Cardiomyopathy in Celiac Disease: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
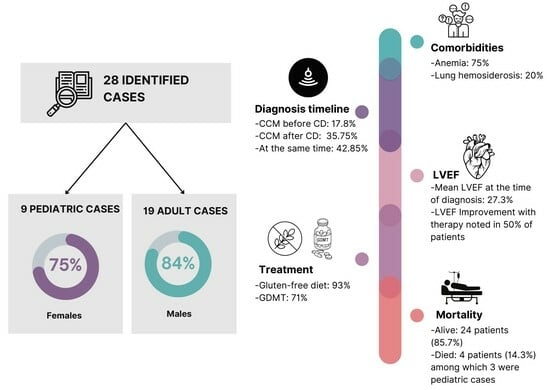
3.1. Demographics and Comorbidities
3.2. Presentation
3.3. Evaluation
3.4. Treatment, Complications, and Outcomes
4. Discussion
4.1. Pathophysiology
4.2. Clinical Presentation
4.3. Diagnosis and Testing
4.4. Treatment
4.5. Complications and Outcomes
5. Conclusions
6. Limitations of Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fasano, A.; Catassi, C. Celiac Disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Oxentenko, A.S.; Rubio-Tapia, A. Celiac Disease. Mayo Clin. Proc. 2019, 94, 2556–2571. [Google Scholar] [CrossRef] [PubMed]
- Laurikka, P.; Nurminen, S.; Kivelä, L.; Kurppa, K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients 2018, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, M.; Ferro, A.; Brascugli, I.; Mattivi, S.; Fagoonee, S.; Pellicano, R. Extra-Intestinal Manifestations of Celiac Disease: What Should We Know in 2022? J. Clin. Med. 2022, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Balaban, D.V.; Popp, A.; Ionita Radu, F.; Jinga, M. Hematologic Manifestations in Celiac Disease—A Practical Review. Medicina 2019, 55, 373. [Google Scholar] [CrossRef] [PubMed]
- Balaban, D.V.; Coman, L.I.; Enache, I.C.; Mardan, C.M.; Dima, A.; Jurcuț, C.; Balaban, M.; Costache, R.S.; Ioniță-Radu, F.; Popp, A.; et al. Prevalence of Coagulopathy in Patients with Celiac Disease: A Single-Center Retrospective Case-Control Study. Gastroenterol. Insights 2023, 14, 463–474. [Google Scholar] [CrossRef]
- Pantic, N.; Pantic, I.; Jevtic, D.; Mogulla, V.; Oluic, S.; Durdevic, M.; Nordin, T.; Jecmenica, M.; Milovanovic, T.; Gavrancic, T.; et al. Celiac Disease and Thrombotic Events: Systematic Review of Published Cases. Nutrients 2022, 14, 2162. [Google Scholar] [CrossRef]
- Maisch, B. Classification of cardiomyopathies according to the WHO/ISFC Task Force—More questions than answers? Med. Klin. Munich 1998, 93, 199–209. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Maron, B.J. The 2006 American Heart Association Classification of Cardiomyopathies Is the Gold Standard. Circ. Heart Fail. 2008, 1, 72–76. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Moroni, F.; Montone, R.A.; Azzalini, L.; Sanna, T.; Abbate, A. Ischemic Cardiomyopathy and Heart Failure After Acute Myocardial Infarction. Curr. Cardiol. Rep. 2022, 24, 1505–1515. [Google Scholar] [CrossRef]
- Dilated Cardiomyopathy—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441911/ (accessed on 14 April 2022).
