Stage 5 Chronic Kidney Disease: Epidemiological Analysis in a NorthEastern District of Italy Focusing on Access to Nephrological Care
Abstract
1. Introduction
2. Materials and Methods
- -
- Belonging to other healthcare system districts;
- -
- The presence of acute kidney injuries with the subsequent improvement of eGFR over 30 mL/min.
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Johansen, K.L.; Chertow, G.M.; Foley, R.N.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Kurella Tamura, M.; Li, S.; et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021, 77 (Suppl. S1), A7–A8. [Google Scholar] [CrossRef]
- United States Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2019. [Google Scholar]
- Freedberg, D.E.; Segall, L.; Liu, B.; Jacobson, J.S.; Mohan, S.; George, V.; Kumar, R.; Neugut, A.I.; Radhakrishnan, J. International Variability in the Epidemiology, Management, and Outcomes of Chronic and End-Stage Kidney Disease: A Systematic Review. Kidney360 2023, 5, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Han, K.D.; Oh, T.R.; Suh, S.H.; Kim, M.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Trends in the incidence and prevalence of end-stage renal disease with hemodialysis in entire Korean population: A nationwide population-based study. Medicine 2021, 100, e25293. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.J. Epidemiology of end stage renal disease and implications for public policy. Public Health Rep. 1984, 99, 492–498. [Google Scholar] [PubMed]
- Registro Italiano di Dialisi e Trapianto. 2019 Dati Forniti dal RIDT, Registro Italiano di Dialisi e Trapianto. Available online: www.sin-ridt.org (accessed on 1 November 2023).
- Martino, F.K.; Novara, G.; Nalesso, F.; Calò, L.A. Conservative Management in End-Stage Kidney Disease between the Dialysis Myth and Neglected Evidence-Based Medicine. J. Clin. Med. 2024, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, L.; Osypuk, T.L.; MacLehose, R.F.; Luepker, R.V.; Drawz, P.E. Neighborhood Socioeconomic Status, Health Insurance, and CKD Prevalence: Findings from a Large Health Care System. Kidney Med. 2021, 3, 555–564. [Google Scholar] [CrossRef]
