Predicting Long-Term Endothelial Cell Loss after Preloaded Descemet Membrane Endothelial Keratoplasty in Fuchs’ Endothelial Corneal Dystrophy: A Mathematical Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria and the Ethical Statement
2.2. DMEK Graft Preparation and Surgery
2.3. Preoperative and Postoperative Analysis
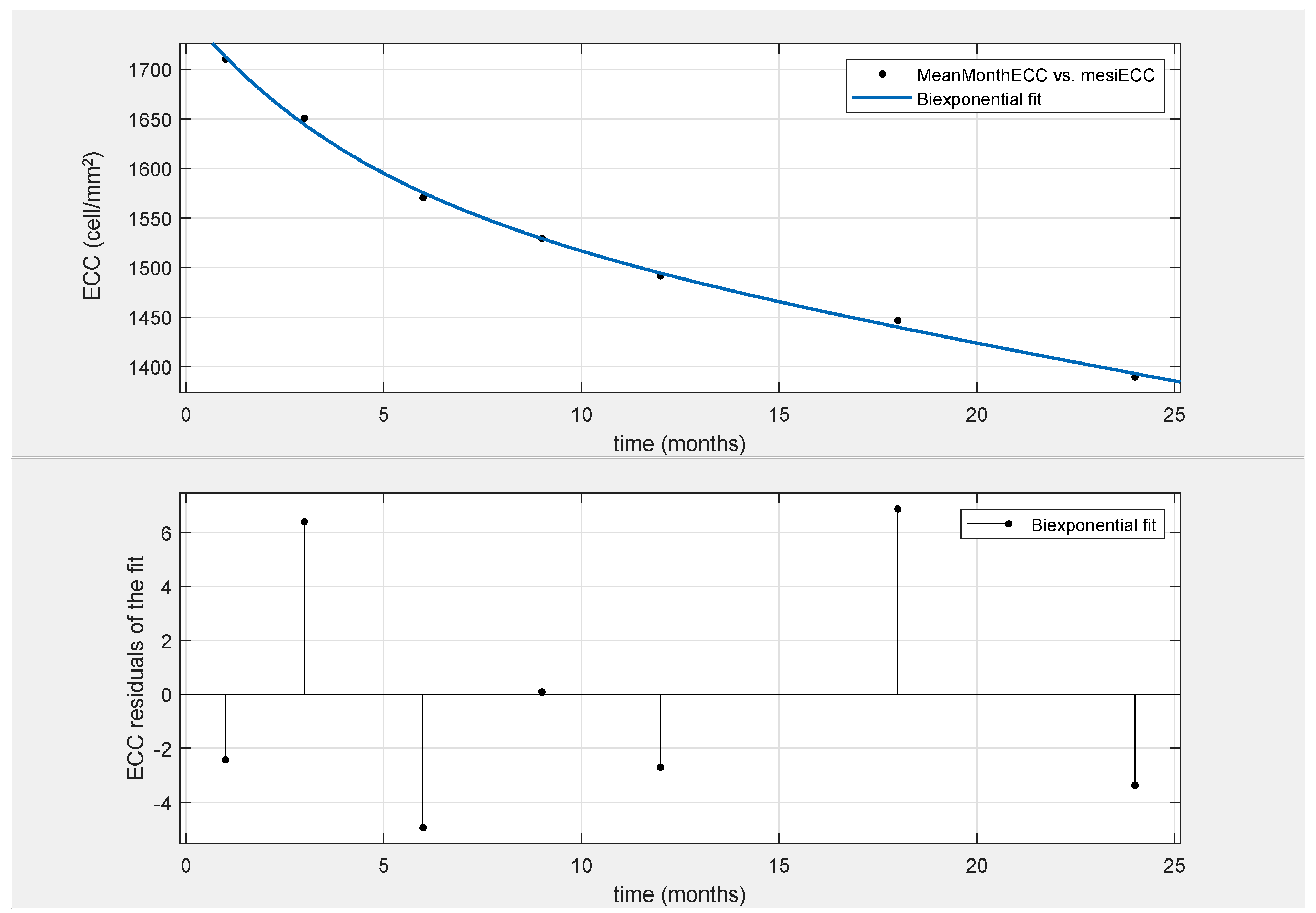
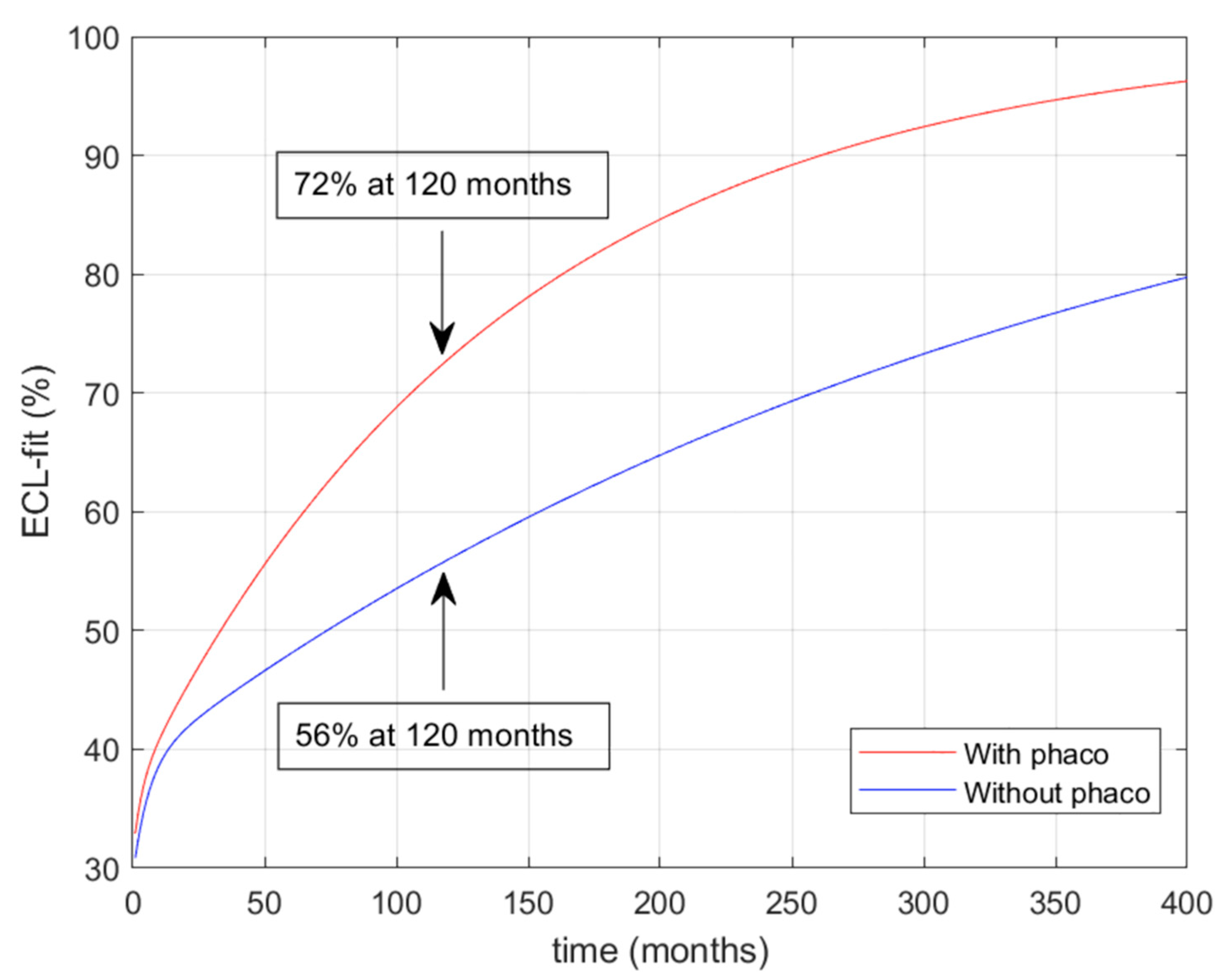
2.4. Mathematical Model
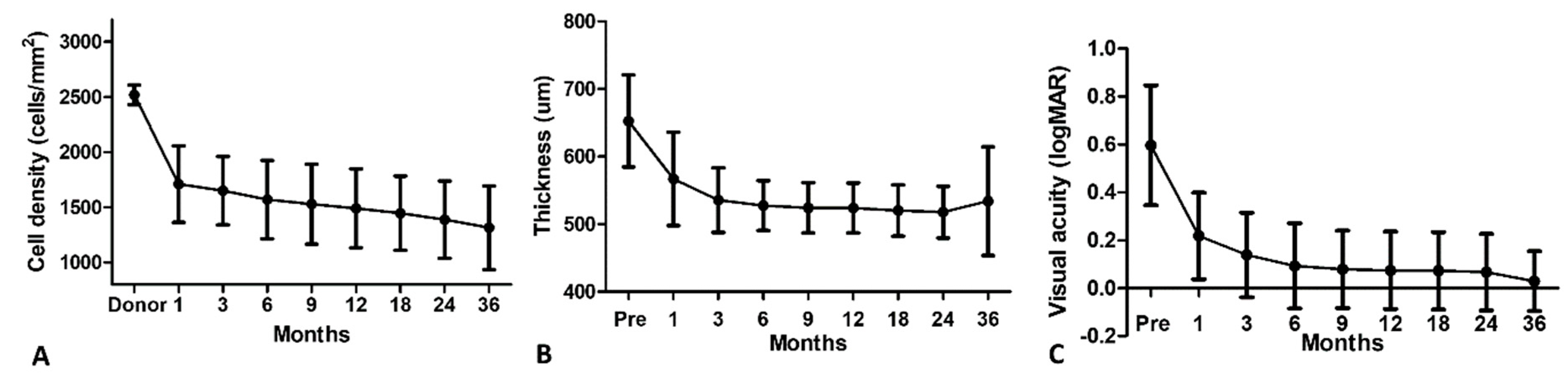
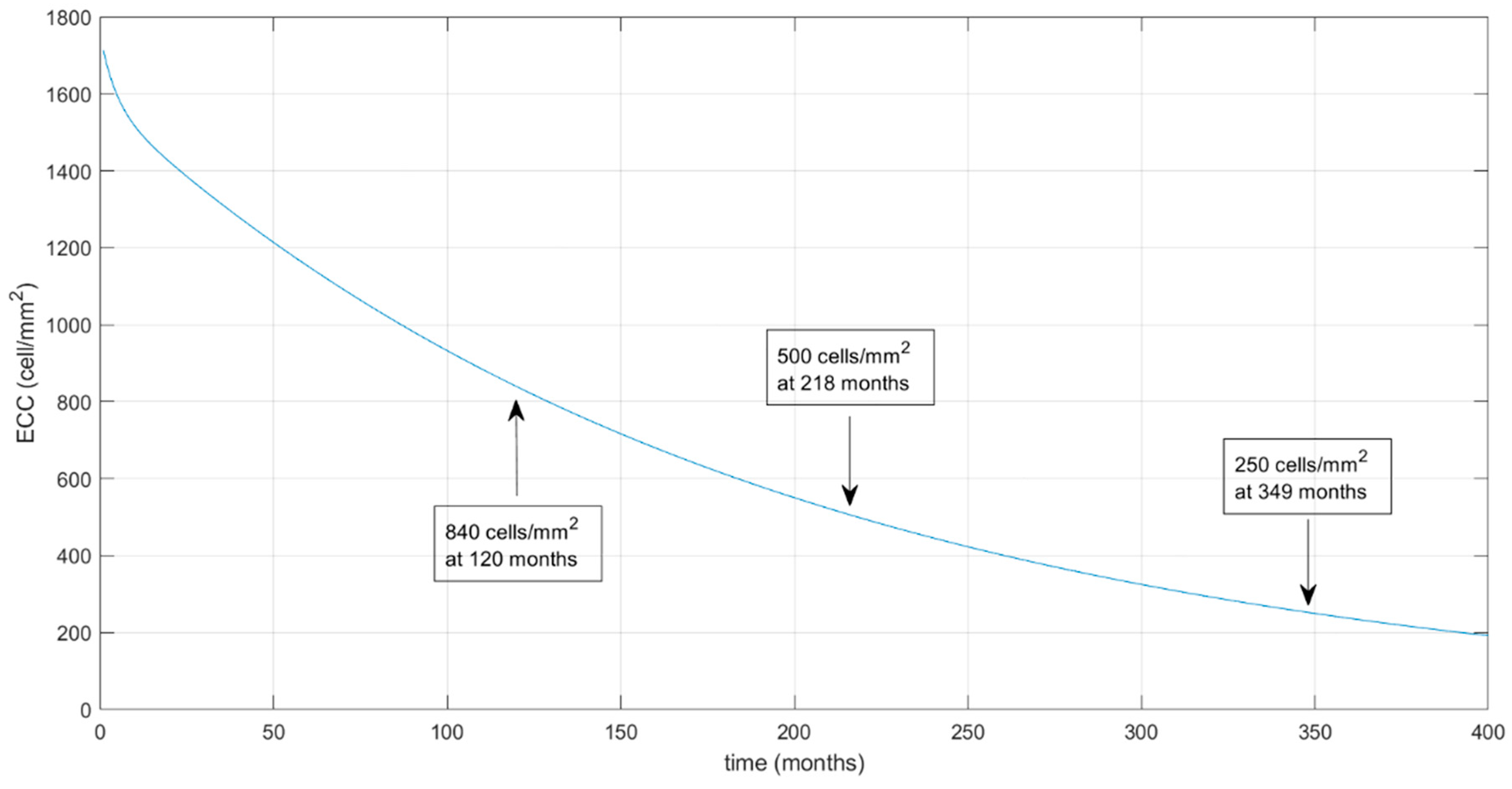
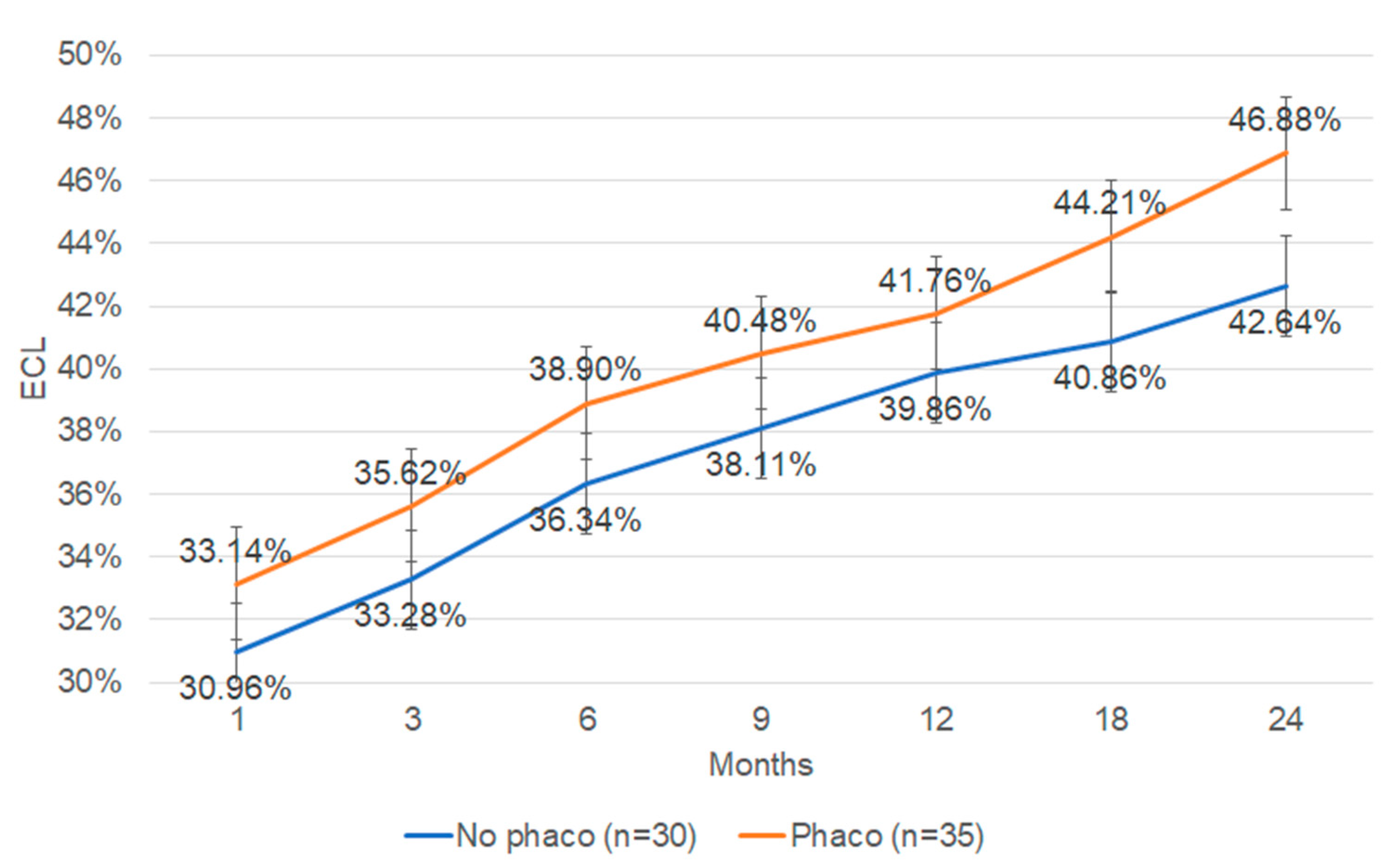
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parekh, M.; Romano, V.; Hassanin, K.; Testa, V.; Wongvisavavit, R.; Ferrari, S.; Haneef, A.; Willoughby, C.; Ponzin, D.; Jhanji, V.; et al. Biomaterials for corneal endothelial cell culture and tissue engineering. J Tissue Eng. 2021, 16, 12. [Google Scholar] [CrossRef]
- Racz, A.; Toth, G.Z.; Tarnoki, A.D.; Tarnoki, D.L.; Littvay, L.; Suveges, I.; Nagy, Z.Z.; Nemeth, J. The inheritance of corneal endothelial cell density. Ophthalmic Genet. 2016, 37, 281–284. [Google Scholar] [CrossRef]
- Thompson, R.W.; Price, M.O.; Bowers, P.J.; Price, F.W. Long-term graft survival after penetrating keratoplasty. Ophthalmology 2003, 110, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Bourne, W.M.; Hodge, D.O.; Nelson, L.R. Corneal endothelium five years after transplantation. Am. J. Ophthalmol. 1994, 118, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, M.A.P.; Hollick, E.J. Modeling Endothelial Cell Loss After Descemet Stripping Endothelial Keratoplasty: Data From 5 Years of Follow-up. Cornea 2017, 36, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Armitage, W.J.; Dick, A.D.; Bourne, W.M. Predicting endothelial cell loss and long-term corneal graft survival. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3326–3331. [Google Scholar] [CrossRef]
- Dooren, B.T.H.V.; Saelens, I.E.Y.; Bleyen, I.; Mulder, P.G.H.; Bartels, M.C.; Rij, G.V. Endothelial cell decay after descemet’s stripping automated endothelial keratoplasty and top hat penetrating keratoplasty. Invest. Ophthalmol. Vis. Sci. 2011, 52, 9226–9231. [Google Scholar] [CrossRef]
- Melles, G.R.; Ong, T.S.; Ververs, B.; van der Wees, J. Descemet membrane endothelial keratoplasty (DMEK). Cornea 2006, 25, 987–990. [Google Scholar]
