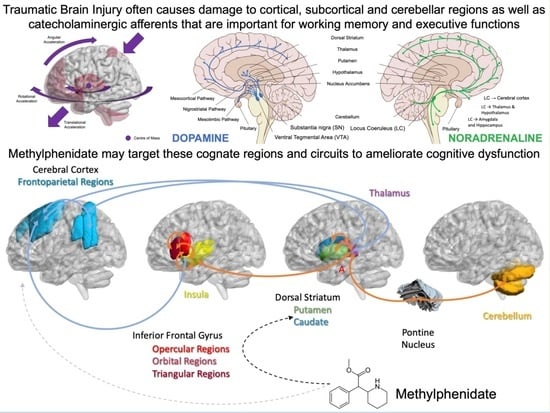
Methylphenidate Ameliorates Behavioural and Neurobiological Deficits in Executive Function for Patients with Chronic Traumatic Brain Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Participants
2.3. Experimental Design
2.4. Tower of London Task Stimulus Presentation
2.5. MRI Data Acquisition and Presentation
2.6. fMRI Data Pre-Processing
2.7. Behavioural Data Analyses
2.8. fMRI Data Analyses and Statistical Modelling
2.9. Task-Modulated Functional Connectivity Analyses
2.10. Behavioural–Functional Correlations
3. Results
3.1. Behavioural Differences
3.1.1. Differences in Mean Reaction Time
3.1.2. Differences in Accuracy
3.2. Activity Differences
Activity Differences Associated with Visuospatial Planning in TBI and with MPh
3.3. Connectivity Differences
3.3.1. Within-Network Task-Modulated Functional Connectivity
3.3.2. Whole-Brain Voxel-Wise Psychophysiological Interaction Analyses
3.4. Functional Brain–Behaviour Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef]
- Iaccarino, M.A.; Bhatnagar, S.; Zafonte, R. Rehabilitation after traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 411–422. [Google Scholar]
- Feigin, V.L.; Theadom, A.; Barker-Collo, S.; Starkey, N.J.; McPherson, K.; Kahan, M.; Dowell, A.; Brown, P.; Parag, V.; Kydd, R.; et al. Incidence of traumatic brain injury in New Zealand: A population-based study. Lancet Neurol. 2013, 12, 53–64. [Google Scholar] [CrossRef]
- Maas, A.I.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Rubiano, A.M.; Carney, N.; Chesnut, R.; Puyana, J.C. Global neurotrauma research challenges and opportunities. Nature 2015, 527, S193–S197. [Google Scholar] [CrossRef]
- Wilson, L.; Horton, L.; Kunzmann, K.; Sahakian, B.J.; Newcombe, V.F.; Stamatakis, E.A.; Von Steinbuechel, N.; Cunitz, K.; Covic, A.; Maas, A.; et al. Understanding the relationship between cognitive performance and function in daily life after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2021, 92, 407–417. [Google Scholar] [CrossRef]
- Te Ao, B.; Brown, P.; Tobias, M.; Ameratunga, S.; Barker-Collo, S.; Theadom, A.; McPherson, K.; Starkey, N.; Dowell, A.; Jones, K.; et al. Cost of traumatic brain injury in New Zealand: Evidence from a population-based study. Neurology 2014, 83, 1645–1652. [Google Scholar] [CrossRef]
- Bombardier, C.H.; Fann, J.R.; Temkin, N.R.; Esselman, P.C.; Barber, J.; Dikmen, S.S. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010, 303, 1938–1945. [Google Scholar] [CrossRef]
- Hillary, F.G.; Genova, H.M.; Chiaravalloti, N.D.; Rypma, B.; DeLuca, J. Prefrontal modulation of working memory performance in brain injury and disease. Hum. Brain Mapp. 2006, 27, 837–847. [Google Scholar] [CrossRef]
- Stuss, D.T. Traumatic brain injury: Relation to executive dysfunction and the frontal lobes. Curr. Opin. Neurol. 2011, 24, 584–589. [Google Scholar] [CrossRef]
- Swick, D.; Honzel, N.; Larsen, J.; Ashley, V.; Justus, T. Impaired response inhibition in veterans with post-traumatic stress disorder and mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2012, 18, 917–926. [Google Scholar] [CrossRef]
- Wammes, J.D.; Good, T.J.; Fernandes, M.A. Autobiographical and episodic memory deficits in mild traumatic brain injury. Brain Cogn. 2017, 111, 112–126. [Google Scholar] [CrossRef]
- De La Plata, C.D.M.; Garces, J.; Kojori, E.S.; Grinnan, J.; Krishnan, K.; Pidikiti, R.; Spence, J.; Devous, M.D.; Moore, C.; McColl, R.; et al. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch. Neurol. 2011, 68, 74–84. [Google Scholar]
- Fortin, S.; Godbout, L.; Braun, C.M. Strategic sequence planning and prospective memory impairments in frontally lesioned head trauma patients performing activities of daily living. Brain Cogn. 2002, 48, 361–365. [Google Scholar]
