Osseodensification vs. Conventional Osteotomy: A Case Series with Cone Beam Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Step
2.3. Reestablishment of Function Step—5–7 Months Later
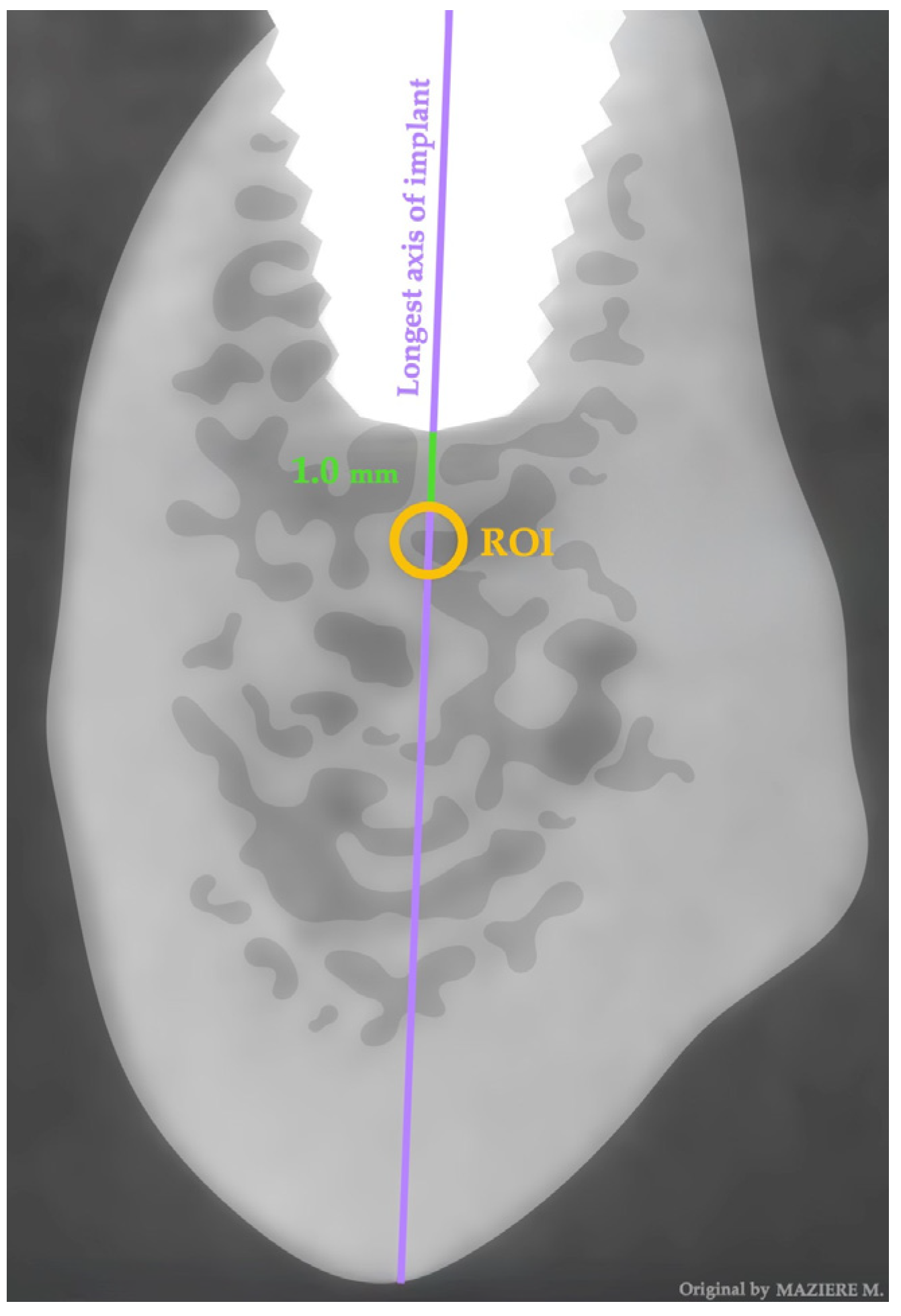
2.4. Data Extration—One Year Later
2.5. Data Analysis
3. Results
3.1. Sample Characterization by Implant Distribution According to Sex and Surgical Technique
3.2. Sample Characterization by Maxillary and Mandibular Implant’s Location
3.3. Sample Characterization by Maxillary and Mandibular Implant’s Location and Surgical Technique
3.4. Sample Characterization by Implant’s Dimensions According Surgical Technique
3.5. Primary Stability According to Surgical Technique
3.6. Correlation between Bone Density in Both Techniques with Area of Location Implant Stability, Implant Dimensions, and Sex
3.7. Bone Density According to Surgical Technique
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiran, S.R.; Bammidi, N.; Kumar, A.K.; Kumar, P.S. Evaluation of the Effect of Topical Melatonin Application on Immediately Placed Dental Implants Using Cone Beam Computed Tomography (CBCT). Cureus 2022, 14, 1–9. [Google Scholar] [CrossRef]
- Tomina, D.; Petrutiu, S.; Crisan, B.; Leucuta, D.; Dinu, C. Influence of Periodontal Status and Prosthetic Treatment on Survival and Success Rates in Implant Therapy: A 5-Year Retrospective Follow-Up Study. J. Clin. Med. 2023, 12, 4275. [Google Scholar] [CrossRef]
- Ivanova, V.; Chenchev, I.; Zlatev, S. Correlation between Primary, Secondary Stability, Bone Density, Percentage of Vital Bone Formation and Implant Size. Int. J. Environ. Res. Public Health 2021, 18, 6994. [Google Scholar] [CrossRef]
- Silva, A.S.; Martins, D.; De Sá, J.; Mendes, J.M. Clinical evaluation of the implant survival rate in patients subjected to immediate implant loading protocols. Dent. Med. Probl. 2021, 58, 61–68. [Google Scholar] [CrossRef]
- Fernández-Olavarria, A.; Gutiérrez-Corrales, A.; González-Martín, M.; Torres-Lagares, D. Influence of different drilling protocols and bone density on the insertion torque of dental implants. Med. Oral Patol. Oral Cir. Bucal 2023, 28, 385–394. [Google Scholar] [CrossRef]
- Nieves, J.W. Sex-Differences in Skeletal Growth and Aging. Curr. Osteoporos. Rep. 2017, 15, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, Y.; Ding, S. Primary stability of implant placement and loading related to dental implant materials and designs: A literature review. J. Dent. Sci. 2023, 18, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Shen, Y.-W.; Fuh, L.-J.; Peng, S.-L.; Tsai, M.-T.; Huang, H.-L.; Hsu, J.