- Price, F.W.; Price, M.O. Evolution of endothelial keratoplasty. Cornea 2013, 32, 28–32. [Google Scholar] [CrossRef]
- Price, M.O.; Gupta, P.; Lass, J.; Price, F.W. EK (DLEK, DSEK, DMEK): New Frontier in Cornea Surgery. Annu. Rev. Vis. Sci. 2017, 3, 69–90. [Google Scholar] [CrossRef]
- Hamill, C.E.; Schmedt, T.; Jurkunas, U. Fuchs endothelial cornea dystrophy: A review of the genetics behind disease development. Semin. Ophthalmol. 2013, 28, 281–286. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Afshari, N.A.; Pittard, A.B.; Siddiqui, A.; Klintworth, G.K. Clinical study of Fuchs corneal endothelial dystrophy leading to penetrating keratoplasty: A 30-year experience. Arch. Ophthalmol. 2006, 124, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Krachmer, J.H.; Purcell, J.J., Jr.; Young, C.W.; Bucher, K.D. Corneal endothelial dystrophy. A study of 64 families. Arch. Ophthalmol. 1978, 96, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Louttit, M.D.; Kopplin, L.J.; Igo, R.P., Jr.; Fondran, J.R.; Tagliaferri, A.; Bardenstein, D.; Aldave, A.J.; Croasdale, C.R.; Price, M.O.; Rosenwasser, G.O.; et al. A multicenter study to map genes for Fuchs endothelial corneal dystrophy: Baseline characteristics and heritability. Cornea 2012, 31, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Minear, M.A.; Li, Y.J.; Rimmler, J.; Balajonda, E.; Watson, S.; Allingham, R.R.; Hauser, M.A.; Klintworth, G.K.; Afshari, N.A.; Gregory, S.G. Genetic screen of African Americans with Fuchs endothelial corneal dystrophy. Mol. Vis. 2013, 19, 2508–2516. [Google Scholar] [PubMed]
- Zhang, X.; Igo, R.P., Jr.; Fondran, J.; Mootha, V.V.; Oliva, M.; Hammersmith, K.; Sugar, A.; Lass, J.H.; Iyengar, S.K. Fuchs’ Genetics Multi-Center Study, G. Association of smoking and other risk factors with Fuchs’ endothelial corneal dystrophy severity and corneal thickness. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5829–5835. [Google Scholar] [CrossRef] [PubMed]
- Jurkunas, U.V. Fuchs Endothelial Corneal Dystrophy Through the Prism of Oxidative Stress. Cornea 2018, 37 (Suppl. S1), S50–S54. [Google Scholar] [CrossRef]
- Schmedt, T.; Silva, M.M.; Ziaei, A.; Jurkunas, U. Molecular bases of corneal endothelial dystrophies. Exp. Eye Res. 2012, 95, 24–34. [Google Scholar] [CrossRef]
- Jun, A.S. One hundred years of Fuchs’ dystrophy. Ophthalmology 2010, 117, 859–860.e814. [Google Scholar] [CrossRef]
