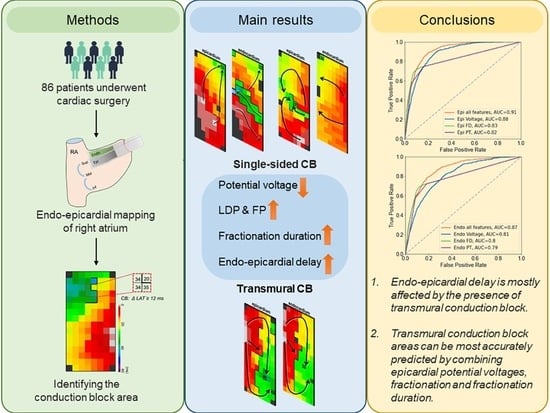
Identification of Atrial Transmural Conduction Inhomogeneity Using Unipolar Electrogram Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Simultaneous Endo-Epicardial Mapping of the RA
2.3. Mapping Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Mapping Data
3.3. Prevalence of Transmural Conduction Block
3.4. Relation between Different Types of CB and EED
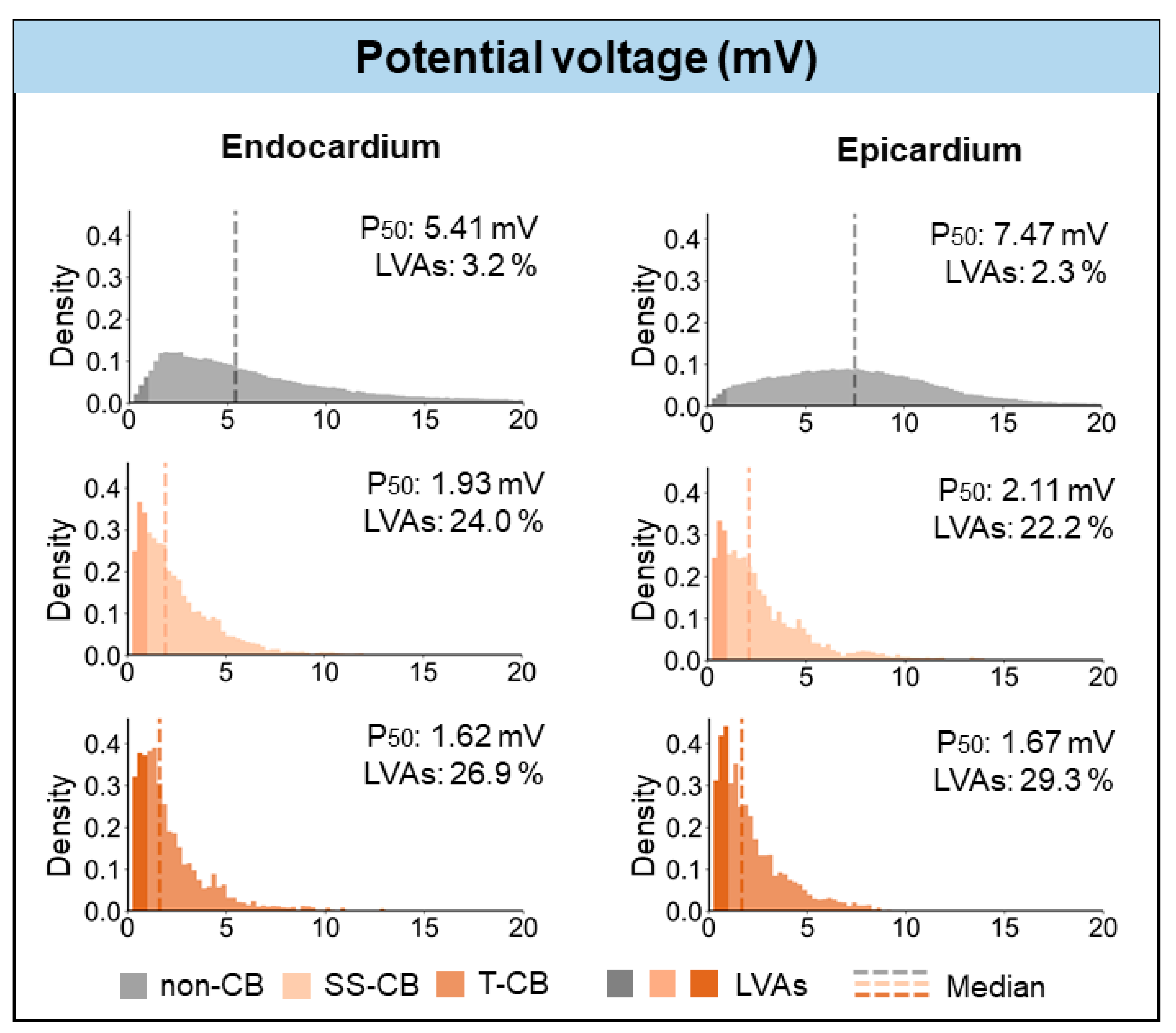
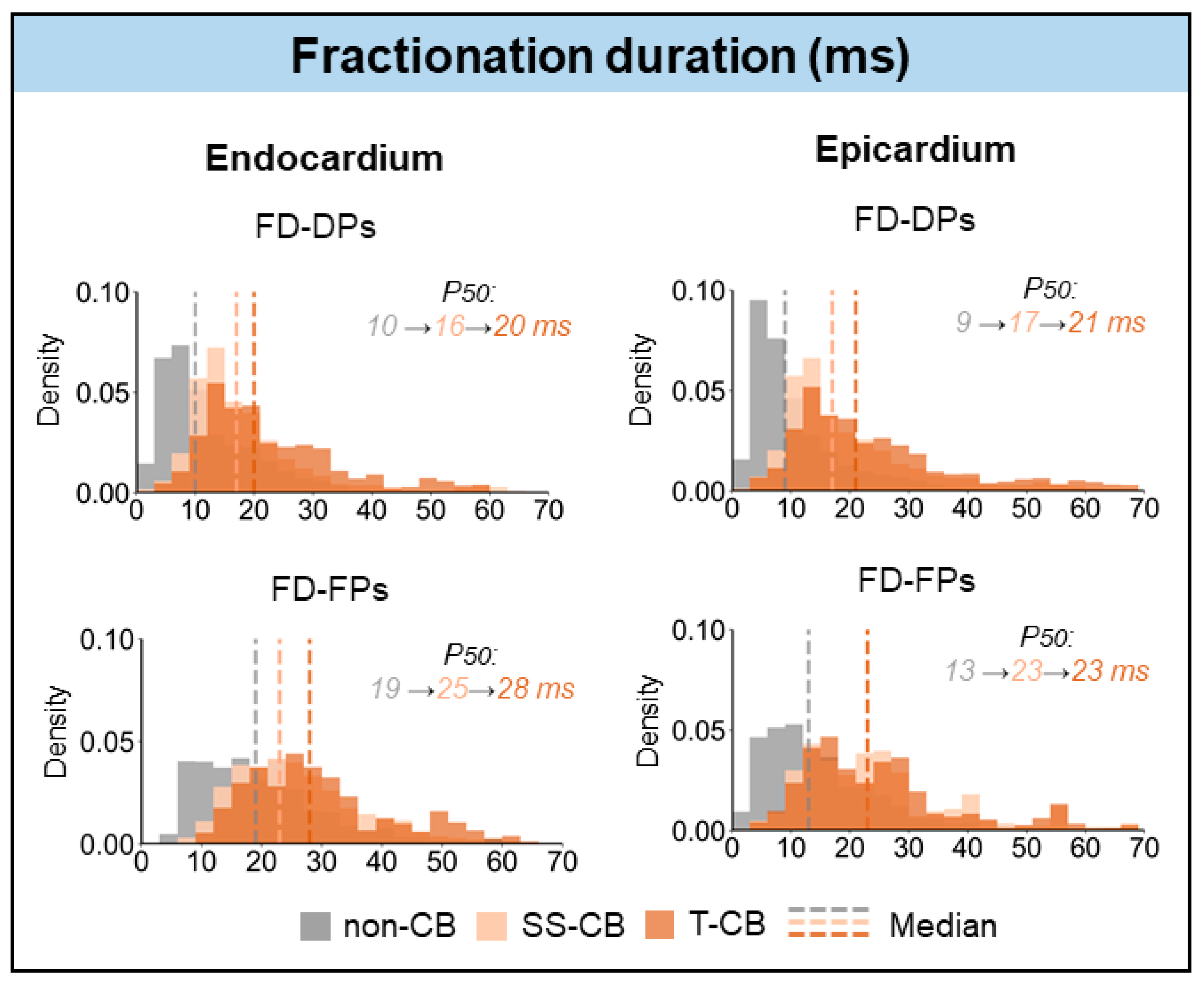
3.5. T-CB and Unipolar Potential Voltages
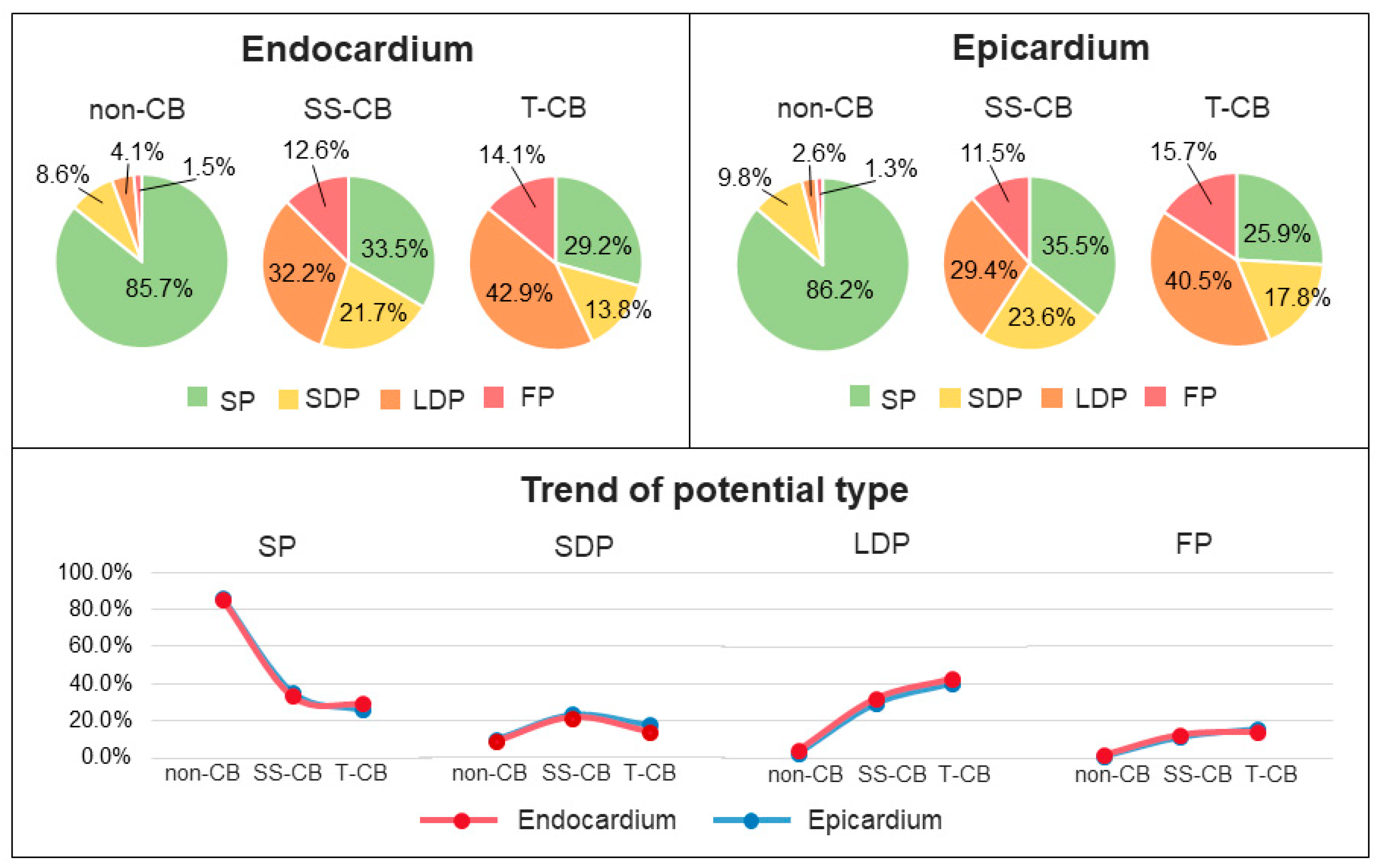
3.6. T-CB and Unipolar Potential Morphology
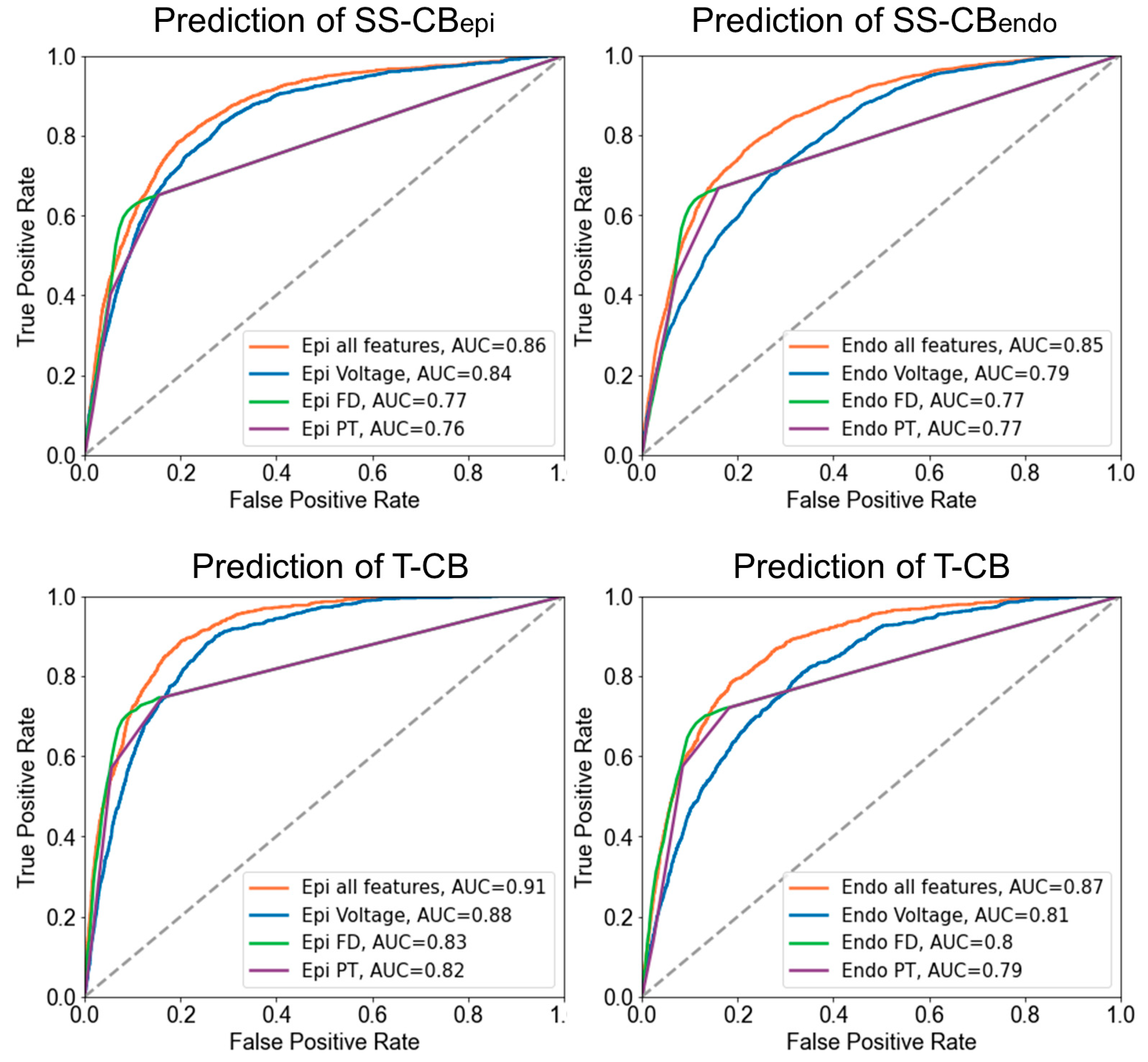
3.7. Prediction of T-CB Areas
4. Discussion
4.1. Endo-Epicardial Asynchrony Related to Transmural Conduction Block
4.2. Potential Morphology as Indicator of Transmural Conduction Block
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pytkowski, M.; Jankowska, A.; Maciag, A.; Kowalik, I.; Sterlinski, M.; Szwed, H.; Saumarez, R.C. Paroxysmal Atrial Fibrillation Is Associated with Increased Intra-Atrial Conduction Delay. Europace 2008, 10, 1415–1420. [Google Scholar] [CrossRef]
- Zaman, J.A.B.; Harling, L.; Ashrafian, H.; Darzi, A.; Gooderham, N.; Athanasiou, T.; Peters, N.S. Post-Operative Atrial Fibrillation Is Associated with a Pre-Existing Structural and Electrical Substrate in Human Right Atrial Myocardium. Int. J. Cardiol. 