- Ciaccio, E.J.; Lewis, S.K.; Biviano, A.B.; Iyer, V.; Garan, H.; Green, P.H. Cardiovascular involvement in celiac disease. World J. Cardiol. 2017, 9, 652–666. [Google Scholar] [CrossRef]
- Zahmatkeshan, M.; Fallahpoor, M.; Amoozgar, H. Prevalence of celiac disease in children with idiopathic dilated cardiomyopathy. Iran. J. Pediatr. 2014, 24, 587–592. [Google Scholar]
- Curione, M.; Barbato, M.; Biase, L.D.; Viola, F.; Russo, L.L.; Cardi, E. Prevalence of coeliac disease in idiopathic dilated cardiomyopathy. Lancet 1999, 354, 222–223. [Google Scholar] [CrossRef]
- Not, T.; Faleschini, E.; Tommasini, A.; Repetto, A.; Pasotti, M.; Baldas, V.; Spano, A.; Sblattero, D.; Marzari, R.; Campana, C.; et al. Celiac disease in patients with sporadic and inherited cardiomyopathies and in their relatives. Eur. Heart J. 2003, 24, 1455–1461. [Google Scholar] [CrossRef]
- Barrio, J.P.; Cura, G.; Ramallo, G.; Diez, M.; Vigliano, C.A.; Katus, H.A.; Mereles, D. Heart transplantation in rapidly progressive end-stage heart failure associated with celiac disease. BMJ Case Rep. 2011, 2011, bcr1220103624. [Google Scholar] [CrossRef]
- Poddar, B.; Shava, U.; Srivastava, A.; Kapoor, A. Severe heart failure, dilated cardiomyopathy and pulmonary haemosiderosis in celiac disease: Report of two cases. Paediatr. Int. Child Health 2014, 34, 142–144. [Google Scholar] [CrossRef]
- De Bem, R.S.T.; Da Ro Sa Utiyama, S.R.; Nisihara, R.M.; Fortunato, J.A.; Tondo, J.A.; Carmes, E.R.; Souza, R.A.E.; Pisani, J.C.; Amarante, H.M.B.D.S. Celiac disease prevalence in Brazilian dilated cardiomyopathy patients. Dig. Dis. Sci. 2006, 51, 1016–1019. [Google Scholar] [CrossRef]
- Vizzardi, E.; Lanzarotto, F.; Carabellese, N.; Mora, A.; Bertolazzi, S.; Benini, F.; Nodari, S.; Dei Cas, L.; Lanzini, A. Lack of association of coeliac disease with idiopathic and ischaemic dilated cardiomyopathies. Scand. J. Clin. Lab. Investig. 2008, 68, 692–695. [Google Scholar] [CrossRef]
- Emilsson, L.; Andersson, B.; Elfström, P.; Green, P.H.R.; Ludvigsson, J.F. Risk of Idiopathic Dilated Cardiomyopathy in 29,000 Patients with Celiac Disease. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2012, 1, e001594. [Google Scholar] [CrossRef]
- Bohra, S.; Shah, A. Celiac Disease Presenting as Cardiomyopathy—A Rare Extra Intestinal Manifestation. Int. J. Celiac Dis. 2020, 8, 56–57. [Google Scholar] [CrossRef]
- Mehra, S.; Gupta, A.; Bhalla, K.; Nanda, S. Recurrent heart failure in a child with underlying dilated cardiomyopathy associated with celiac disease: An unusual presentation. J. Fam. Med. Prim. Care 2022, 11, 5689–5691. [Google Scholar] [CrossRef]
- Elnour, S.; Hashim, M.; Ibrahim, H. Dilated cardiomyopathy associated with celiac disease: A case report. Clin. Case Rep. 2021, 9, e04990. [Google Scholar] [CrossRef]
- Meyer, S.; Nourkami-Tutdibi, N.; Poryo, M.; Casper, M.; Geipel, M.; Zemlin, M. Pericardial effusion, cardiomegaly, oedema, and IgA deficiency in a child: Coeliac disease. Lancet 2021, 397, 1576. [Google Scholar] [CrossRef]
- Myrmel, G.M.S.; Lunde, T.; Dizdar, V.; Larsen, T.H.; Saeed, S. Myocarditis in a young patient with celiac disease; a case report and literature review. Open Cardiovasc. Med. J. 2021, 15, 1–5. [Google Scholar] [CrossRef]