- Vedana, G.; Villarreal, G., Jr.; Jun, A.S. Fuchs endothelial corneal dystrophy: Current perspectives. Clin. Ophthalmol. 2016, 10, 321–330. [Google Scholar]
- Vogt, A. Weitere Ergebnisse der Spaltlampenmikroskopie des vordern Bulbusabschnittes. Arch. Ophthalmol. 1921, 106, 63–113. [Google Scholar]
- Matthaei, M.; Hribek, A.; Clahsen, T.; Bachmann, B.; Cursiefen, C.; Jun, A.S. Fuchs Endothelial Corneal Dystrophy: Clinical, Genetic, Pathophysiologic, and Therapeutic Aspects. Annu. Rev. Vis. Sci. 2019, 5, 151–175. [Google Scholar] [CrossRef]
- Vasiliauskaitė, I.; Oellerich, S.; Ham, L.; Dapena, I.; Baydoun, L.; van Dijk, K.; Melles, G.R.J. Descemet Membrane Endothelial Keratoplasty: Ten-Year Graft Survival and Clinical Outcomes. Am. J. Ophthalmol. 2020, 217, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.M.; Kruse, F.E.; Tourtas, T. Descemet membrane endothelial keratoplasty: Analysis of clinical outcomes of patients with 8–10 years follow-up. Int. Ophthalmol 2022, 42, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Ruzza, A.; Ferrari, S.; Busin, M.; Ponzin, D. Preloaded Tissues for Descemet Membrane Endothelial Keratoplasty. Am. J. Ophthalmol. 2016, 166, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Busin, M.; Leon, P.; D’Angelo, S.; Ruzza, A.; Ferrari, S.; Ponzin, D.; Parekh, M. Clinical Outcomes of Preloaded Descemet Membrane Endothelial Keratoplasty Grafts With Endothelium Tri-Folded Inwards. Am. J. Ophthalmol. 2018, 193, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Pedrotti, E.; Viola, P.; Leon, P.; Neri, E.; Bosio, L.; Bonacci, E.; Ruzza, A.; Kaye, S.B.; Ponzin, D.; et al. Factors Affecting the Success Rate of Preloaded Descemet Membrane Endothelial Keratoplasty With Endothelium-Inward Technique: A Multicenter Clinical Study. Am. J. Ophthalmol. 2022, 241, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Ruzza, A.; Ferrari, S.; Ahmad, S.; Kaye, S.; Ponzin, D.; Romano, V. Endothelium-in versus endothelium-out for Descemet membrane endothelial keratoplasty graft preparation and implantation. Acta Ophthalmol. 2017, 95, 194–198. [Google Scholar] [CrossRef]
- Viola, P.; Neri, E.; Testa, V.; Parekh, M.; Cian, R.; Grassetto, A.; Romano, V. Clinical Outcomes of Preloaded Descemet Membrane Endothelial Keratoplasty With Endothelium Inward: A 24-Month Comparative Analysis Between Fuchs Endothelial Corneal Dystrophy and Bullous Keratopathy. Cornea 2023, 42, 1133–1139. [Google Scholar] [CrossRef]