2016, 220, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- De Groot, N.; van der Does, L.; Yaksh, A.; Lanters, E.; Teuwen, C.; Knops, P.; van de Woestijne, P.; Bekkers, J.; Kik, C.; Bogers, A.; et al. Direct Proof of Endo-Epicardial Asynchrony of the Atrial Wall During Atrial Fibrillation in Humans. Circ. Arrhythmia Electrophysiol. 2016, 9, e003648. [Google Scholar] [CrossRef] [PubMed]
- Schuessler, R.B.; Kawamoto, T.; Hand, D.E.; Mitsuno, M.; Bromberg, B.I.; Cox, J.L.; Boineau, J.P. Simultaneous Epicardial and Endocardial Activation Sequence Mapping in the Isolated Canine Right Atrium. Circulation 1993, 88, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, J.; Maesen, B.; Linz, D.; Zeemering, S.; van Hunnik, A.; Verheule, S.; Allessie, M.; Schotten, U. Time Course and Mechanisms of Endo-Epicardial Electrical Dissociation during Atrial Fibrillation in the Goat. Cardiovasc. Res. 2011, 89, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Niwano, S.; Abe, H.; Rudy, Y.; Johnson, N.J.; Waldo, A.L. Mapping the Conversion of Atrial Flutter to Atrial Fibrillation and Atrial Fibrillation to Atrial Flutter. Insights into Mechanisms. Circ. Res. 1994, 74, 882–894. [Google Scholar] [CrossRef]
- Allessie, M.A.; de Groot, N.M.S.; Houben, R.P.M.; Schotten, U.; Boersma, E.; Smeets, J.L.; Crijns, H.J. Electropathological Substrate of Long-Standing Persistent Atrial Fibrillation in Patients with Structural Heart Disease: Longitudinal Dissociation. Circ. Arrhythmia Electrophysiol. 2010, 3, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Heida, A.; van der Does, W.F.B.; van Staveren, L.N.; Taverne, Y.J.H.J.; Roos-Serote, M.C.; Bogers, A.J.J.C.; de Groot, N.M.S. Conduction Heterogeneity. JACC Clin. Electrophysiol. 2020, 6, 1844–1854. [Google Scholar] [CrossRef]
