Steatotic Liver Disease and Sepsis Outcomes—A Prospective Cohort Study (SepsisFAT)
Abstract
:1. Introduction
2. Materials and Methods
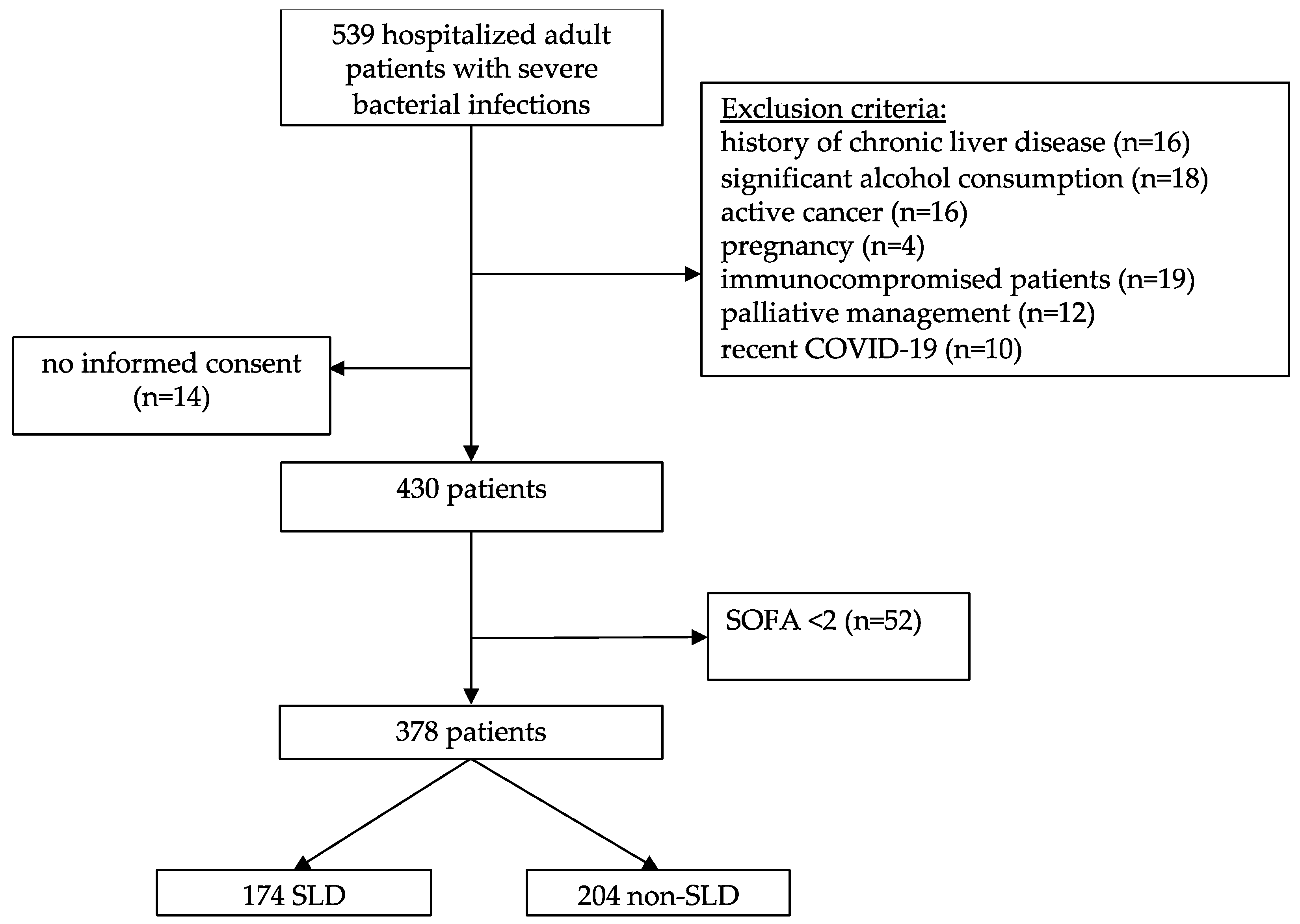
2.1. Study Design and Patients
2.2. Data Collection, Definitions and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Baseline Patient’s Characteristics
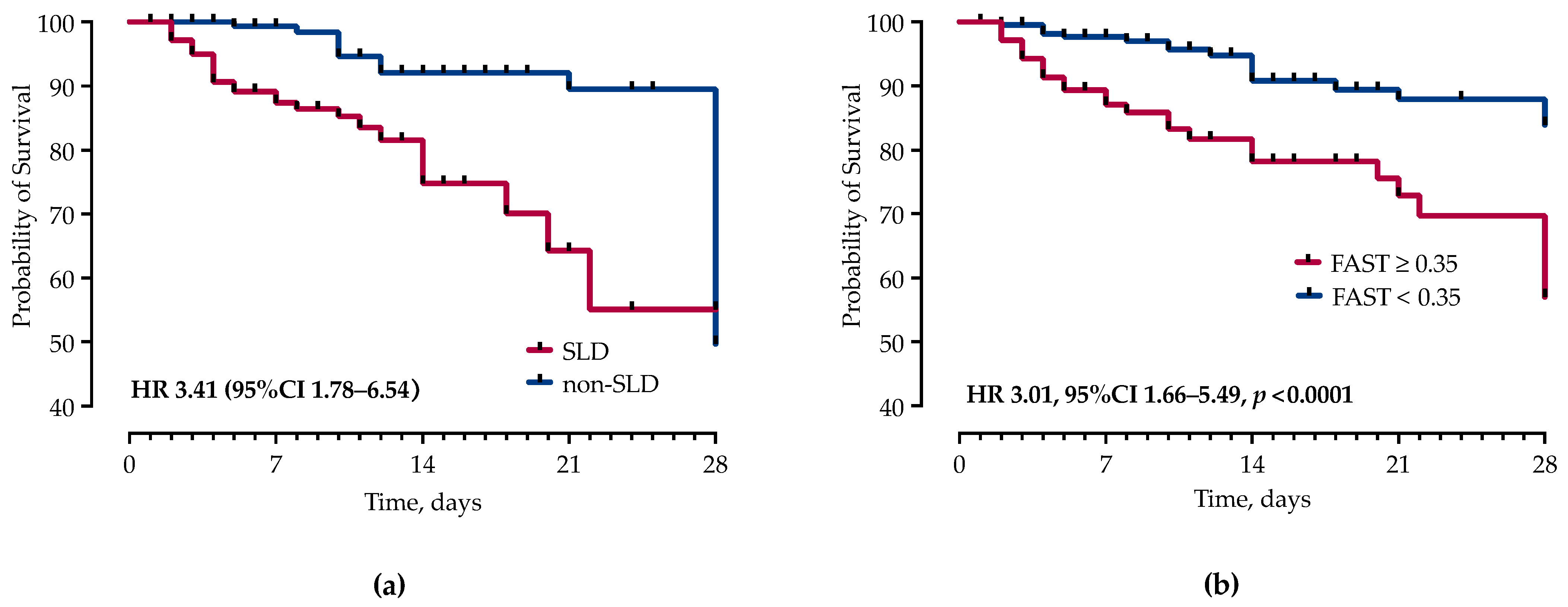
3.2. Clinical Course and Outcome of Sepsis
3.3. Factors Associated with Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Dugani, S.; Veillard, J.; Kissoon, N. Reducing the global burden of sepsis. CMAJ 2017, 189, E2–E3. [Google Scholar] [CrossRef]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef]
- Arvaniti, V.; D’Amico, G.; Fede, G.; Manousou, P.; Tsochatzis, E.; Pleguezuelo, M.; Burroughs, A.K. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010, 139, 1246–1256.e5. [Google Scholar] [CrossRef]
- Wong, F.; Bernardi, M.; Balk, R.; Christman, B.; Moreau, R.; Garcia-Tsao, G.; Patch, D.; Soriano, G.; Hoefs, J.; Navasa, M.; et al. Sepsis in cirrhosis: Report on the 7th meeting of the International Ascites Club. Gut 2005, 54, 718–725. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Eskridge, W.; Vierling, J.M.; Gosbee, W.; Wan, G.A.; Hyunh, M.L.; Chang, H.E. Screening for undiagnosed non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): A population-based risk factor assessment using vibration controlled transient elastography (VCTE). PLoS ONE 2021, 16, e0260320. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Nasiri-Ansari, N.; Androutsakos, T.; Flessa, C.M.; Kyrou, I.; Siasos, G.; Randeva, H.S.; Kassi, E.; Papavassiliou, A.G. Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review. Cells 2022, 11, 2511. [Google Scholar] [CrossRef]
- Brandl, K.; Schnabl, B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr. Opin. Gastroenterol. 2017, 33, 128–133. [Google Scholar] [CrossRef]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of Inflammatory Cytokines With Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef] [PubMed]