- Patel, P.; Smith, F.; Kilcullen, N.; Artis, N. Dilated cardiomyopathy as the first presentation of coeliac disease: Association or causation? Clin. Med. J. R. Coll. Physicians Lond. 2018, 18, 177–179. [Google Scholar] [CrossRef]
- Anderson, B.; Rizvi, S.; Lin, G.; Nehra, V. A case of heart failure and diarrhoea. Gut 2017, 66, 1778. [Google Scholar] [CrossRef]
- McGrath, S.; Thomas, A.; Gorard, D.A. Cardiomyopathy responsive to gluten withdrawal in a patient with coeliac disease. BMJ Case Rep. 2016, 2016, bcr2015213301. [Google Scholar] [CrossRef]
- Khilnani, G.C.; Jain, N.; Tiwari, P.; Hadda, V.; Singh, L. A young man with hemoptysis: Rare association of idiopathic pulmonary hemosiderosis, celiac disease and dilated cardiomyopathy. Lung India 2015, 32, 70–72. [Google Scholar] [CrossRef]
- Milisavljević, N.; Cvetković, M.; Nikolić, G.; Filipović, B.; Milinić, N. Dilated cardiomyopathy associated with celiac disease: Case report and literature review. Srp. Arh. Celok. Lek. 2012, 140, 641–643. [Google Scholar] [CrossRef]
- Boskovic, A.; Kitic, I.; Prokic, D.; Stankovic, I. Cardiomyopathy associated with celiac disease in childhood. Case Rep. Gastrointest. Med. 2012, 2012, 170760. [Google Scholar] [CrossRef]
- Romagnoli, E.; Boldrini, E.; Pietrangelo, A. Association between celiac disease and idiopathic dilated cardiomyopathy: A case report. Intern. Emerg. Med. 2011, 6, 125–128. [Google Scholar] [CrossRef]
- Narula, N.; Rawal, P.; Manoj Kumar, R.; Thapa, B.R. Association of celiac disease with cardiomyopathy and pulmonary hemosiderosis. J. Trop. Pediatr. 2010, 56, 201–203. [Google Scholar] [CrossRef]
- Uslu, N.; Demir, H.; Karagöz, T.; Saltik-Temizel, I.N. Dilated cardiomyopathy in celiac disease: Role of carnitine deficiency. Acta Gastro-Enterol. 2010, 73, 530–531. [Google Scholar]
- Lodha, A.; Haran, M.; Hollander, G.; Frankel, R.; Shani, J. Celiac disease associated with dilated cardiomyopathy. South. Med. J. 2009, 102, 1052–1054. [Google Scholar] [CrossRef]
- Glover, B.M.; Treanor, N.J.; McEneaney, D.J. A case of dilated cardiomyopathy associated with coeliac disease. Arch. Med. Sci. 2007, 3, 272–273. [Google Scholar]
- Gelfond, D.; Fasano, A. Dilated Cardiomyopathy and Type 1 Diabetes in a Patient with Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2006, 43, E43. [Google Scholar] [CrossRef]
- Goel, N.K.; McBane, R.D.; Kamath, P.S. Cardiomyopathy associated with celiac disease. Mayo Clin. Proc. 2005, 80, 674–676. [Google Scholar] [CrossRef]
- Curione, M.; Barbato, M.; Viola, F.; Francia, P.; De Biase, L.; Cucchiara, S. Idiopathic dilated cardiomyopathy associated with coeliac disease: The effect of a gluten-free diet on cardiac performance. Dig. Liver Dis. 2002, 34, 866–869. [Google Scholar] [CrossRef]
- Makhdoom, Z.A.; Randall, N.W. Dilated cardiomyopathy due to anticardiolipin syndrome in association with celiac sprue. J. Clin. Gastroenterol. 2000, 31, 91–92. [Google Scholar] [CrossRef]
- Chuaqui, B.; Garrido, J.; Casanegra, P. Actin-deficient cardiomyopathy coexisting with celiac disease: A chance association? Pathol. Res. Pract. 1986, 181, 604–609. [Google Scholar] [CrossRef]
- Doǧan, M.; Peker, E.; Cagan, E.; Akbayram, S.; Acikgoz, M.; Caksen, H.; Uner, A.; Cesur, Y. Stroke and dilated cardiomyopathy associated with celiac disease. World J. Gastroenterol. 2010, 16, 2302–2304. [Google Scholar] [CrossRef]