- Wojcik, G.; Parekh, M.; Romano, V.; Ferrari, S.; Ruzza, A.; Ahmad, S.; Ponzin, D. Preloaded Descemet Membrane Endothelial Keratoplasty Grafts With Endothelium Outward: A Cross-Country Validation Study of the DMEK Rapid Device. Cornea 2021, 40, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, G.; Parekh, M.; Romano, V.; Ruzza, A.; Scorcia, V.; Viola, P.; Leon, P.; Franch, A.; Gadhvi, K.A.; Ponzin, D.; et al. Preloaded DMEK With Endothelium Outward: A Multicenter Clinical Study Using DMEK Rapid Device. Cornea 2023, 43, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.R.; DeMill, D.L.; Zeidenweber, D.A.; Mayko, Z.M.; Bauer, A.J.; Tran, K.D.; Straiko, M.D.; Terry, M.A. Preloaded Descemet Membrane Endothelial Keratoplasty Donor Tissue: Surgical Technique and Early Clinical Results. Cornea 2018, 37, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Parekh, M.; Ruzza, A.; Willoughby, C.E.; Ferrari, S.; Ponzin, D.; Kaye, S.B.; Levis, H.J. Comparison of preservation and transportation protocols for preloaded Descemet membrane endothelial keratoplasty. Br. J. Ophthalmol. 2018, 102, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Pagano, L.; Gadhvi, K.A.; Coco, G.; Titley, M.; Fenech, M.T.; Kaye, S. Clinical outcomes of pre-loaded ultra-thin DSAEK and pre-loaded DMEK. BMJ Open Ophthalmol. 2020, 5, e000546. [Google Scholar] [CrossRef]
- Parekh, M.; Borroni, D.; Ruzza, A.; Levis, H.J.; Ferrari, S.; Ponzin, D.; Romano, V. A comparative study on different Descemet membrane endothelial keratoplasty graft preparation techniques. Acta Ophthalmol. 2018, 96, e718–e726. [Google Scholar] [CrossRef]
- Parekh, M.; Ruzza, A.; Rovati, M.; Tzamalis, A.; Romano, D.; Gupta, N.; Vaddavalli, P.; Bhogal, M.; Jhanji, V.; Sawant, O.; et al. DMEK surgical training: An instructional guide on various wet-lab methods. Surv. Ophthalmol. 2023, 68, 1129–1152. [Google Scholar] [CrossRef]
- Romano, V.; Kazaili, A.; Pagano, L.; Gadhvi, K.A.; Titley, M.; Steger, B.; Levis, J.B. Eye bank versus surgeon prepared DMEK tissues: Influence on adhesion and re-bubbling rate. Br. J. Ophthalmol. 2022, 106, 177–183. [Google Scholar] [CrossRef]