- Gjurasin, B.; Jelicic, M.; Kutlesa, M.; Papic, N. The Impact of Nonalcoholic Fatty Liver Disease on Severe Community-Acquired Pneumonia Outcomes. Life 2022, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.B.; Mograbi, J.M.; Amara, A.E.; Abu Elheja, O.H.; Mahamid, M.N. Non-alcoholic fatty liver disease and 30-day all-cause mortality in adult patients with community-acquired pneumonia. QJM 2019, 112, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.; Artul, S.; Nasrallah, N.; Mahamid, M. The association between primary bacteremia of presumed gastrointestinal origin and nonalcoholic fatty liver disease. Dig. Liver Dis. 2016, 48, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Papic, N.; Jelovcic, F.; Karlovic, M.; Maric, L.S.; Vince, A. Nonalcoholic fatty liver disease as a risk factor for Clostridioides difficile infection. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Samadan, L.; Jelicic, M.; Vince, A.; Papic, N. Nonalcoholic Fatty Liver Disease—A Novel Risk Factor for Recurrent Clostridioides difficile Infection. Antibiotics 2021, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.; Amara, A.; Farah, R.; Ahmad, H.S.; Mograbi, J.; Mahamid, M. Non-alcoholic Fatty Liver Disease is Associated with Recurrent Urinary Tract Infection in Premenopausal Women Independent of Metabolic Syndrome. Isr. Med. Assoc. J. 2019, 21, 386–389. [Google Scholar] [PubMed]
- Vrsaljko, N.; Samadan, L.; Viskovic, K.; Mehmedovic, A.; Budimir, J.; Vince, A.; Papic, N. Association of Nonalcoholic Fatty Liver Disease With COVID-19 Severity and Pulmonary Thrombosis: CovidFAT, a Prospective, Observational Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac073. [Google Scholar] [CrossRef]
- Hegyi, P.J.; Vancsa, S.; Ocskay, K.; Dembrovszky, F.; Kiss, S.; Farkas, N.; Eross, B.; Szakacs, Z.; Hegyi, P.; Par, G. Metabolic Associated Fatty Liver Disease Is Associated With an Increased Risk of Severe COVID-19: A Systematic Review With Meta-Analysis. Front. Med. (Lausanne) 2021, 8, 626425. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Ledinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, Q.; Wang, T.; Wen, J.; Wang, H.; Zhang, T. Controlled attenuation parameter for assessment of hepatic steatosis grades: A diagnostic meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 17654–17663. [Google Scholar] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.M.; Venkatesh, S.K.; Ehman, R.L.; Jhaveri, K.; Kamath, P.; Ohliger, M.A.; Samir, A.E.; Silva, A.C.; Taouli, B.; Torbenson, M.S.; et al. Evaluation of hepatic fibrosis: A review from the society of abdominal radiology disease focus panel. Abdom Radiol (NY) 2017, 42, 2037–2053. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Chen, V.L.; Hawa, F.; Berinstein, J.A.; Reddy, C.A.; Kassab, I.; Platt, K.D.; Hsu, C.Y.; Steiner, C.A.; Louissaint, J.; Gunaratnam, N.T.; et al. Hepatic Steatosis Is Associated with Increased Disease Severity and Liver Injury in Coronavirus Disease-19. Dig. Dis. Sci. 2021, 66, 3192–3198. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Simon, T.G.; Hagstrom, H.; Soderling, J.; Wester, A.; Roelstraete, B.; Ludvigsson, J.F. Risk of Severe Infection in Patients With Biopsy-proven Nonalcoholic Fatty Liver Disease—A Population-based Cohort Study. Clin. Gastroenterol. Hepatol. 2023, 21, 3346–3355.e19. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- Caussy, C.; Aubin, A.; Loomba, R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr. Diab Rep. 2021, 21, 15. [Google Scholar] [CrossRef]
- Dewidar, B.; Kahl, S.; Pafili, K.; Roden, M. Metabolic liver disease in diabetes—From mechanisms to clinical trials. Metabolism 2020, 111S, 154299. [Google Scholar] [CrossRef]
- Balintescu, A.; Lind, M.; Franko, M.A.; Oldner, A.; Cronhjort, M.; Svensson, A.M.; Eliasson, B.; Martensson, J. Glycemic Control and Risk of Sepsis and Subsequent Mortality in Type 2 Diabetes. Diabetes Care 2022, 45, 127–133. [Google Scholar] [CrossRef]
- Bertoni, A.G.; Saydah, S.; Brancati, F.L. Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care 2001, 24, 1044–1049. [Google Scholar] [CrossRef]
- Jiang, L.; Cheng, M. Impact of diabetes mellitus on outcomes of patients with sepsis: An updated systematic review and meta-analysis. Diabetol. Metab. Syndr. 2022, 14, 39. [Google Scholar] [CrossRef]
- Chang, C.W.; Kok, V.C.; Tseng, T.C.; Horng, J.T.; Liu, C.E. Diabetic patients with severe sepsis admitted to intensive care unit do not fare worse than non-diabetic patients: A nationwide population-based cohort study. PLoS ONE 2012, 7, e50729. [Google Scholar] [CrossRef] [PubMed]