- Leary, J.B.; Kim, G.Y.; Bradley, C.L.; Hussain, U.Z.; Sacco, M.; Bernad, M.; Collins, J.; Dsurney, J.; Chan, L. The association of cognitive reserve in chronic-phase functional and neuropsychological outcomes following traumatic brain injury. J. Head Trauma Rehabil. 2018, 33, E28. [Google Scholar] [CrossRef]
- Rigon, A.; Duff, M.C.; McAuley, E.; Kramer, A.F.; Voss, M.W. Is traumatic brain injury associated with reduced inter-hemispheric functional connectivity? A study of large-scale resting state networks following traumatic brain injury. J. Neurotrauma 2016, 33, 977–989. [Google Scholar] [CrossRef]
- Rigon, A.; Voss, M.W.; Turkstra, L.S.; Mutlu, B.; Duff, M.C. Frontal and temporal structural connectivity is associated with social communication impairment following traumatic brain injury. J. Int. Neuropsychol. Soc. 2016, 22, 705–716. [Google Scholar] [CrossRef]
- Chang, Y.K.; Tsai, C.L.; Hung, T.M.; So, E.C.; Chen, F.T.; Etnier, J. Effects of acute exercise on executive function: A study with a Tower of London Task. J. Sport Exerc. Psychol. 2011, 33, 847–865. [Google Scholar] [CrossRef]
- Rainville, C.; Lepage, E.; Gauthier, S.; Kergoat, M.J.; Belleville, S. Executive function deficits in persons with mild cognitive impairment: A study with a Tower of London task. J. Clin. Exp. Neuropsychol. 2012, 34, 306–324. [Google Scholar] [CrossRef]
- Sullivan, J.R.; Riccio, C.A.; Castillo, C.L. Concurrent validity of the tower tasks as measures of executive function in adults: A meta-analysis. Appl. Neuropsychol. 2009, 16, 62–75. [Google Scholar] [CrossRef]
- Baker, S.C.; Rogers, R.D.; Owen, A.M.; Frith, C.D.; Dolan, R.J.; Frackowiak, R.S.J.; Robbins, T.W. Neural systems engaged by planning: A PET study of the Tower of London task. Neuropsychologia 1996, 34, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Cazalis, F.; Valabregue, R.; Pélégrini-Issac, M.; Asloun, S.; Robbins, T.W.; Granon, S. Individual differences in prefrontal cortical activation on the Tower of London planning task: Implication for effortful processing. Eur. J. Neurosci. 2003, 17, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G.; Ahmed, S.; Syed, G.M.; Toone, B.K. Neural correlates of planning ability: Frontal lobe activation during the Tower of London test. Neuropsychologia 1993, 31, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.D.; Pittman, G. The Tower of London: A study of the effect of problem structure on planning. J. Clin. Exp. Neuropsychol. 2007, 29, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.D.; Carpenter, P.A.; Varma, S.; Just, M.A. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia 2003, 41, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.D.; Greco, J.A.; Lee, D. An fMRI study of the Tower of London: A look at problem structure differences. Brain Res. 2009, 1286, 123–132. [Google Scholar] [CrossRef]
- Nitschke, K.; Köstering, L.; Finkel, L.; Weiller, C.; Kaller, C.P. A meta-analysis on the neural basis of planning: Activation likelihood estimation of functional brain imaging results in the tower of London task. Hum. Brain Mapp. 2017, 38, 396–413. [Google Scholar] [CrossRef]
- Shallice, T. Specific impairments of planning. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982, 298, 199–209. [Google Scholar]
- Unterrainer, J.M.; Rahm, B.; Kaller, C.P.; Leonhart, R.; Quiske, K.; Hoppe-Seyler, K.; Meier, C.; Müller, C.; Halsband, U. Planning abilities and the Tower of London: Is this task measuring a discrete cognitive function? J. Clin. Exp. Neuropsychol. 2004, 26, 846–856. [Google Scholar] [CrossRef]
