Navigating Social Waters: Understanding Theory-of-Mind Challenges in Patients with Mesial Temporal Lobe Epilepsy
Abstract
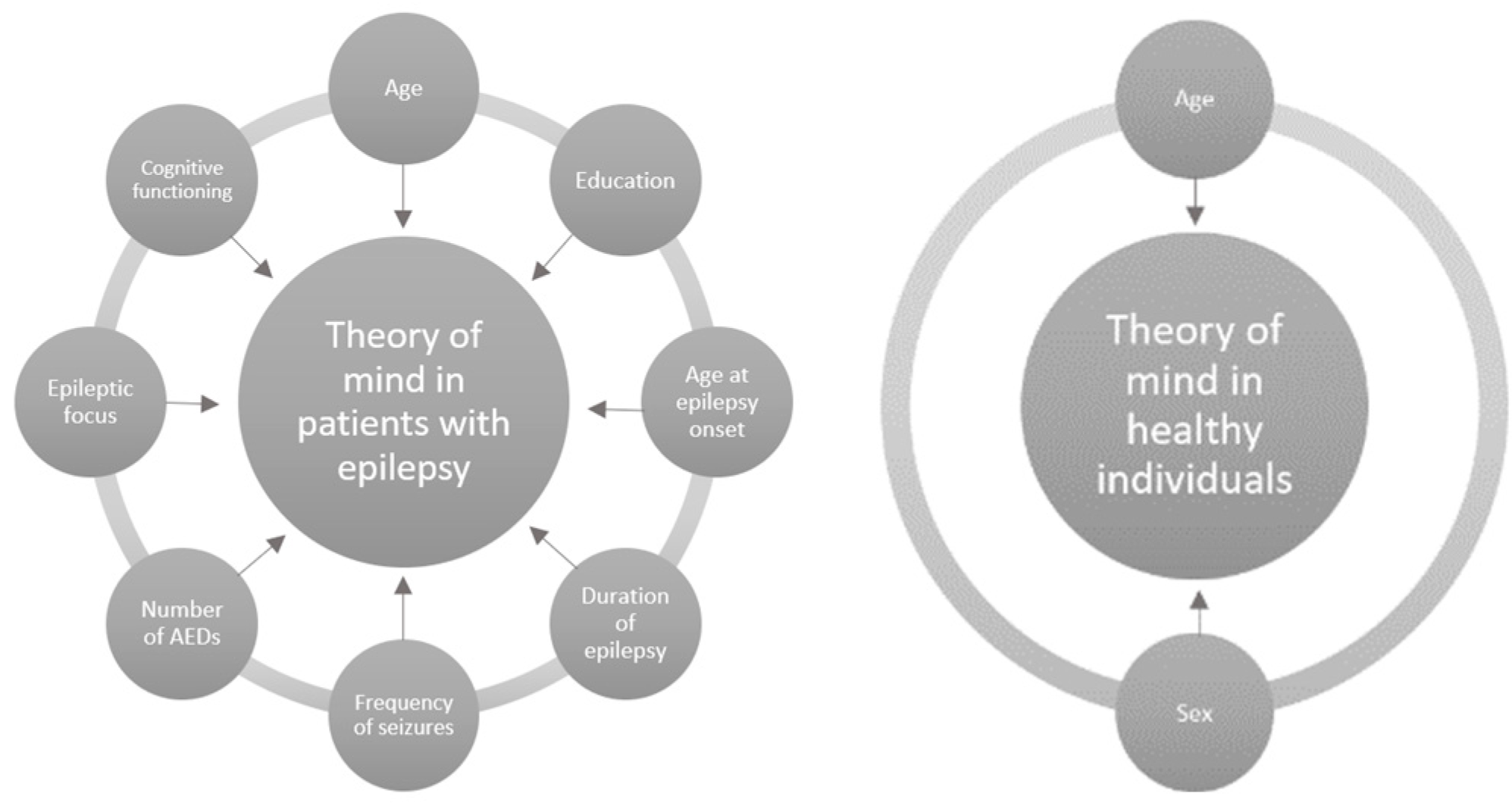
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Diagnostic Tools
2.3. Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef]
- Ngugi, A.; Kariuki, S.; Bottomley, C.; Kleinschmidt, I.; Sander, J.; Newton, C. Incidence of epilepsy: A systematic review and meta-analysis. Neurology 2011, 77, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Bertram, E.H. Temporal lobe epilepsy: Where do the seizures really begin? Epilepsy Behav. 2009, 14 (Suppl. 1), 32–37. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, W.C.; MDas, J. Temporal Seizure; StatPearls. StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yang, L.; Zhang, R.; Zhu, H.; Chen, F.; Yu, N.; Di, Q. Factors influencing the long-term prognosis of patients with temporal lobe epilepsy: A single center study. Ann. Palliat. Med. 2020, 9, 3194–3203. [Google Scholar] [CrossRef]
- Giovagnoli, A.R.; Avanzini, G. Quality of life and memory performance in patients with temporal lobe epilepsy. Acta Neurol. Scand. 2000, 101, 295–300. [Google Scholar] [CrossRef]
- Green, M.F.; Penn, D.L.; Bentall, R.; Carpenter, W.T.; Gaebel, W.; Gur, R.C.; Kring, A.M.; Park, S.; Silverstein, S.M.; Heinssen, R.; et al. Social cognition in schizophrenia: An NIMH workshop on definitions, assessment, and research opportunities. Schizophr. Bull. 2008, 34, 1211–1220. [Google Scholar] [CrossRef]
- Adolphs, R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001, 11, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Frith, C.D. The social brain? Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 671–678. [Google Scholar] [CrossRef]
- Bala, A.; Okruszek, Ł.; Piejka, A.; Głębicka, A.; Szewczyk, E.; Bosak, K.; Szantroch, M.; Hyniewska, S.; Rysz, A.; Marchel, A. Social Perception in Mesial Temporal Lobe Epilepsy: Interpreting Social Information From Moving Shapes and Biological Motion. J. Neuropsychiatry 2018, 30, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Meletti, S. Social cognition in temporal lobe epilepsy: A systematic review and meta-analysis. Epilepsy Behav. 2016, 60, 50–57. [Google Scholar] [CrossRef]
- Cristinzio, C.; Vuilleumier, P. The role of amygdala in emotional and social functions: Implications for temporal lobe epilepsy. Epileptologie 2007, 24, 78–89. [Google Scholar]
- Okruszek, Ł.; Bala, A.; Dziekan, M.; Szantroch, M.; Rysz, A.; Marchel, A.; Hyniewska, S. Gaze matters! The effect of gaze direction on emotional enhancement of memory for faces in patients with mesial temporal lobe epilepsy. Epilepsy Behav. 2017, 72, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhao, J.; Zhao, P.; Zhang, H.; Zhong, J.; Pan, P.; Wang, G.; Yi, Z.; Xie, L. Theory of mind and facial emotion recognition in adults with temporal lobe epilepsy: A meta-analysis. Front. Psychiatry 2022, 13, 976439. [Google Scholar] [CrossRef] [PubMed]
- Premack, D.; Woodruff, G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1978, 1, 515–526. [Google Scholar] [CrossRef]