- Parekh, M.; Baruzzo, M.; Favaro, E.; Borroni, D.; Ferrari, S.; Ponzin, D.; Ruzza, A. Standardizing Descemet Membrane Endothelial Keratoplasty Graft Preparation Method in the Eye Bank-Experience of 527 Descemet Membrane Endothelial Keratoplasty Tissues. Cornea 2017, 36, 1458–1466. [Google Scholar] [CrossRef]
- Busin, M.; Leon, P.; Scorcia, V.; Ponzin, D. Contact Lens-Assisted Pull-Through Technique for Delivery of Tri-Folded (Endothelium in) DMEK Grafts Minimizes Surgical Time and Cell Loss. Ophthalmology 2016, 123, 476–483. [Google Scholar] [CrossRef]
- Deng, S.X.; Lee, W.B.; Hammersmith, K.M.; Kuo, A.N.; Li, J.Y.; Shen, J.F.; Weikert, M.P.; Shtein, R.M. Descemet Membrane Endothelial Keratoplasty: Safety and Outcomes: A Report by the American Academy of Ophthalmology. Ophthalmology 2018, 125, 295–310. [Google Scholar] [CrossRef]
- Tillett, C.W. Posterior lamellar keratoplasty. Am. J. Ophthalmol. 1956, 41, 530–533. [Google Scholar] [CrossRef]
- Gorovoy, M.S. Descemet-stripping automated endothelial ker- atoplasty. Cornea 2006, 25, 886–889. [Google Scholar] [CrossRef]
- Price, F.W., Jr.; Price, M.O. Descemet’s stripping with endothelial keratoplasty in 200 eyes: Early challenges and techniques to enhance donor adherence. J. Cataract. Refract. Surg. 2006, 32, 411–418. [Google Scholar] [CrossRef]
- Dapena, I.; Moutsouris, K.; Droutsas, K.; Ham, L.; van Disk, K.V.; Melles, G.R.J. Standardized “no- touch” technique for Descemet membrane endothelial kerato- plasty. Arch. Ophthalmol. 2011, 129, 88–94. [Google Scholar]
- Price, M.O.; Giebel, A.W.; Fairchild, K.M.; Price, F.W., Jr. Desce- met’s membrane endothelial keratoplasty: Prospective multi- center study of visual and refractive outcomes and endothelial survival. Ophthalmology 2009, 116, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Guell, J.L.; Morral, M.; Gris, O.; Elies, D.; Manero, F. Comparison of sulfur hexafluoride 20% versus air tamponade in Descemet mem- brane endothelial keratoplasty. Ophthalmology 2015, 122, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Kruse, F.E.; Laaser, K.; Cursiefen, C.; Heindl, L.M.; Schlotzer-Schrehardt, U.; Riss, S.; Bachmann, B.O. A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea 2011, 30, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Veldman, P.B.; Dye, P.K.; Holiman, J.D.; Mayko, Z.M.; Sales, C.S.; Straiko, M.D.; Galloway, J.D.; Terry, M.A. The