- Winitzki, D.; Zacharias, H.U.; Nadal, J.; Baid-Agrawal, S.; Schaeffner, E.; Schmid, M.; Busch, M.; Bergmann, M.M.; Schultheiss, U.; Kotsis, F.; et al. Educational Attainment Is Associated with Kidney and Cardiovascular Outcomes in the German CKD (GCKD) Cohort. Kidney Int. Rep. 2022, 7, 1004–1015. [Google Scholar] [CrossRef]
- Hara, A.; Hirata, T.; Okamura, T.; Kimura, S.; Urushihara, H. Lifestyle behaviors associated with the initiation of renal replacement therapy in Japanese patients with chronic kidney disease: A retrospective cohort study using a claims database linked with specific health checkup results. Environ. Health Prev. Med. 2021, 26, 102. [Google Scholar] [CrossRef]
- Ni, W.; Yuan, X.; Zhang, Y.; Zhang, H.; Zheng, Y.; Xu, J. Sociodemographic and lifestyle determinants of multimorbidity among community-dwelling older adults: Findings from 346,760 SHARE participants. BMC Geriatr. 2023, 23, 419. [Google Scholar] [CrossRef] [PubMed]
- Milkowski, A.; Prystacki, T.; Marcinkowski, W.; Dryl-Rydzynska, T.; Zawierucha, J.; Malyszko, J.S.; Zebrowski, P.; Zuzda, K.; Małyszko, J. Lack or insufficient predialysis nephrology care worsens the outcomes in dialyzed patients—Call for action. Ren. Fail. 2022, 44, 946–957. [Google Scholar] [CrossRef]
- Gambaro, G.; Yabarek, T.; Graziani, M.S.; Gemelli, A.; Abaterusso, C.; Frigo, A.C.; Marchionna, N.; Citron, L.; Bonfante, L.; Grigoletto, F.; et al. Prevalence of CKD in northeastern Italy: Results of the INCIPE study and comparison with NHANES. Clin. J. Am. Soc. Nephrol. 2010, 5, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Donfrancesco, C.; Palleschi, S.; Palmieri, L.; Rossi, B.; Lo Noce, C.; Pannozzo, F.; Spoto, B.; Tripepi, G.; Zoccali, C.; Giampaoli, S. Estimated glomerular filtration rate, all-cause mortality and cardiovascular diseases incidence in a low risk population: The MATISS study. PLoS ONE 2013, 8, e78475. [Google Scholar] [CrossRef]