- Angriman, F.; Lawler, P.R.; Shah, B.R.; Martin, C.M.; Scales, D.C.; Sepsis Canada Network. Prevalent diabetes and long-term cardiovascular outcomes in adult sepsis survivors: A population-based cohort study. Crit. Care 2023, 27, 302. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huang, J.; Wang, D.; Zhu, D.; Zhao, Q.; Li, T.; Zhou, X.; Xu, Y. Association of body mass index with mortality of sepsis or septic shock: An updated meta-analysis. J. Intensive Care 2023, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Jagan, N.; Morrow, L.E.; Walters, R.W.; Plambeck, R.W.; Wallen, T.J.; Patel, T.M.; Malesker, M.A. Sepsis and the Obesity Paradox: Size Matters in More than One Way. Crit. Care Med. 2020, 48, e776–e782. [Google Scholar] [CrossRef] [PubMed]
- Janini, E.; Fteiha, B.; Ramlawi, I.; Mahamid, M. Clinical Trajectory and Predictors of Intensive Care Unit Mortality Among Nonalcoholic Fatty Liver Disease Patients: A Retrospective Case-Control Study. J. Clin. Exp. Hepatol. 2023, 13, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.H.; Wong, V.W.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Zelenika, M.; Lucijanic, M.; Bokun, T.; Bozin, T.; Barisic Jaman, M.; Tjesic Drinkovic, I.; Pastrovic, F.; Madir, A.; Luksic, I.; Piskac Zivkovic, N.; et al. FibroScan-AST Score Predicts 30-Day Mortality or Need for Mechanical Ventilation among Patients Hospitalized with COVID-19. J. Clin. Med. 2021, 10, 4355. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Bamidele, A.O.; Wang, H.; Povero, D.; Revelo, X.S. Emerging Roles of T Cells in the Pathogenesis of Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Front. Endocrinol. 2021, 12, 760860. [Google Scholar] [CrossRef]
- Papic, N.; Samadan, L.; Vrsaljko, N.; Radmanic, L.; Jelicic, K.; Simicic, P.; Svoboda, P.; Lepej, S.Z.; Vince, A. Distinct Cytokine Profiles in Severe COVID-19 and Non-Alcoholic Fatty Liver Disease. Life 2022, 12, 795. [Google Scholar] [CrossRef]
- Susak, F.; Vrsaljko, N.; Vince, A.; Papic, N. TGF Beta as a Prognostic Biomarker of COVID-19 Severity in Patients with NAFLD-A Prospective Case-Control Study. Microorganisms 2023, 11, 1571. [Google Scholar] [CrossRef]
| SLD (n = 174) | Non-SLD (n = 204) | p-Value * | |
|---|---|---|---|
| Age, years, median (IQR) | 69 (57–78) | 64 (50–77) | 0.0156 |