- Unterrainer, J.M.; Rahm, B.; Leonhart, R.; Ruff, C.C.; Halsband, U. The Tower of London: The impact of instructions, cueing, and learning on planning abilities. Cogn. Brain Res. 2003, 17, 675–683. [Google Scholar] [CrossRef]
- Wagner, G.; Koch, K.; Reichenbach, J.R.; Sauer, H.; Schlösser, R.G. The special involvement of the rostrolateral prefrontal cortex in planning abilities: An event-related fMRI study with the Tower of London paradigm. Neuropsychologia 2006, 44, 2337–2347. [Google Scholar] [CrossRef]
- Basso, D.; Saracini, C. Differential involvement of left and right frontoparietal areas in visuospatial planning: An rTMS study. Neuropsychologia 2020, 136, 107260. [Google Scholar] [CrossRef]
- Lazeron, R.H.; Rombouts, S.A.; Machielsen, W.C.; Scheltens, P.; Witter, M.P.; Uylings, H.B.; Barkhof, F. Visualizing brain activation during planning: The tower of London test adapted for functional MR imaging. Am. J. Neuroradiol. 2000, 21, 1407–1414. [Google Scholar]
- Rasmussen, I.A.; Antonsen, I.K.; Berntsen, E.M.; Xu, J.; Lagopoulos, J.; Håberg, A.K. Brain activation measured using functional magnetic resonance imaging during the Tower of London task. Acta Neuropsychiatr. 2006, 18, 216–225. [Google Scholar] [CrossRef]
- Alchihabi, A.; Ekmekci, O.; Kivilcim, B.B.; Newman, S.D.; Yarman Vural, F.T. Analyzing Complex Problem Solving by Dynamic Brain Networks. Front. Neuroinform. 2021, 15, 670052. [Google Scholar] [CrossRef] [PubMed]
- Schall, U.; Johnston, P.; Lagopoulos, J.; Jüptner, M.; Jentzen, W.; Thienel, R.; Dittmann-Balçar, A.; Bender, S.; Ward, P.B. Functional brain maps of Tower of London performance: A positron emission tomography and functional magnetic resonance imaging study. NeuroImage 2003, 20, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Christophel, T.B.; Klink, P.C.; Spitzer, B.; Roelfsema, P.R.; Haynes, J.D. The distributed nature of working memory. Trends Cogn. Sci. 2017, 21, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.A.; Calloway, P.; Sahakian, B.J. Amelioration of specific working memory deficits by methylphenidate in a case of adult attention deficit/hyperactivity disorder. J. Psychopharmacol. 2000, 14, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.A.; Owen, A.M.; Sahakian, B.J.; Mavaddat, N.; Pickard, J.D.; Robbins, T.W. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J. Neurosci. 2000, 20, RC65. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; Doyon, J.; Petrides, M.; Evans, A.C. Planning and spatial working memory: A positron emission tomography study in humans. Eur. J. Neurosci. 1996, 8, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.F.; Dotson, N.M.; Bressler, S.L.; Gray, C.M. Content-specific fronto-parietal synchronization during visual working memory. Science 2012, 338, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Campbell, Z.; Zakzanis, K.K.; Jovanovski, D.; Joordens, S.; Mraz, R.; Graham, S.J. Utilizing virtual reality to improve the ecological validity of clinical neuropsychology: An FMRI case study elucidating the neural basis of planning by comparing the Tower of London with a three-dimensional navigation task. Appl. Neuropsychol. 2009, 16, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, T.; O’Sullivan, B.T.; Roland, P.E. Bilateral activation of fronto-parietal networks by incrementing demand in a working memory task. Cereb. Cortex 1997, 7, 465–471. [Google Scholar] [CrossRef]
- Nissim, N.R.; O’Shea, A.M.; Bryant, V.; Porges, E.C.; Cohen, R.; Woods, A.J. Frontal structural neural correlates of working memory performance in older adults. Front. Aging Neurosci. 2017, 8, 328. [Google Scholar] [CrossRef]