- Capuano, V.; Lamaida, N.; Borrelli, M.I.; Capuano, E.; Fasolino, A.; Capuano, E.; Sonderegger, M.; Capuano, R.; Citro, V.; Franculli, F. Prevalenza e trend (1998–2008) dell’insufficienza renale cronica in un’area dell’Italia meridionale: I dati del progetto VIP [Chronic kidney disease prevalence and trends (1998–2008) in an area of southern Italy. The data of the VIP project]. G. Ital. Nefrol. Organo Uff. Della Soc. Ital. Nefrol. 2012, 29, 445–451. [Google Scholar]
- Pani, A.; Bragg-Gresham, J.; Masala, M.; Piras, D.; Atzeni, A.; Pilia, M.G.; Ferreli, L.; Balaci, L.; Curreli, N.; Delitala, A.; et al. Prevalence of CKD and its relationship to eGFR-related genetic loci and clinical risk factors in the SardiNIA study cohort. J. Am. Soc. Nephrol. 2014, 25, 1533–1544. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter. Suppl. 2012, 2, 1–138. [Google Scholar]
- De Jong, P.E.; van der Velde, M.; Gansevoort, R.T.; Zoccali, C. Screening for chronic kidney disease: Where does Europe go? Clin. J. Am. Soc. Nephrol. 2008, 3, 616–623. [Google Scholar] [CrossRef]
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020. [Google Scholar]
- Kampmann, J.D.; Heaf, J.G.; Mogensen, C.B.; Mickley, H.; Wolff, D.L.; Brandt, F. Prevalence and incidence of chronic kidney disease stage 3–5—Results from KidDiCo. BMC Nephrol. 2023, 24, 17. [Google Scholar] [CrossRef]
- Schlanger, L.E.; Bailey, J.L.; Sands, J.M. Geriatric nephrology: Old or new subspecialty. Clin. Geriatr. Med. 2009, 25, 311–324. [Google Scholar] [CrossRef]
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef]
- Kurella, M.; Chertow, G.M.; Fried, L.F.; Cummings, S.R.; Harris, T.; Simonsick, E.; Satterfield, S.; Ayonayon, H.; Yaffe, K. Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J. Am. Soc. Nephrol. 2005, 16, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.E.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.B.; Essa, H.; Wattoo, A.; Gulab, A.; Akhtar, H.; Sudani, H.A.; Angelim, L.; Helfman, B.; Peterson, E.; Brousas, S.; et al. Representation of Chronic Kidney Disease in Randomized Controlled Trials among Patients with Heart failure with Reduced Ejection Fraction: A Systematic Review. Curr. Probl. Cardiol. 2023, 48, 101047. [Google Scholar] [CrossRef] [PubMed]