- Isįkay, S.; Yilmaz, K.; Kilinç, M. Celiac disease with pulmonary haemosiderosis and cardiomyopathy. BMJ Case Rep. 2012, 2012, bcr2012007262. [Google Scholar] [CrossRef]
- Prati, D.; Bardella, M.T.; Peracchi, M.; Porretti, L.; Scalamogna, M.; Conte, D. Antiendomysial antibodies in patients with end-stage heart failure. Am. J. Gastroenterol. 2002, 97, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Fonager, K.; Sørensen, H.T.; Nørgård, B.; Thulstrup, A.M. Cardiomyopathy in Danish patients with coeliac disease. Lancet 1999, 354, 1561. [Google Scholar] [CrossRef] [PubMed]
- Curione, M.; Barbato, M.; Cugini, P.; Amato, S.; Da Ros, S.; Di Bona, S. Association of cardiomyopathy and celiac disease: An almost diffuse but still less know entity. A review. Arch. Med. Sci. 2008, 4, 103–107. [Google Scholar]
- Polat, T.B.; Urganci, N.; Yalcin, Y.; Zeybek, C.; Akdeniz, C.; Erdem, A.; Imanov, E.; Celebi, A. Cardiac functions in children with coeliac disease during follow-up: Insights from tissue Doppler imaging. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2008, 40, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Menezes, T.M.G.A.L.D.; Motta, M.E.F.A. Celiac disease prevalence in children and adolescents with myocarditis and dilated cardiomiopathy. J. Pediatr. 2012, 88, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, L.; Carlsson, R.; Holmqvist, M.; James, S.; Ludvigsson, J.F. The characterisation and risk factors of ischaemic heart disease in patients with coeliac disease. Aliment. Pharmacol. Ther. 2013, 37, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Elfström, P.; Hamsten, A.; Montgomery, S.M.; Ekbom, A.; Ludvigsson, J.F. Cardiomyopathy, pericarditis and myocarditis in a population-based cohort of inpatients with coeliac disease. J. Intern. Med. 2007, 262, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Mannarino, S.; Santacesaria, S.; Raso, I.; Fini, G.; Pozzi, E.; Cocuccio, C.; Calcaterra, V.; Zuccotti, G. Atrioventricular Block in Celiac Disease: An Unusual Clinical Presentation in a Child. A Case-Based Review. Children 2022, 9, 1627. [Google Scholar] [CrossRef]
- Saleh, F.; Greene, E.A.; Mathison, D. Evaluation and management of atrioventricular block in children. Curr. Opin. Pediatr. 2014, 26, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Curione, M. Dilated cardiomyopathy and celiac disease. Ital. Heart J. Off. J. Ital. Fed. Cardiol. 2002, 3, 384–385. [Google Scholar]
- Kalliokoski, S.; Sulic, A.-M.; Korponay-Szabó, I.R.; Szondy, Z.; Frias, R.; Perez, M.A.; Martucciello, S.; Roivainen, A.; Pelliniemi, L.J.; Esposito, C.; et al. Celiac Disease–Specific TG2-Targeted Autoantibodies Inhibit Angiogenesis Ex Vivo and In Vivo in Mice by Interfering with Endothelial Cell Dynamics. PLoS ONE 2013, 8, e65887. [Google Scholar] [CrossRef] [PubMed]
- Myrsky, E.; Caja, S.; Simon-Vecsei, Z.; Korponay-Szabo, I.R.; Nadalutti, C.; Collighan, R.; Mongeot, A.; Griffin, M.; Mäki, M.; Kaukinen, K.; et al. Celiac disease IgA modulates vascular permeability in vitro through the activity of transglutaminase 2 and RhoA. Cell. Mol. Life Sci. 2009, 66, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Boucelma, M.; Saadi, M.; Boukrara, H.; Bensalah, D.; Hakem, D.; Berrah, A. Association of celiac disease and cerebral venous thrombosis: Report of two cases. J. Mal. Vasc. 2013, 38, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Cuoco, L.; Chimenti, C.; Pieroni, M.; Fioravanti, G.; Gentiloni, N.; Maseri, A.; Gasbarrini, G. Celiac disease associated with autoimmune myocarditis. Circulation 2002, 105, 2611–2618. [Google Scholar] [CrossRef]