-T. Relationship between Cortical Bone Thickness and Cancellous Bone Density at Dental Implant Sites in the Jawbone. Diagnostics 2020, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Stoilov, M.; Shafaghi, R.; Stark, H.; Marder, M.; Kraus, D.; Enkling, N. Influence of Implant Macro-Design, Length, and Diameter on Primary Implant Stability Depending on Different Bone Qualities Using Standard Drilling Protocols—An In Vitro Analysis. J. Funct. Biomater. 2023, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Antonacci, D.; Del, M.; Bollero, P.; Stocchero, M.; Jinno, Y.; Canullo, L. Clinical effects of conventional and underprepared drilling preparation of the implant site based on bone density: A systematic review and meta-regression. J. Prosthod. Res. 2023, 67, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A. Influence of Stepped Osteotomy on Primary Stability of Implants Inserted in Low-Density Bone Sites: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2017, 32, 37–41. [Google Scholar] [CrossRef]
- Summers, R. A new concept in maxillary implant surgery: The osteotome technique. Compendium 1994, 15, 152, 154–156. [Google Scholar] [PubMed]
- Attanasio, F.; Antonelli, A.; Brancaccio, Y.; Averta, F.; Figliuzzi, M.M.; Fortunato, L.; Giudice, A. Primary Stability of Three Different Osteotomy Techniques in Medullary Bone: An in Vitro Study. Dent. J. 2020, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Tolstunov, L.; Hamrick, J.F.E. Bone Augmentation Techniques for Horizontal and Vertical Alveolar Ridge Deficiency in Oral Implantology. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Huwais, S. Fluted Osteotome and Surgical Method for Use. U.S. Patent US2013/0004918, 3 March 2013. granted 12 May 2015. [Google Scholar]
- Huwais, S.; Meyer, E.G. A Novel Osseous Densification Approach in Implant Osteotomy Preparation to Increase Biomechanical Primary. Int. J. Oral Maxillofac. Implant. 2017, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.; Mendes, J.M.; Salazar, F.; Pacheco, J.J.; Rompante, P.; Câmara, M.I. Analysis of peri–implant bone defects by using cone beam computed tomography (CBCT): An integrative review. Oral Radiol. 2023, 39, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.; Cavalcanti, Y.; Costa, F.; Peixoto, L.; Maia, A.; Rovaris, K.; Melo, D.P. Assessment of artefacts produced by metal posts on CBCT images. Int. Endod. J. 2019, 52, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Salmon, B.; Codari, M.; Hassan, B.; Bornstein, M.M. Cone beam computed tomography in implant dentistry: Recommendations for clinical use. BMC Oral Health 2018, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, E.; Elluru, S.V. Cone Beam Computed Tomography: Basics and Applications in Dentistry. J. Istanbul Univ. Fac. Dent. 2017, 51, 102–121. [Google Scholar] [CrossRef]