- Frith, U. The 38th Sir Frederick Bartlett Lecture Why we need cognitive explanations of autism. Q. J. Exp. Psychol. 2012, 65, 2073–2092. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, A.R.; Parente, A.; Villani, F.; Franceschetti, S.; Spreafico, R. Theory of mind and epilepsy: What clinical implications? Epilepsia 2013, 54, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Washburn, D.; Wilson, G.; Roes, M.; Rnic, K.; Harkness, K.L. Theory of mind in social anxiety disorder, depression, and comorbid conditions. J. Anxiety Disord. 2016, 37, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Preckel, K.; Kanske, P.; Singer, T. On the interaction of social affect and cognition: Empathy, compassion and theory of mind. Curr. Opin. Behav. Sci. 2018, 19, 1–6. [Google Scholar] [CrossRef]
- Banati, M.; Sandor, J.; Mike, A.; Illes, E.; Bors, L.; Feldmann, A.; Herold, R.; Illes, Z. Social cognition and theory of mind in patients with relapsing-remitting multiple sclerosis. Eur. J. Neurol. 2010, 17, 426–433. [Google Scholar] [CrossRef]
- Moreau, N.; Rauzy, S.; Viallet, F.; Champagne-Lavau, M. Theory of mind in Alzheimer disease: Evidence of authentic impairment during social interaction. Neuropsychology 2016, 30, 312–321. [Google Scholar] [CrossRef]
- Martín-Rodríguez, J.F.; León-Carrión, J. Theory of mind deficits in patients with acquired brain injury: A quantitative review. Neuropsychologia 2010, 48, 1181–1191. [Google Scholar] [CrossRef]
- Suurmeijer, T.P.; Reuvekamp, M.F.; Aldenkamp, B.P. Social functioning, psychological functioning, and quality of life in epilepsy. Epilepsia 2001, 42, 1160–1168. [Google Scholar] [CrossRef]
- Thompson, P. The importance of theory of mind in epilepsy. Epilepsy Behav. 2014, 39, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, Y.; Wang, Y.; Zhou, N.; Zhu, L.; Wang, K. Empathy and emotion recognition in patients with idiopathic generalized epilepsy. Epilepsy Behav. 2014, 37, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Schacher, M.; Winkler, R.; Grunwald, T.; Kraemer, G.; Kurthen, M.; Reed, V.; Jokeit, H. Mesial temporal lobe epilepsy impairs advanced social cognition. Epilepsia 2006, 47, 2141–2146. [Google Scholar] [CrossRef]
- Giovagnoli, A.R.; Franceschetti, S.; Reati, F.; Parente, A.; Maccagnano, C.; Villani, F.; Spreafico, R. Theory of mind in frontal and temporal lobe epilepsy: Cognitive and neural aspects. Epilepsia 2011, 52, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Hennion, S.; Delbeuck, X.; Koelkebeck, K.; Brion, M.; Tyvaert, L.; Plomhause, L.; Derambure, P.; Lopes, R.; Szurhaj, W. A functional magnetic resonance imaging investigation of theory of mind impairments in patients with temporal lobe epilepsy. Neuropsychologia 2016, 93, 271–279. [Google Scholar] [CrossRef]
- Hennion, S.; Delbeuck, X.; Duhamel, A.; Lopes, R.; Semah, F.; Tyvaert, L.; Derambure, P.; Szurhaj, W. Characterization and prediction of theory of mind disorders in temporal lobe epilepsy. Neuropsychology 2015, 29, 485–492. [Google Scholar] [CrossRef]
- Shaw, P.; Lawrence, E.J.; Radbourne, C.; Bramham, J.; Polkey, C.E.; David, A.S. The impact of early and late damage to the human amygdala on ‘theory of mind’ reasoning. Brain 2004, 127 Pt B, 1535–1548. [Google Scholar] [CrossRef]