- Curione, M.; Danese, C.; Viola, F.; Di Bona, S.; Anastasia, A.; Cugini, P.; Barbato, M. Carnitine deficiency in patients with coeliac disease and idiopathic dilated cardiomyopathy. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 279–283. [Google Scholar] [CrossRef]
- van Elburg, R.M.; Uil, J.J.; Mulder, C.J.; Heymans, H.S. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut 1993, 34, 354–357. [Google Scholar] [CrossRef]
- Posner, E.B.; Haseeb, M. Celiac Disease; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Mehta, P.A.; Dubrey, S.W. High output heart failure. QJM Int. J. Med. 2009, 102, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.K.A.; Clark, A.L.; Cleland, J.G.F. Chronic heart failure and micronutrients. J. Am. Coll. Cardiol. 2001, 37, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Parzanese, I.; Qehajaj, D.; Patrinicola, F.; Aralica, M.; Chiriva-Internati, M.; Stifter, S.; Elli, L.; Grizzi, F. Celiac disease: From pathophysiology to treatment. World J. Gastrointest. Pathophysiol. 2017, 8, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef]
- Bhadada, S.K.; Rastogi, A.; Agarwal, A.; Kochhar, R.; Kochhar, R.; Bhansali, A. Comparative study of clinical features of patients with celiac disease & those with concurrent celiac disease & type 1 diabetes mellitus. Indian J. Med. Res. 2017, 145, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Huang, M.; Chen, S. Primary Carnitine Deficiency and Cardiomyopathy. Korean Circ. J. 2013, 43, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Yüce, A.; Demir, H.; Temizel, I.N.S.; Koçak, N. Serum carnitine and selenium levels in children with celiac disease. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2004, 23, 87–88. [Google Scholar]
- Lee, S.K.; Lo, W.; Memeo, L.; Rotterdam, H.; Green, P.H.R. Duodenal histology in patients with celiac disease after treatment with a gluten-free diet. Gastrointest. Endosc. 2003, 57, 187–191. [Google Scholar] [CrossRef]
- Manavalan, J.S.; Hernandez, L.; Shah, J.G.; Konikkara, J.; Naiyer, A.J.; Lee, A.R.; Ciaccio, E.; Minaya, M.T.; Green, P.H.R.; Bhagat, G. Serum cytokine elevations in celiac disease: Association with disease presentation. Hum. Immunol. 2010, 71, 50–57. [Google Scholar] [CrossRef]
- Marriott, J.B.; Goldman, J.H.; Keeling, P.J.; Baig, M.K.; Dalgleish, A.G.; McKenna, W.J. Abnormal cytokine profiles in patients with idiopathic dilated cardiomyopathy and their asymptomatic relatives. Heart 1996, 75, 287–290. [Google Scholar] [CrossRef]
- Mason, J.W. Myocarditis and dilated cardiomyopathy: An inflammatory link. Cardiovasc. Res. 2003, 60, 5–10. [Google Scholar] [CrossRef]
- Sategna-Guidetti, C.; Franco, E.; Martini, S.; Bobbio, M. Binding by serum IgA antibodies from patients with coeliac disease to monkey heart tissue. Scand. J. Gastroenterol. 2004, 39, 540–543. [Google Scholar] [CrossRef]
- Vives-Pi, M.; Takasawa, S.; Pujol-Autonell, I.; Planas, R.; Cabre, E.; Ojanguren, I.; Montraveta, M.; Santos, A.L.; Ruiz-Ortiz, E. Biomarkers for diagnosis and monitoring of celiac disease. J. Clin. Gastroenterol. 2013, 47, 308–313. [Google Scholar] [CrossRef]