- Steiger-Ronay, V.; Krcmaric, Z.; Schmidlin, P.R.; Sahrmann, P.; Wiedemeier, D.B.; Benic, G.I. Assessment of peri-implant defects at titanium and zirconium dioxide implants by means of periapical radiographs and cone beam computed tomography: An in-vitro examination. Clin. Oral Implants Res. 2018, 29, 1195–1201. [Google Scholar] [CrossRef]
- Kaasalainen, T.; Ekholm, M.; Siiskonen, T.; Kortesniemi, M. Physica Medica Dental cone beam CT: An updated review. Phys. Med. 2021, 88, 193–217. [Google Scholar] [CrossRef]
- Kunz, A.S.; Patzer, T.S.; Grunz, J.P.; Luetkens, K.S.; Hartung, V.; Hendel, R.; Huflage, H.; Fieber, T.; Genest, F.; Ergün, S.; et al. Metal artifact reduction in ultra—High resolution cone beam CT imaging with a twin robotic X-ray system. Sci. Rep. 2022, 12, 15549. [Google Scholar] [CrossRef] [PubMed]
- Pessôa de Oliveira, P.G.F.; Bergamo, E.T.P.; Neiva, R.; Bonfante, E.A.; Witek, L.; Tovar, N.; Coelho, P.G. Osseodensification outperforms conventional implant subtractive instrumentation: A study in sheep. Mater. Sci. Eng. C 2018, 90, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.D.; Alifarag, A.M.; Torroni, A.; Tovar, N.; Rodrigo, J.; Witek, L.; Rodriguez, E.D.; Coelho, P.G. Osseodensification for enhancement of spinal surgical hardware fixation. J. Mech. Behav. Biomed. Mater. 2017, 69, 275–281. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. New Osseodensification Implant Site Preparation Method to Increase Bone Density in Low-Density Bone: In Vivo. Implant Dent. Dent. 2016, 25, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lahens, B.; Neiva, R.; Tovar, N.; Alifarag, A.M.; Jimbo, R.; Bonfante, E.A.; Bowers, M.M.; Cuppini, M.; Freitas, H.; Witek, L. Biomechanical and histologic basis of osseodensification drilling for endosteal implant placement in low density bone. An experimental study in sheep. J. Mech. Behav. Biomed. Mater. 2016, 63, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Moon, S.; You, J.; Lee, W. The Effect of Under-Drilling and Osseodensification Drilling on Low-Density Bone: A Comparative Ex Vivo Study. Appl. Sci. 2022, 12, 1163. [Google Scholar] [CrossRef]
- Sultana, A.; Makkar, S.; Saxena, D.; Wadhawan, A.; Kusum, C.K. To compare the stability and crestal bone loss of implants placed using osseodensification and traditional drilling protocol: A clinicoradiographical study. J. Indian Prosthodont. Soc. 2020, 20, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Aloorker, S.; Shetty, M.; Hegde, C. Effect of Osseodensification on Bone Density and Crestal Bone Levels: A Split-mouth Study. J. Contemp. Dent. Pract. 2022, 23, 62–68. [Google Scholar] [CrossRef]
- Hassan, M.A.; El-zefzaf, E.A.K.; Mohamed, M. Comparative assessment for osseodensification versus conventional surgical technique in low density bone. J. Dent. Med. Sci. 2021, 20, 25–30. [Google Scholar] [CrossRef]