- Stone, V.E.; Baron-Cohen, S.; Knight, R.T. Frontal lobe contributions to theory of mind. J. Cogn. Neurosci. 1998, 10, 640–656. [Google Scholar] [CrossRef]
- Corcoran, R.; Mercer, G.; Frith, C.D. Schizophrenia, symptomatology and social inference: Investigating “theory of mind” in people with schizophrenia. Schizophr. Res. 1995, 17, 5–13. [Google Scholar] [CrossRef]
- Matczak, A.; Piekarska, A. Test Rozumienia Emocji TRE. Podręcznik; Pracownia Testów Psychologicznych Polskiego Towarzystwa Psychologicznego: Warsaw, Poland, 2011. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Bottiroli, S.; Cavallini, E.; Ceccato, I.; Vecchi, T.; Lecce, S. Theory of Mind in aging: Comparing cognitive and affective components in the faux pas test. Arch. Gerontol. Geriatr. 2016, 62, 152–162. [Google Scholar] [CrossRef]
- Lindgren, M.; Torniainen-Holm, M.; Heiskanen, I.; Voutilainen, G.; Pulkkinen, U.; Mehtälä, T.; Jokela, M.; Kieseppä, T.; Suvisaari, J.; Therman, S. Theory of mind in a first-episode psychosis population using the Hinting Task. Psychiatry Res. 2018, 263, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Chiu, M.-J.; Yeh, Z.-T.; Liou, H.-H.; Cheng, T.-W.; Hua, M.-S. Theory of mind in patients with temporal lobe epilepsy. J. Int. Neuropsychol. Soc. 2013, 19, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Morou, N.; Papaliagkas, V.; Markouli, E.; Karagianni, M.; Nazlidou, E.; Spilioti, M.; Afrantou, T.; Kimiskidis, V.K.; Foroglou, N.; Kosmidis, M.H. Theory of Mind impairment in focal versus generalized epilepsy. Epilepsy Behav. 2018, 88, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ives-Deliperi, V.L.; Jokeit, H. Impaired Social Cognition in Epilepsy: A Review of What We Have Learnt From Neuroimaging Studies. Front. Neurol. 2019, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, P.; Zhao, J.; Zhong, J.; Pan, P.; Wang, G.; Yi, Z. Theory of Mind and Empathy in Adults with Epilepsy: A Meta-Analysis. Front. Psychiatry 2022, 13, 877957. [Google Scholar] [CrossRef]
- Novak, A.; Vizjak, K.; Rakusa, M. Cognitive Impairment in People with Epilepsy. J. Clin. Med. 2022, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Whitten, A.; Jacobs, M.L.; Englot, D.J.; Rogers, B.P.; Levine, K.K.; González, H.F.; Morgan, V.L. Resting-state hippocampal networks related to language processing reveal unique patterns in temporal lobe epilepsy. Epilepsy Behav. 2021, 117, 107834. [Google Scholar] [CrossRef] [PubMed]
- Broicher, S.D.; Frings, L.; Huppertz, H.-J.; Grunwald, T.; Kurthen, M.; Krämer, G.; Jokeit, H. Alterations in functional connectivity of the amygdala in unilateral mesial temporal lobe epilepsy. J. Neurol. 