correction Method | Femtosecond Laser Firing Frequency (kHz) | Exam Method | Follow-Up Time (Mean, SD, Range) (Months) | Ablation Depth (Mean, SD, Range) (µm) | SE (Mean, SD) (Diopters) | ECD (Mean, SD) | CV (Mean, SD) | HEX (Mean, SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Eyes | Pre-Op | Pre-Op | Post-Op | Pre-Op | Post-Op | Pre-Op | Post-Op | |||||||
| Kamiya K. et al., 2012 [26] | 20 | 38 | 33 ± 8 (20–48) | Femtosecond Lenticule Extraction | 500 | SM | 6 N/S, N/S | N/S | −4.26 ± 1.39 N/S, (−2.00–−7.00) | 2814 ± 199 | 2762 ± 213 | N/S | N/S | N/S | N/S |
| Zhang H. et al., 2015 [27] | 30 | 56 | 26 ± 6 (18–40) | Femtosecond Lenticule Extraction | 500 | SM | 12 N/S, N/S | N/S | −5.50 ± 1.10 (−3.50–−8.25) | 2840 ± 322 | 2837 ± 311 | 31.90 ± 4.90 | 32.10 ± 4.60 | 58.7 ± 9.3 | 58.5 ± 8.5 |
| Shaaban Y.M. et al., 2020 [34] | 20 | 40 | 28 ± 5 (19–37) | Femtosecond Lenticule Extraction | N/S | SM | 6 N/S, N/S | N/S | −5.18 ± 1.86 N/S | 3038 ± 161 | 2913 ± 170 * | 28.38 ± 2.24 | 33.03 ± 2.47 * | 61.7 ± 2.6 | 56.7 ±2.5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juda, M.; Bedliński, M.; Roszkowska, A.M.; Wierzbowska, J. Clinical Evaluation of Corneal Endothelial Parameters following Laser Refractive Surgery in Myopic Eyes: A Review. J. Clin. Med. 2024, 13, 1665. https://doi.org/10.3390/jcm13061665
Juda M, Bedliński M, Roszkowska AM, Wierzbowska J. Clinical Evaluation of Corneal Endothelial Parameters following Laser Refractive Surgery in Myopic Eyes: A Review. Journal of Clinical Medicine. 2024; 13(6):1665. https://doi.org/10.3390/jcm13061665
Chicago/Turabian StyleJuda, Maciej, Maciej Bedliński, Anna Maria Roszkowska, and Joanna Wierzbowska. 2024. "Clinical Evaluation of Corneal Endothelial Parameters following Laser Refractive Surgery in Myopic Eyes: A Review" Journal of Clinical Medicine 13, no. 6: 1665. https://doi.org/10.3390/jcm13061665