S-stamp in Descemet membrane endothelial keratoplasty safely eliminates upside-down graft implantation. Ophthalmology 2016, 123, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Goshe, J.; Dupps, W.J.; Kaiser, P.K.; Singh, R.P.; Gans, R.; Eisengart, J.; Srivastava, S.K. Determination of feasi- bility and utility of microscope-integrated optical coherence tomography during ophthalmic surgery: The DISCOVER study RESCAN results. JAMA Ophthalmol. 2015, 133, 1124–1132. [Google Scholar] [CrossRef]
- Dirisamer, M.; van Dijk, K.; Dapena, I.; Ham, L.; Oganes, O.; Frank, L.E.; Melles, G.R.J. Prevention and management of graft detachment in Descemet membrane endothelial keratoplasty. Arch. Ophthalmol. 2012, 130, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Patefield, A.; Meng, Y.; Airaldi, M.; Coco, G.; Vaccaro, S.; Parekh, M.; Semeraro, F.; Gadhvi, K.A.; Kaye, S.B.; Zheng, Y.; et al. Deep learning using preoperative AS-OCT predicts graft detachment in DMEK. Transl. Vis. Sci. Technol. 2023, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Dirisamer, M.; Naveiras, M.; Tse, W.H.; van Dijk, K.; Frank, L.E.; Ham, L.; Melles, G.R. Outcomes of Descemet membrane endothelial keratoplasty in phakic eyes. J. Cataract. Refract. Surg. 2012, 38, 871–877. [Google Scholar] [CrossRef]
- Maier, A.K.; Wolf, T.; Gundlach, E.; Klamann, M.K.J.; Gonnermann, J.; Bertelmann, E.; Joussen, A.M.; Torun, N. Intraocular pressure elevation and post-DMEK glaucoma following Descemet membrane endothelial keratoplasty. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1947–1954. [Google Scholar] [CrossRef]
- Basak, S.K.; Basak, S.; Pradhan, V.R. Outcomes of Descemet Membrane Endothelial Keratoplasty (DMEK) Using Surgeon’s Prepared Donor DM-Roll in Consecutive 100 Indian Eyes. Open Ophthalmol. J. 2018, 12, 134–142. [Google Scholar] [CrossRef]
- Hos, D.; Tuac, O.; Schaub, F.; Stanzel, T.P.; Schrittenlocher, S.; Hellmich, M.; Bachmann, B.; Cursiefen, C. Incidence and clinical course of immune reactions after Descemet membrane endothelial keratoplasty: Retrospective analysis of 1000 consecutive eyes. Ophthalmology 2017, 124, 512–518. [Google Scholar] [CrossRef]
- Price, M.O.; Scanameo, A.; Feng, M.T.; Price, F.W., Jr. Descemet’s membrane endothelial keratoplasty: Risk of immunologic rejection episodes after discontinuing topical corticosteroids. Ophthalmology 2016, 123, 1232–1236. [Google Scholar] [CrossRef]