- Nurkic, J.; Numanovic, F.; Arnautalic, L.; Tihic, N.; Halilovic, D.; Jahic, M. Diagnostic Significance of Reduced IgA in Children. Med. Arch. 2015, 69, 236–239. [Google Scholar] [CrossRef]
- Counsell, C.E.; Taha, A.; Ruddell, W.S. Coeliac disease and autoimmune thyroid disease. Gut 1994, 35, 844–846. [Google Scholar] [CrossRef]
- Komatireddy, G.R.; Marshall, J.B.; Aqel, R.; Spollen, L.E.; Sharp, G.C. Association of systemic lupus erythematosus and gluten enteropathy. South. Med. J. 1995, 88, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.J.; Kelly, C.P. Celiac Sprue. N. Engl. J. Med. 2002, 346, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K. Idiopathic pulmonary hemosiderosis: A state of the art review. Respir. Med. 2021, 176, 106234. [Google Scholar] [CrossRef] [PubMed]
- Ploier, R.; Emhofer, J.; Dorninger, L.; Kranzl, G.; Feichtinger, J.; Müller, K.M.; Brandtzaeg, P. Immunological aspects of a child with idiopathic pulmonary hemosiderosis and celiac disease. Klin. Padiatr. 1998, 210, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Stolfo, D.; Caiffa, T.; Pivetta, A.; Sinagra, G. Clinical Presentation, Spectrum of Disease, and Natural History. In Dilated Cardiomyopathy; Springer: Cham, Switzerland, 2019; pp. 71–82. [Google Scholar] [CrossRef]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Davies, S.L.; Butler, M.; Scott, D.; Clark, M.; Kumar, P. Endomysial antibody: Is it the best screening test for coeliac disease? Gut 1992, 33, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primer 2019, 5, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Cenk, S.; Aylin, D.B.; Fatma Ebru, A.; Nihal, A.B.; Sevil, Ö.S.; Serdal, B.; Emine, B.; Hüseyin, A.; Telat, K.; Tahir, D.; et al. Assessment of left ventricular function by strain-strain rate echocardiography in patients with celiac disease. Turk. J. Med. Sci. 2014, 44, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Deveci, M.; Uncuoğlu Aydoğan, A.; Altun, G.; Kayabey, Ö.; Tuğral, O.; Babaoğlu, K. Left ventricular mechanics are affected in children with celiac disease: A study based on two-dimensional speckle tracking echocardiography. Echocardiography 2017, 34, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- El Amrousy, D.; Elshehaby, W.; Elsharaby, R.; Badr, S.; Hamza, M.; Elbarky, A. Myocardial function using two dimension speckle-tracking echocardiography in children with celiac disease. Eur. J. Pediatr. 2023, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J. Am. Coll. Cardiol. 2007, 50, 1914–1931. [Google Scholar] [CrossRef]
- Haines, M.L.; Anderson, R.P.; Gibson, P.R. Systematic review: The evidence base for long-term management of coeliac disease. Aliment. Pharmacol. Ther. 2008, 28, 1042–1066. [Google Scholar] [CrossRef]
- Lebwohl, B.; Cao, Y.; Zong, G.; Hu, F.B.; Green, P.H.R.; Neugut, A.I.; Rimm, E.B.; Sampson, L.; Dougherty, L.W.; Giovannucci, E.; et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: Prospective cohort study. BMJ 2017, 357, j1892. [Google Scholar] [CrossRef]
- Machado, M.V. New Developments in Celiac Disease Treatment. Int. J. Mol. Sci. 2023, 24, 945. [Google Scholar] [CrossRef]
- Gass, J.; Bethune, M.T.; Siegel, M.; Spencer, A.; Khosla, C. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology 2007, 133, 472–480. [Google Scholar] [CrossRef]