- Verheule, S.; Tuyls, E.; Gharaviri, A.; Hulsmans, S.; van Hunnik, A.; Kuiper, M.; Serroyen, J.; Zeemering, S.; Kuijpers, N.H.L.; Schotten, U. Loss of Continuity in the Thin Epicardial Layer Because of Endomysial Fibrosis Increases the Complexity of Atrial Fibrillatory Conduction. Circ. Arrhythmia Electrophysiol. 2013, 6, 202–211. [Google Scholar] [CrossRef]
- Gharaviri, A.; Bidar, E.; Potse, M.; Zeemering, S.; Verheule, S.; Pezzuto, S.; Krause, R.; Maessen, J.G.; Auricchio, A.; Schotten, U. Epicardial Fibrosis Explains Increased Endo–Epicardial Dissociation and Epicardial Breakthroughs in Human Atrial Fibrillation. Front. Physiol. 2020, 11, 68. [Google Scholar] [CrossRef]
- Ramos, K.S.; Li, J.; Wijdeveld, L.F.J.; van Schie, M.S.; Taverne, Y.J.H.J.; Boon, R.A.; de Groot, N.M.S.; Brundel, B.J.J.M. Long Noncoding RNA UCA1 Correlates with Electropathology in Patients With Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9 Pt 2, 1097–1107. [Google Scholar] [CrossRef]
- Kharbanda, R.K.; Knops, P.; van der Does, L.J.M.E.; Kik, C.; Taverne, Y.J.H.J.; Roos-Serote, M.C.; Heida, A.; Oei, F.B.S.; Bogers, A.J.J.C.; de Groot, N.M.S. Simultaneous Endo-Epicardial Mapping of the Human Right Atrium: Unraveling Atrial Excitation. J. Am. Heart Assoc. 2020, 9, e017069. [Google Scholar] [CrossRef]