- Resnik, R.R.; Misch, C. Prophylactic Antibiotic Regimens in Oral Implantology: Rationale and Protocol. Implant Dent. 2008, 7, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.D.; Zauszniewski, J.A.; Musil, C.M. How to determine whether a convenience sample represents the population. Appl. Nurs. Res. 2004, 17, 130–133. [Google Scholar] [CrossRef]
- Marupudi, S.; Cao, Q.; Samala, R.; Petrick, N. Characterization of mechanical stiffness using additive manufacturing and finite element analysis: Potential tool for bone health assessment. 3D Print Med. 2023, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Pinhata-Baptista, O.; Goulart, I.; Ferraço, R.; Kim, J.H.; Abdala, R., Jr.; Arita, E.S.; Cortes, A.R.G.; Ackerman, J.L. Correlation among alveolar bone assessments provided by CBCT, micro-CT, and 14 MRI. Dentomaxillofacial Radiol. 2022, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, A.-P.; Ali, R.; Al-Gburi, A.; Liimatainen, T.; Matikka, H. A dose—Neutral image quality comparison of different CBCT and CT systems using paranasal sinus imaging protocols and phantoms. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Sghaireen, M.G.; Ganji, K.K.; Alam, M.K.; Srivastava, K.C.; Shrivastava, D.; Rahman, S.A. Comparing the Diagnostic Accuracy of CBCT Grayscale Values with DXA Values for the Detection of Osteoporosis. Appl. Sci. 2020, 10, 4584. [Google Scholar] [CrossRef]
- Pereira, J.F.; Costa, R.; Vasques, M.N.; Salazar, F.; Mendes, J.M.; Cãmara, M.I. Osseodensification: An Alternative to Conventional Osteotomy in Implant Site Preparation: A Systematic Review. J. Clin. Med. 2023, 12, 7046. [Google Scholar] [CrossRef]
- Alhamdani, F.; Abdulla, A.E. Influence of Patient’s Age and Gender on Dental Implant Treatment Five Year retrospective study. J. Med. Res. Health Sci. 2021, 4, 1461–1467. [Google Scholar] [CrossRef]
- Morar, L.; Băciuț, G.; Băciuț, M.; Bran, S.; Colosi, H.; Manea, A.; Almășan, O.; Dinu, C. Analysis of CBCT Bone Density Using the Hounsfield Scale. Prosthesis 2022, 1000, 414–423. [Google Scholar] [CrossRef]
| Soft Bone | Hard Bone (Mandible) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Implant Diameter | Drill | Bur 1 | Bur 2 | Bur 3 | Bur 4 | Drill | Bur 1 | Bur 2 | Bur 3 | Bur 4 |
| 3.3 | Pilot Drill | VT1828 | - | - | - | Pilot Drill | VT1828 | VS2228 | - | - |
| 4.1 | Pilot Drill | VT1525 | VT2535 | - | - | Pilot Drill | VT1828 | VT2535 | VT2838 | - |
| Technique | Sex | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Male | 12 | 57.1 | |
| Osseodensification | Female | 9 | 42.9 |
| Total | 21 | 100 | |
| Male | 10 | 50 | |
| Conventional | Female | 10 | 50 |
| Total | 20 | 100 |
| Jaw | Location | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Maxilla | Anterior | 7 | 17.1 |