- Laffin, L.J.; Bakris, G.L. Intersection between Chronic Kidney Disease and Cardiovascular Disease. Curr. Cardiol. Rep. 2021, 23, 117. [Google Scholar] [CrossRef]
- Piko, N.; Bevc, S.; Ekart, R.; Petreski, T.; Vodošek Hojs, N.; Hojs, R. Diabetic patients with chronic kidney disease: Non-invasive assessment of cardiovascular risk. World J. Diabetes 2021, 12, 975–996. [Google Scholar] [CrossRef]
- Collins, A.J.; Gilbertson, D.T.; Snyder, J.J.; Chen, S.C.; Foley, R.N. Chronic kidney disease awareness, screening and prevention: Rationale for the design of a public education program. Nephrology 2010, 15 (Suppl. S2), 37–42. [Google Scholar] [CrossRef]
- Stolpe, S.; Kowall, B.; Scholz, C.; Stang, A.; Blume, C. High Unawareness of Chronic Kidney Disease in Germany. Int. J. Environ. Res. Public Health 2021, 18, 11752. [Google Scholar] [CrossRef]
- Zdrojewski, Ł.; Król, E.; Rutkowski, B.; Piotrowski, W.; Pająk, A.; Drygas, W.; Zdrojewski, T. Chronic kidney disease in Polish elderly population aged 75+: Results of the WOBASZ Senior Survey. Int. Urol. Nephrol. 2017, 49, 669–676. [Google Scholar] [CrossRef]
- Hödlmoser, S.; Winkelmayer, W.C.; Zee, J.; Pecoits-Filho, R.; Pisoni, R.L.; Port, F.K.; Robinson, B.M.; Ristl, R.; Krenn, S.; Kurnikowski, A.; et al. Sex differences in chronic kidney disease awareness among US adults, 1999 to 2018. PLoS ONE 2020, 15, e0243431. [Google Scholar] [CrossRef]
- Morton, R.L.; Turner, R.M.; Howard, K.; Snelling, P.; Webster, A.C. Patients who plan for conservative care rather than dialysis: A national observational study in Australia. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2012, 59, 419–427. [Google Scholar] [CrossRef] [PubMed]
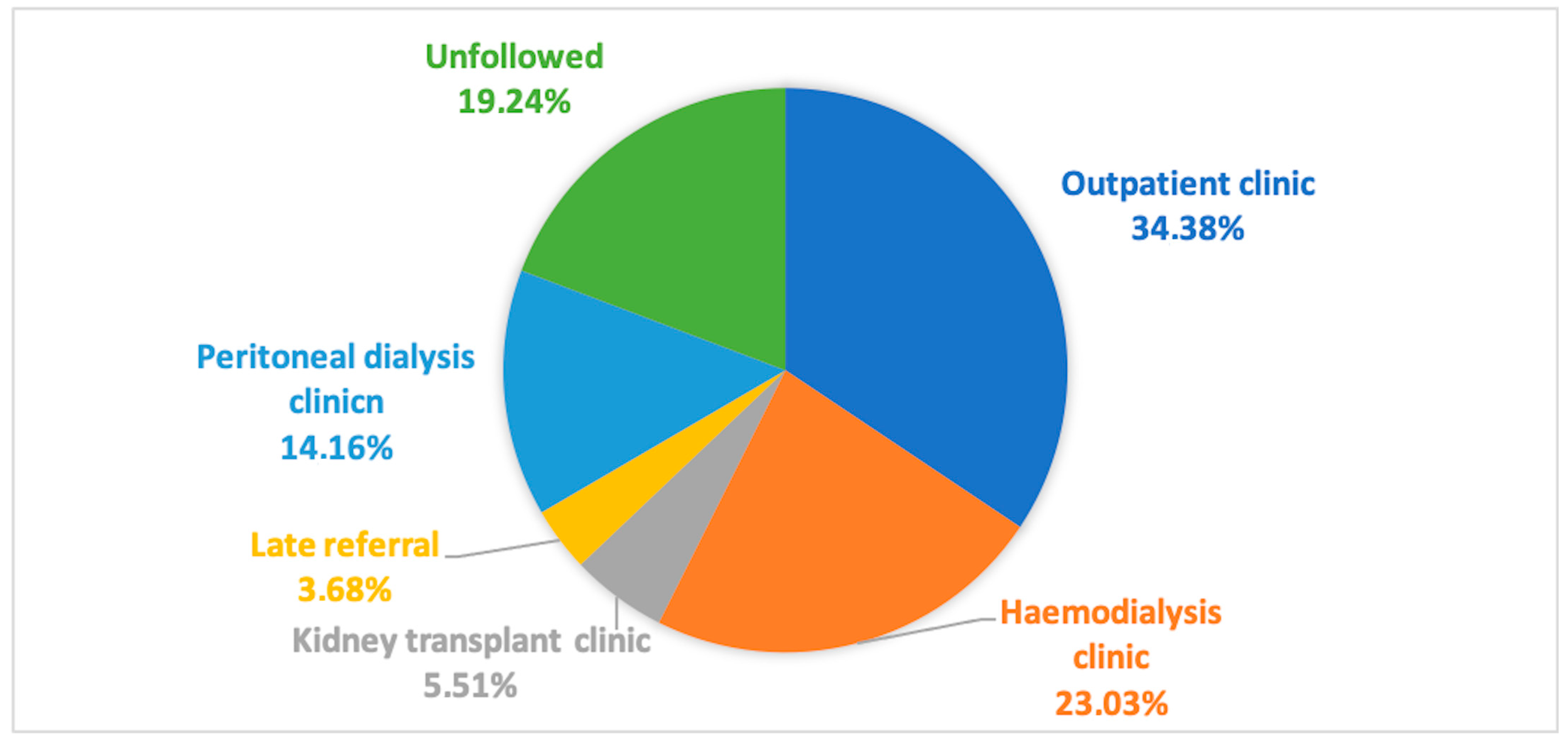