- Jadidi, A.S.; Duncan, E.; Miyazaki, S.; Lellouche, N.; Shah, A.J.; Forclaz, A.; Nault, I.; Wright, M.; Rivard, L.; Liu, X.; et al. Functional Nature of Electrogram Fractionation Demonstrated by Left Atrial High-Density Mapping. Circ. Arrhythm. Electrophysiol. 2012, 5, 32–42. [Google Scholar] [CrossRef]
- Ghoraani, B.; Dalvi, R.; Gizurarson, S.; Das, M.; Ha, A.; Suszko, A.; Krishnan, S.; Chauhan, V.S. Localized Rotational Activation in the Left Atrium during Human Atrial Fibrillation: Relationship to Complex Fractionated Atrial Electrograms and Low-Voltage Zones. Heart Rhythm. 2013, 10, 1830–1838. [Google Scholar] [CrossRef]
- Ye, Z.; van Schie, M.S.; de Groot, N.M.S. Signal Fingerprinting as a Novel Diagnostic Tool to Identify Conduction Inhomogeneity. Front. Physiol. 2021, 12, 652128. [Google Scholar] [CrossRef]
- Tung, R.; Burris, R.; Salazar, P.; Aziz, Z. Human Recordings of Left Atrial Epicardial-Endocardial Asynchrony During Persistent Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2022, 15, e010605. [Google Scholar] [CrossRef] [PubMed]
- Knops, P.; Kik, C.; Bogers, A.J.J.C.; de Groot, N.M.S. Simultaneous Endocardial and Epicardial High-Resolution Mapping of the Human Right Atrial Wall. J. Thorac. Cardiovasc. Surg. 2016, 152, 929–931. [Google Scholar] [CrossRef] [PubMed]
- de Groot, N.M.S.; Shah, D.; Boyle, P.M.; Anter, E.; Clifford, G.D.; Deisenhofer, I.; Deneke, T.; van Dessel, P.; Doessel, O.; Dilaveris, P.; et al. Critical Appraisal of Technologies to Assess Electrical Activity during Atrial Fibrillation: A Position Paper from the European Heart Rhythm Association and European Society