- Hoerster, R.; Stanzel, T.P.; Bachmann, B.O.; Siebelmann, S.; Felsch, M.; Cursiefen, C. Intensified topical steroids as prophylaxis for macular edema after pos- terior lamellar keratoplasty combined with cataract surgery. Am. J. Ophthalmol. 2016, 163, 174–179. [Google Scholar] [CrossRef]
- Feng, M.T.; Price, M.O.; Miller, J.M.; Price, F.W., Jr. Air reinjection and endothelial cell density in Descemet membrane endothe- lial keratoplasty: Five-year follow-up. J. Cataract. Refract. Surg. 2014, 40, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Ham, L.; Dapena, I.; Liarakos, V.S.; Baydoun, L.; van Dijk, K.; Ilyas, A.; Oellerich, S.; Melles, G.R.J. Midterm results of Descemet membrane endothelial keratoplasty: 4 to 7 years clinical outcome. Am. J. Ophthalmol. 2016, 171, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Schlögl, A.; Tourtas, T.; Kruse, F.E.; Weller, J.M. Long-term clin- ical outcome after Descemet membrane endothelial kerato- plasty. Am. J. Ophthalmol. 2016, 169, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Hoerster, R.; Stanzel, T.P.; Bachmann, B.O.; Siebelmann, S.; Cursiefen, C. Intensified early postoperative topical steroids do not influence endothelial cell density after Descemet membrane endothelial keratoplasty combined with cataract surgery (Triple-DMEK). Cornea 2016, 35, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Aravena, C.; Yu, F.; Deng, S.X. Outcomes of Descemet mem- brane endothelial keratoplasty in patients with previous glau- coma surgery. Cornea 2017, 36, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Böhringer, D.; Böhringer, S.; Poxleitner, K.; Birnbaum, F.; Schwartzkopff, J.; Maier, P.; Sundmacher, R.; Reinhard, T. Long-term graft survival in penetrating keratoplasty: The biexponential model of chronic endothelial cell loss revisited. Cornea 2010, 29, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Yee, R.W.; Matsuda, M.; Schultz, R.O.; Edelhauser, H.F. Changes in the normal corneal endothelial cellular pattern as a function of age. Curr. Eye Res. 1985, 4, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Bigar, F. Specular microscopy of the corneal endothelium. Optical solutions and clinical results. Dev. Ophthalmol. 1982, 6, 1–94. [Google Scholar] [PubMed]