- Cicerone, K.; Levin, H.; Malec, J.; Stuss, D.; Whyte, J. Cognitive rehabilitation interventions for executive function: Moving from bench to bedside in patients with traumatic brain injury. J. Cogn. Neurosci. 2006, 18, 1212–1222. [Google Scholar] [CrossRef]
- Elliott, R. Executive functions and their disorders: Imaging in clinical neuroscience. Br. Med. Bull. 2003, 65, 49–59. [Google Scholar] [CrossRef]
- Scheibel, R.S.; Pearson, D.A.; Faria, L.P.; Kotrla, K.J.; Aylward, E.; Bachevalier, J.; Levin, H.S. An fMRI study of executive functioning after severe diffuse TBI. Brain Inj. 2003, 17, 919–930. [Google Scholar] [CrossRef]
- Sharp, D.J.; Scott, G.; Leech, R. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 2014, 10, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Jacobsen, A.; Chen, Z.; Wang, Y. Detecting social-cognitive deficits after traumatic brain injury: An ALE meta-analysis of fMRI studies. Brain Inj. 2017, 31, 1331–1339. [Google Scholar] [CrossRef]
- Konrad, C.; Geburek, A.J.; Rist, F.; Blumenroth, H.; Fischer, B.; Husstedt, I.; Arolt, V.; Schiffbauer, H.; Lohmann, H. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol. Med. 2011, 41, 1197–1211. [Google Scholar] [CrossRef]
- Cole, J.H.; Jolly, A.; de Simoni, S.; Bourke, N.; Patel, M.C.; Scott, G.; Sharp, D.J. Spatial patterns of progressive brain volume loss after moderate-severe traumatic brain injury. Brain 2018, 141, 822–836. [Google Scholar] [CrossRef]
- Levin, H.; Hanten, G.; Max, J.; Li, X.; Swank, P.; Ewing-Cobbs, L.; Dennis, M.; Menefee, D.S.; Schachar, R. Symptoms of attention-deficit/hyperactivity disorder following traumatic brain injury in children. J. Dev. Behav. Pediatr. 2007, 28, 108–118. [Google Scholar] [CrossRef]
- Levin, H.; Troyanskaya, M.; Petrie, J.; Wilde, E.A.; Hunter, J.V.; Abildskov, T.J.; Scheibel, R.S. Methylphenidate treatment of cognitive dysfunction in adults after mild to moderate traumatic brain injury: Rationale, efficacy, and neural mechanisms. Front. Neurol. 2019, 10, 925. [Google Scholar] [CrossRef] [PubMed]
- Levin, H.S.; Hanten, G.; Zhang, L.; Swank, P.R.; Ewing-Cobbs, L.; Dennis, M.; Barnes, M.A.; Max, J.; Schachar, R.; Chapman, S.B.; et al. Changes in working memory after traumatic brain injury in children. Neuropsychology 2004, 18, 240. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Voss, M.W.; Pence, A.; McAuley, E.; Kramer, A.F.; Cohen, N.J. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front. Aging Neurosci. 2013, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Valera, E.M.; Faraone, S.V.; Murray, K.E.; Seidman, L.J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol. Psychiatry 2007, 61, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Huang, C.C.; Sun, C.K.; Lin, G.H.; Hou, W.H. Methylphenidate on cognitive improvement in patients with traumatic brain injury: A meta-analysis. Curr. Neuropharmacol. 2016, 14, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Schachar, R. Methylphenidate treatment of attention-defit/hyperactivity disorder secondary to traumatic brain injury: A critical appraisal of treatment studies. CNS Spectr. 2004, 9, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Whyte, J.; Patel, S.; Europa, E.; Wang, J.; Coslett, H.B.; Detre, J.A. Methylphenidate modulates sustained attention and cortical activation in survivors of traumatic brain injury: A perfusion fMRI study. Psychopharmacology 2012, 222, 47–57. [Google Scholar] [CrossRef]
- Slomine, B.S.; Salorio, C.F.; Grados, M.A.; Vasa, R.A.; Christensen, J.R.; Gerring, J.P. Differences in attention, executive functioning, and memory in children with and without ADHD after severe traumatic brain injury. J. Int. Neuropsychol. Soc. 2005, 11, 645–653. [Google Scholar] [CrossRef]