| Posterior | 23 | 56.1 | |
| Mandible | Anterior | 2 | 4.9 |
| Posterior | 9 | 22.0 | |
| Total | 41 | 100 |
| Technique | Location | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Osseodensification | Anterior Maxilla | 3 | 14.3 |
| Posterior Maxilla | 12 | 57.1 | |
| Anterior Mandible | 1 | 4.8 | |
| Posterior Mandible | 5 | 23.8 | |
| Total | 21 | 100 | |
| Conventional | Anterior Maxilla | 4 | 20.0 |
| Posterior Maxilla | 11 | 55.0 | |
| Anterior Mandible | 1 | 5.0 | |
| Posterior Mandible | 4 | 20.0 | |
| Total | 20 | 100 |
| Implant Dimensions | Implant Dimensions (mm) | Osseodensification (n) | Conventional (n) | Total (n) | Total (%) |
|---|---|---|---|---|---|
| Width | 3.3 | 7 | 5 | 12 | 29.3 |
| 4.1 | 14 | 15 | 29 | 70.7 | |
| 41 | 100 | ||||
| Length | 8 | 4 | 4 | 8 | 19.5 |
| 10 | 7 | 10 | 17 | 41.4 | |
| 12 | 7 | 5 | 12 | 29.3 | |
| 14 | 3 | 1 | 4 | 9.8 | |
| 41 | 100 |
| Torque Insertion (N/cm) | Osteotomy (n) | Osseodensification (n) | Total (n) | Total (%) |
|---|---|---|---|---|
| 35 | 6 | 5 | 11 | 26.8 |
| ˃35 | 14 | 16 | 30 | 73.2 |
| 41 | 100 |
| Correlation Variables | Insertion Torque (p-Value) | Implant Dimension Width (p-Value) | Implant Dimension Length (p-Value) | Areas of Location (p-Value) | Sex (p-Value) |
|---|---|---|---|---|---|
| Bone density with Osseodensification | 0.892 * | 0.474 * | 0.098 * | 0.749 ** | 0.644 *** |
| Bone density with Conventional Osteotomy | 0.995 * | 0.585 * | 0.662 * | 0.918 ** | 0.290 *** |
| Technique | Parameters | Density Values | p-Value |
|---|---|---|---|
| Osseodensification | Median | 1020 | 0.028 * |
| IQR | 396.5 | ||
| Q1 | 781.5 | ||
| Q3 | 1178 | ||
| Conventional | Median | 732 | |
| IQR | 393.5 | ||
| Q1 | 615.3 | ||
| Q3 | 1008.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.A.; Mendes, J.M.; Salazar, F.; Pacheco, J.J.; Rompante, P.; Moreira, J.F.; Mesquita, J.D.; Adubeiro, N.; da Câmara, M.I. Osseodensification vs. Conventional Osteotomy: A Case Series with Cone Beam Computed Tomography. J. Clin. Med. 2024, 13, 1568. https://doi.org/10.3390/jcm13061568
Costa JA, Mendes JM, Salazar F, Pacheco JJ, Rompante P, Moreira JF, Mesquita JD, Adubeiro N, da Câmara MI. Osseodensification vs. Conventional Osteotomy: A Case Series with Cone Beam Computed Tomography. Journal of Clinical Medicine. 2024; 13(6):1568. https://doi.org/10.3390/jcm13061568
Chicago/Turabian StyleCosta, José Adriano, José Manuel Mendes, Filomena Salazar, José Júlio Pacheco, Paulo Rompante, Joaquim Ferreira Moreira, José Diogo Mesquita, Nuno Adubeiro, and Marco Infante da Câmara. 2024. "Osseodensification vs. Conventional Osteotomy: A Case Series with Cone Beam Computed Tomography" Journal of Clinical Medicine 13, no. 6: 1568. https://doi.org/10.3390/jcm13061568