- Leffler, D.A.; Kelly, C.P.; Abdallah, H.Z.; Colatrella, A.M.; Harris, L.A.; Leon, F.; Arterburn, L.A.; Paterson, B.M.; Lan, Z.H.; Murray, J.A. A Randomized, Double-Blind Study of Larazotide Acetate to Prevent the Activation of Celiac Disease During Gluten Challenge. Am. J. Gastroenterol. 2012, 107, 1554. [Google Scholar] [CrossRef] [PubMed]
- Büchold, C.; Hils, M.; Gerlach, U.; Weber, J.; Pelzer, C.; Heil, A.; Aeschlimann, D.; Pasternack, R. Features of ZED1227: The First-In-Class Tissue Transglutaminase Inhibitor Undergoing Clinical Evaluation for the Treatment of Celiac Disease. Cells 2022, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Lähdeaho, M.-L.; Scheinin, M.; Vuotikka, P.; Taavela, J.; Popp, A.; Laukkarinen, J.; Koffert, J.; Koivurova, O.-P.; Pesu, M.; Kivelä, L.; et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: A phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol. Hepatol. 2019, 4, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Vanuytsel, T.; Hiele, M. Celiac Disease Remission with Tofacitinib: A Case Report. Ann. Intern. Med. 2020, 173, 585. [Google Scholar] [CrossRef]
- Maadarani, O.; Bigdelu, L.; Bitar, Z.; Alhabibi, M.; Kabbara, H. Spontaneous Recovery of Isolated Advanced Heart Block in Patient with Celiac Disease by Starting a Strict Gluten Free Diet: A Case Report and Review of the Literature. Eur. J. Case Rep. Intern. Med. 2023, 10, 004012. [Google Scholar] [CrossRef]



| Demographic Characteristics | n | Age Range (Years) | Mean Age ± SD (Years) | M:F Ratio |
|---|---|---|---|---|
| Adult | 19 (67.9%) | 18–70 | 35.8 ± 15.5 | 16:3 |
| Pediatric | 9 (31.1%) | 3–17 | 9.7 ± 4.7 | 2:6 (1 NR) |
| Total | 28 (100%) | 3–70 | 27.4 ± 18.0 | 18:9 (1 NR) |
| Comorbidities | ||||
| Reported | 20 (71.4%) | |||
| Anemia | 15 (75%) | |||
| Idiopathic lung hemosiderosis | 4 (20%) | |||
| Atrial fibrillation, diabetes type 1, stroke, hypothyroidism, depression | Each 1 (5%) | |||
| Not reported | 8 (28.6%) | |||
| Clinical Presentation | ||||
| Dyspnea | 16 (64%) | |||
| Diarrhea | 9 (36%) | |||
| Palpitations | 5 (20%) | |||
| Weakness/malaise, fatigue | 5 (20%) | |||
| Chest pain | 3 (12%) | |||
| Orthopnea | 3 (12%) | |||
| PND | 3 (12%) | |||
| Syncope | 2 (8%) | |||
| Not reported | 3 (10.7%) | |||
| Left Ventricular Ejection Fraction at Diagnosis | n | EF Range (%) | Mean EF ± SD (%) |
|---|---|---|---|
| Total reported | 25 (89.3%) | 10–50 | 27.3 ± 11.5 |
| Adult | 17 | 12–50 | 27.6 ± 11.3 |
| Pediatric | 8 | 10–45 | 27.6 ± 12.2 |
| Not reported | 3 (10.7%) | - | - |
| New York Heart Association Classification | |||
| NYHA I | 3 | ||
| NYHA II | 1 | ||
| NYHA III | 3 | ||
| NYHA IV | 0 | ||
| Not reported | 21 (75%) | ||
| Electrocardiography | |||
| Conduction abnormalities | 13 (57.1%) | ||
| LBBB | 10 (62.5%) | ||
| RBBB | 1 (6.3%) | ||
| IVCD | 1 (6.3%) | ||
| AV block | 1 (6.3%) | ||
| Tachyarrhythmias | 4 (25%) | ||
| T-wave abnormalities | 2 (12.5%) | ||
| Not reported or no abnormalities | 12 (42.8%) | ||
| Reference/Year | Sex/Age | Timeline of Diagnosis | LVEF at Diagnosis | GFD | GDMT | Follow-Up LVEF |
|---|---|---|---|---|---|---|
| Mehra, 2022 [23] | M, 10 | CMP | 25–30% | Yes | Ivabradine | NR |
| Elnour, 2021 [24] | F, 33 | Same time | 15–20% | NR | BB, ARB, MRA, Ivabradine | NR |