| Male sex, n (%) | 92 (52.87%) | 95 (46.57%) | 0.2563 |
| Body mass index, kg/m2 | 30 (27–36) | 25 (22–28) | <0.0001 |
| Waist-hip ratio | 1.0 (0.94–1.0) | 0.95 (0.84–1.0) | <0.0001 |
| Waist-height ratio | 1.7 (1.5–1.9) | 2.0 (1.8–2.2) | <0.0001 |
| Controlled Attenuation Parameter (dB/m) | 300 (273–335) | 202 (170–225) | <0.0001 |
| Liver stiffness (kPa) | 6.2 (5.1–9) | 5.2 (4–8.1) | 0.0001 |
| Smoking | 37 (21.26%) | 38 (18.63%) | 0.6050 |
| Moderate alcohol consumption | 22 (12.64%) | 18 (8.82%) | 0.2442 |
| Charlson comorbidity index | 4 (2–5.8) | 3 (1–5) | 0.0078 |
| Comorbidities, n (%) | |||
| Diabetes mellitus type 2 | 59 (33.91%) | 33 (16.18%) | 0.0001 |
| Arterial hypertension | 126 (72.41%) | 90 (44.12%) | 0.0001 |
| Dyslipidemia | 54 (31.03%) | 36 (17.65%) | 0.0025 |
| Chronic obstructive pulmonary disease | 21 (12.07%) | 9 (4.41%) | 0.0072 |
| Gastritis/gastroesophageal reflux disease | 18 (10.34%) | 19 (9.31%) | 0.8624 |
| Cardiovascular diseases | 46 (26.44%) | 48 (23.53%) | 0.5515 |
| Chronic renal insufficiency | 13 (7.47%) | 9 (4.41%) | 0.2707 |
| Peripheral vascular disease | 11 (6.32%) | 12 (5.88%) | 1.0000 |
| Neurological diseases | 29 (16.67%) | 42 (20.59%) | 0.3570 |
| Metabolic syndrome | 110 (63.22%) | 47 (23.04%) | <0.0001 |
| MetS median score | 3 (2–4) | 2 (1–2) | <0.0001 |
| Chronic medications | |||
| Beta blockers | 47 (27.01%) | 48 (23.53%) | 0.4761 |
| ACE inhibitors | 78 (44.83%) | 59 (28.92%) | 0.0018 |
| Other antihypertensive drugs | 75 (43.10%) | 55 (29.96%) | 0.0011 |
| Statins | 44 (25.29%) | 24 (11.76%) | 0.0007 |
| Metformin | 34 (19.54%) | 18 (8.82%) | 0.0028 |
| Another oral anti-diabetic | 37 (21.26%) | 18 (8.82%) | 0.0007 |
| Insulin | 25 (14.37%) | 21 (10.29%) | 0.0465 |
| Antiplatelet agent | 25 (14.37%) | 21 (10.29%) | 0.2697 |
| Duration of symptom onset to hospital admission | 4 (2–6) | 4 (2–7) | 0.2722 |
| Sepsis severity scores | |||
| SIRS | 2 (2–3) | 2 (2–3) | 0.9919 |
| SOFA | 3 (2–5) | 3 (2–4) | 0.0159 |
| APACHE II | 13 (9–20) | 12 (8–18) | 0.0766 |
| Liver related scores | |||
| FAST score | 0.31 (0.15–0.52) | 0.1 (0.04–0.27) | <0.0001 |
| APRI score | 0.46 (0.23–1.1) | 0.36 (0.22–0.73) | 0.0429 |
| FIB-4 score | 2.1 (1.2–3.4) | 1.6 (0.91–2.7) | 0.0056 |