- Willmott, C.; Ponsford, J. Efficacy of methylphenidate in the rehabilitation of attention following traumatic brain injury: A randomised, crossover, double blind, placebo controlled inpatient trial. J. Neurol. Neurosurg. Psychiatry 2009, 80, 552–557. [Google Scholar] [CrossRef]
- Willmott, C.J.; Ponsford, J.L. Methylphenidate improves cognitive function during inpatient rehabilitation after traumatic brain injury. Nat. Rev. Neurol. 2009, 5, 125. [Google Scholar]
- Whyte, J.; Hart, T.; Vaccaro, M.; Grieb-Neff, P.; Risser, A.; Polansky, M.; Coslett, H.B. Effects of methylphenidate on attention deficits after traumatic brain injury: A multidimensional, randomized, controlled trial. Am. J. Phys. Med. Rehabil. 2004, 83, 401–420. [Google Scholar] [CrossRef]
- Berridge, C.W.; Devilbiss, D.M.; Andrzejewski, M.E.; Arnsten, A.F.; Kelley, A.E.; Schmeichel, B.; Hamilton, C.; Spencer, R.C. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry 2006, 60, 1111–1120. [Google Scholar] [CrossRef]
- Engert, V.; Pruessner, J.C. Dopaminergic and noradrenergic contributions to functionality in ADHD: The role of methylphenidate. Curr. Neuropharmacol. 2008, 6, 322–328. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Logan, J.; Gerasimov, M.; Maynard, L.; Ding, Y.S.; Gatley, S.J.; Gifford, A.; Franceschi, D. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J. Neurosci. 2001, 21, RC121. [Google Scholar] [CrossRef] [PubMed]
- Wilens, T.E. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 2008, 28, S46–S53. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.; Wentzel, A.P.; Andréll, P.; Mannheimer, C.; Rönnbäck, L. Methylphenidate reduces mental fatigue and improves processing speed in persons suffered a traumatic brain injury. Brain Inj. 2015, 29, 758–765. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ko, M.H.; Na, S.Y.; Park, S.H.; Kim, K.W. Effects of single-dose methylphenidate on cognitive performance in patients with traumatic brain injury: A double-blind placebo-controlled study. Clin. Rehabil. 2006, 20, 24–30. [Google Scholar] [CrossRef]
- Zhang, W.T.; Wang, Y.F. Efficacy of methylphenidate for the treatment of mental sequelae after traumatic brain injury. Medicine 2017, 96, e6960. [Google Scholar] [CrossRef]
- Dorer, C.L.; Manktelow, A.E.; Allanson, J.; Sahakian, B.J.; Pickard, J.D.; Bateman, A.; Menon, D.K.; Stamatakis, E.A. Methylphenidate-mediated motor control network enhancement in patients with traumatic brain injury. Brain Inj. 2018, 32, 1040–1049. [Google Scholar] [CrossRef]
- Manktelow, A.E.; Menon, D.K.; Sahakian, B.J.; Stamatakis, E.A. Working memory after traumatic brain injury: The neural basis of improved performance with methylphenidate. Front. Behav. Neurosci. 2017, 11, 58. [Google Scholar] [CrossRef]
- Moreno-López, L.; Manktelow, A.E.; Sahakian, B.J.; Menon, D.K.; Stamatakis, E.A. Anything goes? Regulation of the neural processes underlying response inhibition in TBI patients. Eur. Neuropsychopharmacol. 2017, 27, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Wang, G.; Ding, Y.; Gatley, S.J. Mechanism of action of methylphenidate: Insights from PET imaging studies. J. Atten. Disord. 2002, 6 (Suppl. 1), 31–43. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Ding, Y.S.; Gatley, S.J. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: Results from imaging studies. Eur. Neuropsychopharmacol. 2002, 12, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Logan, J.; Franceschi, D.; Maynard, L.; Ding, Y.S.; Gatley, S.J.; Gifford, A.; Zhu, W.; et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: Therapeutic implications. Synapse 2002, 43, 181–187. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Ding, Y.S. Imaging the effects of methylphenidate on brain dopamine: New model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.K.; Drewencki, L.L.; Chen, X.; Santos, F.R.; Khan, A.S.; Harun, R.; Torres, G.E.; Michael, A.C.; Dixon, C.E. Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J. Neurochem. 2009, 108, 986–997. [Google Scholar] [CrossRef]