of Cardiology Working Group on eCardiology in Collaboration with the Heart Rhythm Society, Asia Pacific Heart Rhythm Society, Latin American Heart Rhythm Society and Computing in Cardiology. EP Eur. 2022, 24, 313–330. [Google Scholar] [CrossRef]
- de Groot, N.M.S.; Houben, R.P.M.; Smeets, J.L.; Boersma, E.; Schotten, U.; Schalij, M.J.; Crijns, H.; Allessie, M.A. Electropathological Substrate of Longstanding Persistent Atrial Fibrillation in Patients with Structural Heart Disease: Epicardial Breakthrough. Circulation 2010, 122, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- van der Does, L.J.M.E.; Knops, P.; Teuwen, C.P.; Serban, C.; Starreveld, R.; Lanters, E.A.H.; Mouws, E.M.J.P.; Kik, C.; Bogers, A.; de Groot, N. Unipolar Atrial Electrogram Morphology from an Epicardial and Endocardial Perspective. Heart Rhythm. 2018, 15, 879–887. [Google Scholar] [CrossRef]
- van der Does, L.J.M.E.; de Groot, N.M.S. Inhomogeneity and Complexity in Defining Fractionated Electrograms. Heart Rhythm. 2017, 14, 616–624. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Otsubo, T.; Takahashi, Y.; Nakashima, K.; Fukui, A.; Hirota, K.; Ishii, Y.; Shinzato, K.; Osako, R.; Tahara, M.; et al. Atrial Structural Remodeling in Patients with Atrial Fibrillation Is a Diffuse Fibrotic Process: Evidence From High-Density Voltage Mapping and Atrial Biopsy. J. Am. Heart Assoc. 2022, 11, e024521. [Google Scholar] [CrossRef]