| NAFLD score | 0.43 (−0.62–2.1) | −0.48 (−1.9–1.1) | <0.0001 |
| Infection source | |||
| Pneumonia | 39 (22.41%) | 48 (23.41) | 0.3597 |
| Skin and soft tissue | 32 (18.39%) | 21 (10.24%) | |
| Gastrointestinal tract | 28 (16.09%) | 28 (13.66%) | |
| Urinary tract | 26 (14.94%) | 45 (21.95%) | |
| Other | 20 (11.49%) | 24 (11.71%) | |
| Unknown | 15 (8.62%) | 21 (10.24%) | |
| Etiology identified | 86 (49.43%) | 118 (57.84) | 0.1017 |
| SLD (n = 174) | Non-SLD (n = 204) | p-Value * | |
|---|---|---|---|
| Primary outcomes | |||
| In-hospital mortality | 32 (18.39%) | 20 (9.80%) | 0.0157 |
| Time to death from hospital admission | 7 (4–17) | 18 (10–28) | 0.0020 |
| 48-h mortality | 4 (2.30%) | 0 (0%) | 0.0295 |
| 7-day mortality | 17 (9.77%) | 2 (0.98%) | <0.0001 |
| 28-day mortality | 27 (15.52%) | 17 (8.33%) | 0.0300 |
| Secondary outcomes | |||
| Length of hospital stay | 10 (7–17) | 12 (7–23) | 0.1233 |
| ICU admission | 64 (36.78%) | 62 (30.39%) | 0.1890 |
| Vasopressor therapy | 53 (30.46%) | 47 (23.04%) | 0.1031 |
| Duration of vasopressor therapy | 3 (1–7) | 2 (1–6) | 0.6191 |
| Moderate/severe ARDS | 40 (22.99%) | 32 (15.69%) | 0.0715 |
| Invasive mechanical ventilation | 51 (29.31%) | 37 (18.14%) | 0.0104 |
| Duration of IMV | 7 (2–14) | 6 (1–16) | 0.8038 |
| Acute kidney injury | 46 (29.31%) | 36 (17.65%) | 0.0388 |
| Continuous renal replacement therapy (CRRT) | 28 (16.09%) | 18 (8.82%) | 0.0312 |
| Duration of CRRT | 3.0 (2–8) | 2 (1–16) | 0.3908 |
| Nosocomial infections | 30 (17.24%) | 24 (11.76%) | 0.1294 |
| Acute heart failure | 16 (9.20%) | 14 (6.86%) | 0.4030 |
| Univariable Analysis | Cox Proportional Regression Analysis | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age ≥ 60 years | 1.07 (0.55–2.06) | 0.8214 | ||
| Male sex | 0.862 (0.64–2.12) | 0.6556 | ||
| CAP ≥ 270 dB/m | 1.173 (1.081–3.597) | 0.0223 | ||
| Liver stiffness ≥ 6.6 kPa | 2.013 (1.115–3.577) | 0.0185 | ||
| SOFA ≥ 5 | 4.271 (2.27–7.69) | <0.0001 | 2.611 (1.493–4.581) | 0.0007 |
| T2DM | 2.19 (1.20–4.02) | 0.0053 | ||
| SLD | 2.08 (1.15–3.82) | 0.0170 | 2.824 (1.40–5.71) | 0.0276 |
| Procalcitonin ≥ 3.0 µg/L | 2.456 (1.37–4.52) | 0.0027 | ||
| Lactate ≥ 2.5 mmol/L | 5.64 (2.96–10.40) | <0.0001 | 2.599 (1.133–6.259) | 0.0265 |