2012, 259, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Broicher, S.D.; Kuchukhidze, G.; Grunwald, T.; Krämer, G.; Kurthen, M.; Jokeit, H. “Tell me how do I feel”—Emotion recognition and theory of mind in symptomatic mesial temporal lobe epilepsy. Neuropsychologia 2012, 50, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Lawrence, E.; Bramham, J.; Brierley, B.; Radbourne, C.; David, A. A prospective study of the effects of anterior temporal lobectomy on emotion recognition and theory of mind. Neuropsychologia 2007, 45, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.; Catroppa, C.; Gonzalez, L.; Gill, D.; Webster, R.; Lawson, J.; Sabaz, M.; Mandalis, A.; Barton, B.; McLean, S.; et al. Facial emotion perception and social competence in children (8 to 16 years old) with genetic generalized epilepsy and temporal lobe epilepsy. Epilepsy Behav. 2019, 100 Pt B, 106301. [Google Scholar] [CrossRef]
- Park, S.-P.; Kwon, S.-H. Cognitive effects of antiepileptic drugs. J. Clin. Neurol. 2008, 4, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.; Catroppa, C.; Lah, S. Theory of Mind in Patients with Epilepsy: A Systematic Review and Meta-analysis. Neuropsychol. Rev. 2016, 26, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.M.; Warrier, V.; Abu-Akel, A.; Allison, C.; Gajos, K.Z.; Reinecke, K.; Rentfrow, P.J.; Radecki, M.A.; Baron-Cohen, S. Sex and age differences in “theory of mind” across 57 countries using the English version of the “Reading the Mind in the Eyes” Test. Proc. Natl. Acad. Sci. USA 2023, 120, e2022385119. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, J.; Neufeld, J.; Bölte, S. What’s the Link Between Theory of Mind and Other Cognitive Abilities—A Co-twin Control Design of Neurodevelopmental Disorders. Front. Psychol. 2021, 12, 575100. [Google Scholar] [CrossRef]
- Giovagnoli, A.R. Theory of mind across lifespan from ages 16 to 81 years. Epilepsy Behav. 2019, 100, 106349. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y. Theory of mind in old adults: The performance on Happe’s stories and faux pas stories. Psychologia 2006, 49, 228–237. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Sharma, H.; Bhaskar, R.; Narayanan, K.B. A Comprehensive Review of Emerging Trends and Innovative Therapies in Epilepsy Management. Brain Sci. 2023, 13, 1305. [Google Scholar] [CrossRef] [PubMed]

| MTLE Group n = 44 | Healthy Controls n = 21 | p | |
|---|---|---|---|
| Sex: male/female | 20/24 | 10/11 | >0.05 |
| Handedness: R/L | 41/3 | 20/1 | >0.05 |
| Age M(SD) | 35.70 (10.61) | 33.23 (11.49) | >0.05 |
| Years of education M(SD) | 13.48 (2.92) | 14.00 (1.51) | >0.05 |
| Epilepsy onset M(SD) | 13.14 (11.43) | N/A | |
| Years of epilepsy M(SD) | 21.96 (11.06) | N/A | |
| Numer of seizures/month M(SD) | 8.43 (10.63) | N/A |
| Clinical Group n = 44 | Control Group n = 21 | Statistics | p Value | Cohen’s d | ||
|---|---|---|---|---|---|---|
| Faux Pas Test | Detection | 13.72 (5.83) | 34.80 (3.54) | t = 17.99 | p < 0.001 | 4.04 |
| Understanding | 10.52 (3.43) | 15.23 (2.38) | t = 6.42 | p < 0.001 | 1.50 | |
| Intentions | 10.29 (3.10) | 15.04 (2.35) | t = 6.20 | p < 0.001 | 1.64 | |
| Bieliefs | 9.70 (3.06) | 15.71 (2.43) | t = 7.86 | p < 0.001 | 2.08 | |
| Empathy | 10.31 (2.73) | 15.85 (2.78) | t = 7.59 | p < 0.001 | 2.01 | |
| Comprehension | 33.18 (4.47) | 35.00 (3.97) | t = 1.58 | p = 0.059 | 0.42 | |
| Emotion Comprehension Test | Situation | 2.22 (1.64) | 4.38 (1.39) | t = 5.18 | p < 0.001 | 1.37 |
| Reaction | 2.25 (1.55) | 3.66 (1.71) | t = 3.32 | p < 0.001 | 0.88 | |