- Hohendanner, F.; Romero, I.; Blaschke, F.; Heinzel, F.R.; Pieske, B.; Boldt, L.-H.; Parwani, A.S. Extent and Magnitude of Low-Voltage Areas Assessed by Ultra-High-Density Electroanatomical Mapping Correlate with Left Atrial Function. Int. J. Cardiol. 2018, 272, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Kurata, N.; Masuda, M.; Kanda, T.; Asai, M.; Iida, O.; Okamoto, S.; Ishihara, T.; Nanto, K.; Tsujimura, T.; Matsuda, Y.; et al. Left Atrial Localized Low-Voltage Areas Indicate Whole Left Atrial Electrophysiological Degeneration in Atrial Fibrillation Patients. Circ. J. 2022, 86, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Van Schie, M.S.; Starreveld, R.; Bogers, A.J.J.C.; de Groot, N.M.S. Sinus Rhythm Voltage Fingerprinting in Patients with Mitral Valve Disease Using a High-Density Epicardial Mapping Approach. Europace 2021, 23, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yamaguchi, T.; Otsubo, T.; Nakashima, K.; Shinzato, K.; Osako, R.; Shichida, S.; Kawano, Y.; Fukui, A.; Kawaguchi, A.; et al. Histological Validation of Atrial Structural Remodelling in Patients with Atrial Fibrillation. Eur. Heart J. 2023, 44, ehad396. [Google Scholar] [CrossRef]
- Van Schie, M.S.; Knops, P.; Zhang, L.; Van Schaagen, F.R.N.; Taverne, Y.J.H.J.; De Groot, N.M.S. Detection of Endo-Epicardial Atrial Low-Voltage Areas Using Unipolar and Omnipolar Voltage Mapping. Front. Physiol. 2022, 13, 1030025. [Google Scholar] [CrossRef]
- Zheng, Z.; Yao, Y.; Li, H.; Zheng, L.; Liu, S.; Lin, H.; Duan, F. Simultaneous Hybrid Maze Procedure for Long-Standing Persistent Atrial Fibrillation with Dilated Atrium. JTCVS Tech. 2021, 5, 34–42. [Google Scholar] [CrossRef]