| Blood urea nitrogen ≥ 9.0 mmol/L | 2.018 (1.12–3.69) | 0.0181 | ||
| Creatinine ≥ 115 µmol/L | 3.075 (1.66–5.43) | 0.0001 | ||
| eGFR ≤ 50 mL/min/1.73 m2 | 2.75 (1.49–5.09) | 0.0006 | ||
| LDH ≥ 260 IU/L | 2.931 (1.62–5.43) | 0.0003 | ||
| Albumin ≤ 30 g/L | 2.156 (1.18–3.96) | 0.0113 | ||
| INR ≥ 1.3 | 6.115 (3.24–11.48) | <0.0001 | 2.225 (1.13–4.42) | <0.0001 |
| D-dimer ≥ 4.0 mg/L | 4.100 (2.19–7.46) | <0.0001 | ||
| Cholesterol ≤ 2.8 mmol/L | 5.149 (2.07–11.88) | <0.0001 | ||
| Triglycerides ≤ 1.7 mmol/L | 4.113 (1.68–9.35) | <0.0001 | ||
| HDL ≤ 0.6 mmol/L | 2.750 (1.17–6.26) | 0.0165 | ||
| NAFLD fibrosis score ≥ 1.3 | 5.545 (2.97–9.97) | <0.0001 | ||
| FIB-4 score ≥ 3.0 | 4.558 (2.46–8.42) | <0.0001 | ||
| FAST score ≥ 0.35 | 3.659 (2.00–6.69) | <0.0001 | 2.244 (1.240–4.124) | 0.0081 |
| Respiratory tract origin | 2.19 (1.20–4.13) | 0.0122 | ||
| Non-urinary tract origin | 10.12 (1.74–10.46) | 0.0055 | 1.529 (1.037–2.217) | 0.028 |
| ICU admission | 14.95 (6.593–29.59) | <0.001 | ||
| Renal insufficiency | 9.078 (4.684–16.72) | <0.001 | 5.586 (2.687–12.02) | <0.0001 |
| CRRT | 22.99 (11.05–48.82) | <0.001 | ||
| IMV | 17.02 (8.542–33.38) | <0.001 | 3.951 (1.065–13.85) | 0.0374 |
| ARDS | 8.328 (4.474–15.54) | <0.001 | ||
| Shock > 24 h | 31.79 (13.87–76.94) | <0.001 | ||
| Nosocomial infections | 5.397 (2.747–10.73) | <0.001 | 3.839 (1.503–10.58) | 0.0066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krznaric, J.; Papic, N.; Vrsaljko, N.; Gjurasin, B.; Kutlesa, M.; Vince, A. Steatotic Liver Disease and Sepsis Outcomes—A Prospective Cohort Study (SepsisFAT). J. Clin. Med. 2024, 13, 798. https://doi.org/10.3390/jcm13030798
Krznaric J, Papic N, Vrsaljko N, Gjurasin B, Kutlesa M, Vince A. Steatotic Liver Disease and Sepsis Outcomes—A Prospective Cohort Study (SepsisFAT). Journal of Clinical Medicine. 2024; 13(3):798. https://doi.org/10.3390/jcm13030798
Chicago/Turabian StyleKrznaric, Juraj, Neven Papic, Nina Vrsaljko, Branimir Gjurasin, Marko Kutlesa, and Adriana Vince. 2024. "Steatotic Liver Disease and Sepsis Outcomes—A Prospective Cohort Study (SepsisFAT)" Journal of Clinical Medicine 13, no. 3: 798. https://doi.org/10.3390/jcm13030798