| All Population (925) | Followed (747) | Unfollowed (178) | Haemodialysis Clinic (213) | Peritoneal Dialysis Clinic (131) | Kidney Transplant Clinic (51) | Outpatient Clinic (318) | Late Referral (34) | p Values | |
|---|---|---|---|---|---|---|---|---|---|
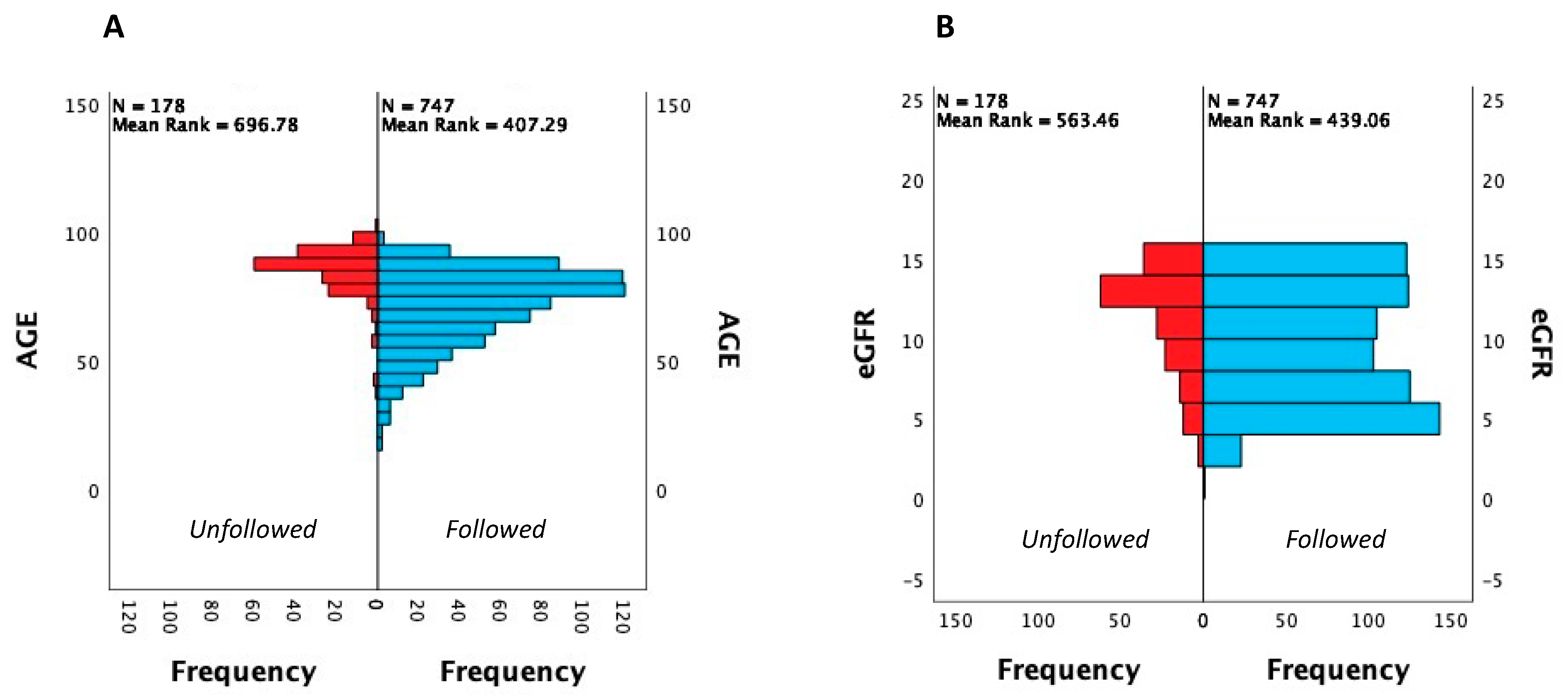
| Age, yrs (median, IQR) | 78 (65.5–86) | 75 (62–83) * | 88 (82–91) * | 70 (61–78) | 64 (53–76) | 51 (41–62) | 82 (75–87) | 75.5 (68–83) | * <0.0001 |
| Female gender (%) | 50.7% | 47% * | 66.3% * | 41.8% | 35.1% | 44.7% | 56.6% | 47.1% | * <0.001 |
| eGFR, ml/min (median, IQR) | 10 (6–13) | 9 (6–12) | 12 (9–13) * | 6 (5–8) | 6 (5–9) | 8 (5–10.5) | 12 (10–14) | 8.5 (5–10.2) | * <0.001 |
| Death (%) | 27.6% | 14.3% * | 83.1% * | 16.9% | 9.9% | 10.5% | 14.5% | 20.6% | * <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, F.K.; Fanton, G.; Zanetti, F.; Carta, M.; Nalesso, F.; Novara, G. Stage 5 Chronic Kidney Disease: Epidemiological Analysis in a NorthEastern District of Italy Focusing on Access to Nephrological Care. J. Clin. Med. 2024, 13, 1144. https://doi.org/10.3390/jcm13041144
Martino FK, Fanton G, Zanetti F, Carta M, Nalesso F, Novara G. Stage 5 Chronic Kidney Disease: Epidemiological Analysis in a NorthEastern District of Italy Focusing on Access to Nephrological Care. Journal of Clinical Medicine. 2024; 13(4):1144. https://doi.org/10.3390/jcm13041144
Chicago/Turabian StyleMartino, Francesca K., Giulia Fanton, Fiammetta Zanetti, Mariarosa Carta, Federico Nalesso, and Giacomo Novara. 2024. "Stage 5 Chronic Kidney Disease: Epidemiological Analysis in a NorthEastern District of Italy Focusing on Access to Nephrological Care" Journal of Clinical Medicine 13, no. 4: 1144. https://doi.org/10.3390/jcm13041144
APA StyleMartino, F. K., Fanton, G., Zanetti, F., Carta, M., Nalesso, F., & Novara, G. (2024). Stage 5 Chronic Kidney Disease: Epidemiological Analysis in a NorthEastern District of Italy Focusing on Access to Nephrological Care. Journal of Clinical Medicine, 13(4), 1144. https://doi.org/10.3390/jcm13041144