- De Martino, G.; Compagnucci, P.; Mancusi, C.; Vassallo, E.; Calvanese, C.; Della Ratta, G.; Librera, M.; Franciulli, M.; Marino, L.; Russo, A.D.; et al. Stepwise Endo-/Epicardial Catheter Ablation for Atrial Fibrillation: The Mediterranea Approach. J. Cardiovasc. Electrophysiol. 2021, 32, 2107–2115. [Google Scholar] [CrossRef]
- Piorkowski, C.; Kronborg, M.; Hourdain, J.; Piorkowski, J.; Kirstein, B.; Neudeck, S.; Wechselberger, S.; Päßler, E.; Löwen, A.; El-Armouche, A.; et al. Endo-/Epicardial Catheter Ablation of Atrial Fibrillation: Feasibility, Outcome, and Insights Into Arrhythmia Mechanisms. Circ. Arrhythm. Electrophysiol. 2018, 11, e005748. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristics | |
|---|---|
| Patients | 86 |
| Male | 68 (79.1%) |
| Age (years) | 67 (61–72) |
| BMI (kg/m2) | 27.9 (24.8–31.0) |
| Underlying heart disease | |
| -iHD | 43 (50.0%) |
| -vHD | 22 (25.6%) |
| -cHD | 20 (23.2%) |
| History of AF | 37 (43.0%) |
| -Paroxysmal | 31 (36.0%) |
| -Persistent | 4 (4.7%) |
| -Longstanding persistent | 2 (2.3%) |
| Cardiovascular risk factors | |
| Hypertension | 54 (62.8%) |
| Hypercholesterolemia | 46 (53.5%) |
| Diabetes mellitus | 28 (32.6%) |
| Left ventricular function | |
| -Mild impairment (>50% LVEF ≥ 40%) | 12 (14.0%) |
| -Moderate impairment (LVEF 30–39%) | 10 (11.6%) |
| -Severe impairment (LVEF < 30%) | 1 (1.2%) |
| Antiarrhythmic drugs | |
| -Class I | 1 (1.2%) |
| -Class II | 59 (68.6%) |