| Meyer, 2021 [25] | F, 4 | Same time | 20% | Yes | NR | NR |
| Myrmel, 2021 [26] | M, 21 | CMP | 25% | Yes | BB, ARNi | 35% |
| Bohra, 2020 [22] | F, 35 | Same time | 20% | Yes | “Inotrops” | 55% |
| Patel, 2018 [27] | M, 19 | CMP | “Severe systolic dysfunction” | Yes | Yes, but not specified | NR |
| Anderson, 2016 [28] | M, 20 | CD | 21% | Yes | NR | 45% (1 y) |
| McGrath, 2016 [29] | M, 57 | CMP | 15% | Yes | BB, ACEi, MRA | 63% (18 m) 70% (2 y) |
| Khilnani GC, 2015 [30] | M, 19 | Same time | 25% | Yes | BB | 35% (2 y) |
| Poddar, 2014 [18] | M, 18 | CMP | 12% | Yes | BB, ACEi, MRA, Digoxin | 25% |
| Poddar, 2014 [18] | F, 13 | CD | 10% | Yes, non-compliant | BB, ACEi, MRA, Digoxin | NR |
| Milisavljevic, 2012 [31] | M, 27 | Same time | 50% | Yes, non-compliant | BB, ACEi | 20–25% (12 m) 15–20% (18 m) |
| Işikay, 2012 [44] | F, 13 | Same time | 32% | Yes | NR | 29% |
| Boskovic, 2012 [32] | F, 3 | Same time | 39-45% | NR | NR | NR |
| Barrio, 2011 [17] | M, 24 | Same time | 24% | Yes | MRA, Digoxin | NR |
| Romagnoli, 2011 [33] | M, 66 | Same time | 25% | Yes | ACEi, Digoxin | NR |
| Dogan, 2010 [43] | F, 8 | Same time | “Dilated Cardiomyopathy” | Yes | NR | NR |
| Narula, 2010 [34] | M, 13 | Same time | 26% | Yes | ACEi, Digoxin | NR |
| Uslu, 2010 [35] | F, 6 | CD | 46% | Yes | ACEi, Digoxin | “WNL” |
| Lodha, 2009 [36] | M, 48 | CD | 40–45% <25% * | Yes, non-compliant | BB, ACEi | “No improvement” |
| Glover, 2007 [37] | M, 36 | Same time | 15% | Yes | NR | 25% |
| Gelfond, 2006 [38] | NR, 17 | CD | 15–20% | Yes | NR | 36% |
| Goel, 2005 [39] | M, 70 | CMP | 45% | Yes | NR | 65% |
| Curione, 2002 [40] | M, 40 | CMP | 38% | Yes | ACEi, Digoxin | 42% |
| Curione, 2002 [40] | M, 32 | CMP | 25% | Yes | ACEi, Digoxin | 30% |
| Curione, 2002 [40] | M, 26 | CMP | 36% | Yes, non-compliant | ACEi, Digoxin, BB added at follow-up | 30% |
| Makhdoom, 2000 [41] | F, 49 | CMP | 30% | Yes | ACEi | 65%, 25% ** |
| Chuaqui, 1986 [42] | M, 34 | Unclear | Diminished | Yes | NR | NR |
| Therapeutic Approach | n (%) |
|---|---|
| Gluten-free diet | 26 (92.9%) |
| Not reported | 2 (7.1%) |
| GDMT | 20 (71.4%) |
| ACEi/ARB/ARNi | 15 (75%) |
| Loop/thiazide diuretics | 14 (70%) |
| Digoxin | 9 (45%) |
| Beta-blocker | 9 (45%) |
| MRA | 5 (25%) |
| Other (dobutamine, ivabradine) | 4 (20%) |
| GDMT started, but not specified | 1 (3.6%) |
| SGLT-2i * | - |
| Not reported | 7 (25%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milutinovic, S.; Jancic, P.; Adam, A.; Radovanovic, M.; Nordstrom, C.W.; Ward, M.; Petrovic, M.; Jevtic, D.; Delibasic, M.; Kotseva, M.; et al. Cardiomyopathy in Celiac Disease: A Systematic Review. J. Clin. Med. 2024, 13, 1045. https://doi.org/10.3390/jcm13041045
Milutinovic S, Jancic P, Adam A, Radovanovic M, Nordstrom CW, Ward M, Petrovic M, Jevtic D, Delibasic M, Kotseva M, et al. Cardiomyopathy in Celiac Disease: A Systematic Review. Journal of Clinical Medicine. 2024; 13(4):1045. https://doi.org/10.3390/jcm13041045
Chicago/Turabian StyleMilutinovic, Stefan, Predrag Jancic, Adam Adam, Milan Radovanovic, Charles W. Nordstrom, Marshall Ward, Marija Petrovic, Dorde Jevtic, Maja Delibasic, Magdalena Kotseva, and et al. 2024. "Cardiomyopathy in Celiac Disease: A Systematic Review" Journal of Clinical Medicine 13, no. 4: 1045. https://doi.org/10.3390/jcm13041045