| Hinting Task | 14.45 (4.23) | 18.47 (1.56) | t = 5.54 | p < 0.001 | 1.11 | |
| Age | Sex | Years of Education | Age at Epilepsy Onset | Seizures/Month | Number of AEDs | Epi Focus | MoCA Scores | ||
|---|---|---|---|---|---|---|---|---|---|
| Faux Pas Test | Detection | p > 0.05 | p > 0.05 | r = 0.75 p < 0.01 | r = 0.65 p < 0.05 | p > 0.05 | r = −0.19 p < 0.05 | p > 0.05 | r = 0.28 p < 0.05 |
| Understanding | p > 0.05 | p > 0.05 | r = 0.53 p < 0.05 | r = 0.48 p < 0.05 | p > 0.05 | p > 0.05 | η = 0.34 p < 0.05 | p > 0.05 | |
| Intentions | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | |
| Bieliefs | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | |
| Empathy | p > 0.05 | p > 0.05 | p > 0.05 | r = 0.69 p < 0.01 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | |
| Comprehension | p > 0.05 | p > 0.05 | r = 0.31 p < 0.0 | p > 0.05 | p > 0.05 | p > 0.05 | η = 0.55 p < 0.05 | r = 0.24 p < 0.05 | |
| Emotion Comprehension Test | Situation | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | r = 0.21 p < 0.05 |
| Reaction | r = 0.54 p < 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | r = 0.31 p < 0.05 | |
| Hinting Task | p > 0.05 | p > 0.05 | r = 0.61 p < 0.05 | p > 0.05 | r = −0.33 p < 0.05 | p > 0.05 | η = 0.42 p < 0.05 | p > 0.05 | |
| Age | Sex | Years of Education | MoCA Scores | ||
|---|---|---|---|---|---|
| Faux Pas Test | Detection | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
| Understanding | p > 0.05 | η = 0.38 p < 0.05 | p > 0.05 | p > 0.05 | |
| Intentions | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | |
| Bieliefs | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | |
| Empathy | p > 0.05 | η = 0.52 p < 0.01 | p > 0.05 | p > 0.05 | |
| Comprehension | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | |
| Emotion Comprehension Test | Situation | p > 0.05 | η = 0.51 p < 0.05 | p > 0.05 | p > 0.05 |
| Reaction | r = 0.29 p < 0.05 | η = 0.43 p < 0.05 | p > 0.05 | p > 0.05 | |
| Hinting Task | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bala, A.; Olejnik, A.; Mojżeszek, M.; Rysz, A.; Kunert, P. Navigating Social Waters: Understanding Theory-of-Mind Challenges in Patients with Mesial Temporal Lobe Epilepsy. J. Clin. Med. 2024, 13, 1410. https://doi.org/10.3390/jcm13051410
Bala A, Olejnik A, Mojżeszek M, Rysz A, Kunert P. Navigating Social Waters: Understanding Theory-of-Mind Challenges in Patients with Mesial Temporal Lobe Epilepsy. Journal of Clinical Medicine. 2024; 13(5):1410. https://doi.org/10.3390/jcm13051410
Chicago/Turabian StyleBala, Aleksandra, Agnieszka Olejnik, Maria Mojżeszek, Andrzej Rysz, and Przemysław Kunert. 2024. "Navigating Social Waters: Understanding Theory-of-Mind Challenges in Patients with Mesial Temporal Lobe Epilepsy" Journal of Clinical Medicine 13, no. 5: 1410. https://doi.org/10.3390/jcm13051410
APA StyleBala, A., Olejnik, A., Mojżeszek, M., Rysz, A., & Kunert, P. (2024). Navigating Social Waters: Understanding Theory-of-Mind Challenges in Patients with Mesial Temporal Lobe Epilepsy. Journal of Clinical Medicine, 13(5), 1410. https://doi.org/10.3390/jcm13051410