| -Class III | 6 (7.0%) |
| -Class IV | 6 (7.0%) |
| SS-CBendo/epi | T-CB | p-Value | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Endocardium | |||
| Voltage (mV) | 1.93 (1.03–3.47) | 1.62 (0.95–2.86) | <0.001 |
| EED (ms) | 9 (4–15) | 13 (6–21) | <0.001 |
| FD (ms) | |||
| FD-DP | 16 (12–22) | 20 (15–30) | <0.001 |
| FD-FP | 25 (19–33) | 28 (20–36) | <0.001 |
| Epicardium | |||
| Voltage (mV) | 2.11 (1.1–3.75) | 1.67 (0.9–3.0) | <0.001 |
| EED (ms) | 8 (4–15) | 13 (6–21) | <0.001 |
| FD (ms) | |||
| FD-DP | 17 (12–25) | 21 (14–31) | <0.001 |
| FD-FP | 23 (15–29) | 23 (16–31) | 0.392 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; van Schie, M.S.; Xiang, H.; Liao, R.; Zheng, J.; Knops, P.; Taverne, Y.J.H.J.; de Groot, N.M.S. Identification of Atrial Transmural Conduction Inhomogeneity Using Unipolar Electrogram Morphology. J. Clin. Med. 2024, 13, 1015. https://doi.org/10.3390/jcm13041015
Zhang L, van Schie MS, Xiang H, Liao R, Zheng J, Knops P, Taverne YJHJ, de Groot NMS. Identification of Atrial Transmural Conduction Inhomogeneity Using Unipolar Electrogram Morphology. Journal of Clinical Medicine. 2024; 13(4):1015. https://doi.org/10.3390/jcm13041015
Chicago/Turabian StyleZhang, Lu, Mathijs S. van Schie, Hongxian Xiang, Rongheng Liao, Jiahao Zheng, Paul Knops, Yannick J. H. J. Taverne, and Natasja M. S. de Groot. 2024. "Identification of Atrial Transmural Conduction Inhomogeneity Using Unipolar Electrogram Morphology" Journal of Clinical Medicine 13, no. 4: 1015. https://doi.org/10.3390/jcm13041015