- Mishima, S. Clinical investigations on the corneal endothelium. Ophthalmology 1982, 89, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Sanchez, J.; de Benito-Llopis, L. Clinical Outcomes of Descemet Membrane Endothelial Keratoplasty in Pseudophakic Eyes Compared With Triple-DMEK at 1-Year Follow-up. Cornea 2021, 40, 420–424. [Google Scholar] [CrossRef]
- Shahnazaryan, D.; Hajjar Sese, A.; Hollick, E.J. Endothelial Cell Loss After Descemet’s Membrane Endothelial Keratoplasty for Fuchs’ Endothelial Dystrophy: DMEK Compared to Triple DMEK. Am. J. Ophthalmol 2020, 218, 1–6. [Google Scholar] [CrossRef]
- Dirisamer, M.; Ham, L.; Dapena, I.; Moutsouris, K.; Droutsas, K.; van Dijk, K.; Frank, L.E.; Oellerich, S.; Melles, G.R.J. Efficacy of descemet membrane endothelial keratoplasty: Clinical outcome of 200 consecutive cases after a learning curve of 25 cases. Arch. Ophthalmol. 2011, 129, 1435–1443. [Google Scholar] [CrossRef]
- Birbal, R.S.; Ni Dhubhghaill, S.; Bourgonje, V.J.A.; Hanko, J.; Ham, L.; Jager, M.J.; Bohringer, S.; Oellerich, S.; Melles, G.R.J. Five-Year Graft Survival and Clinical Outcomes of 500 Consecutive Cases After Descemet Membrane Endothelial Keratoplasty. Cornea 2020, 39, 290–297. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viola, P.; Neri, E.; Occhipinti, T.; Parekh, M.; Cian, R.; Ponzin, D.; Moramarco, A.; Iovieno, A. Predicting Long-Term Endothelial Cell Loss after Preloaded Descemet Membrane Endothelial Keratoplasty in Fuchs’ Endothelial Corneal Dystrophy: A Mathematical Model. J. Clin. Med. 2024, 13, 877. https://doi.org/10.3390/jcm13030877
Viola P, Neri E, Occhipinti T, Parekh M, Cian R, Ponzin D, Moramarco A, Iovieno A. Predicting Long-Term Endothelial Cell Loss after Preloaded Descemet Membrane Endothelial Keratoplasty in Fuchs’ Endothelial Corneal Dystrophy: A Mathematical Model. Journal of Clinical Medicine. 2024; 13(3):877. https://doi.org/10.3390/jcm13030877
Chicago/Turabian StyleViola, Pietro, Enrico Neri, Tommaso Occhipinti, Mohit Parekh, Roberto Cian, Diego Ponzin, Antonio Moramarco, and Alfonso Iovieno. 2024. "Predicting Long-Term Endothelial Cell Loss after Preloaded Descemet Membrane Endothelial Keratoplasty in Fuchs’ Endothelial Corneal Dystrophy: A Mathematical Model" Journal of Clinical Medicine 13, no. 3: 877. https://doi.org/10.3390/jcm13030877