- Blennow, K.; Hardy, J.; Zetterberg, H. The neuropathology and neurobiology of traumatic brain injury. Neuron 2012, 76, 886–899. [Google Scholar] [CrossRef]
- Bigler, E.D.; Maxwell, W.L. Neuropathology of mild traumatic brain injury: Relationship to neuroimaging findings. Brain Imaging Behav. 2012, 6, 108–136. [Google Scholar] [CrossRef]
- Mayer, C.L.; Huber, B.R.; Peskind, E. Traumatic brain injury, neuroinflammation, and post-traumatic headaches. Headache J. Head Face Pain 2013, 53, 1523–1530. [Google Scholar] [CrossRef]
- Mckee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef]
- Bales, J.W.; Kline, A.E.; Wagner, A.K.; Dixon, C.E. Targeting dopamine in acute traumatic brain injury. Open Drug Discov. J. 2010, 2, 119. [Google Scholar]
- Chen, Y.H.; Huang, E.Y.K.; Kuo, T.T.; Miller, J.; Chiang, Y.H.; Hoffer, B.J. Impact of traumatic brain injury on dopaminergic transmission. Cell Transplant. 2017, 26, 1156–1168. [Google Scholar] [CrossRef]
- De Simoni, S.; Jenkins, P.O.; Bourke, N.J.; Fleminger, J.J.; Hellyer, P.J.; Jolly, A.E.; Patel, M.C.; Cole, J.H.; Leech, R.; Sharp, D.J. Altered caudate connectivity is associated with executive dysfunction after traumatic brain injury. Brain 2018, 141, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.O.; De Simoni, S.; Fleminger, J.; Bourke, N.; Jolly, A.; Cole, J.; Darian, D.; Sharp, D. Disruption to the dopaminergic system after traumatic brain injury. J. Neurol. Neuropsychiatry Psychiatry 2016, 87, e1. [Google Scholar] [CrossRef]
- Jenkins, P.O.; De Simoni, S.; Bourke, N.J.; Fleminger, J.; Scott, G.; Towey, D.J.; Svensson, W.; Khan, S.; Patel, M.; Greenwood, R.; et al. Dopaminergic abnormalities following traumatic brain injury. Brain 2018, 141, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.O.; De Simoni, S.; Bourke, N.J.; Fleminger, J.; Scott, G.; Towey, D.J.; Svensson, W.; Khan, S.; Patel, M.C.; Greenwood, R.; et al. Stratifying drug treatment of cognitive impairments after traumatic brain injury using neuroimaging. Brain 2019, 142, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Logue, S.F.; Gould, T.J. The neural and genetic basis of executive function: Attention, cognitive flexibility, and response inhibition. Pharmacol. Biochem. Behav. 2014, 123, 45–54. [Google Scholar] [CrossRef]
- Repantis, D.; Schlattmann, P.; Laisney, O.; Heuser, I. Modafinil and methylphenidate for neuroenhancement in healthy individuals: A systematic review. Pharmacol. Res. 2010, 62, 187–206. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D.; Wang, G.J.; Wang, R.; Telang, F.; Caparelli, E.C.; Wong, C.; Jayne, M.; Fowler, J.S. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. NeuroImage 2011, 54, 3101–3110. [Google Scholar] [CrossRef]
- Johansson, B.; Andréll, P.; Rönnbäck, L.; Mannheimer, C. Follow-up after 5.5 years of treatment with methylphenidate for mental fatigue and cognitive function after a mild traumatic brain injury. Brain Inj. 2020, 34, 229–235. [Google Scholar] [CrossRef]
- Al-Adawi, S.; Al-Naamani, A.; Jaju, S.; Al-Farsi, Y.M.; Dorvlo, A.S.; Al-Maashani, A.; Al-Adawi, S.S.; Moustafa, A.A.; Al-Sibani, N.; Essa, M.M.; et al. Methylphenidate improves executive functions in patients with traumatic brain injuries: A feasibility trial via the idiographic approach. BMC Neurol. 2020, 20, 103. [Google Scholar] [CrossRef]
- Incoccia, C.; Formisano, R.; Muscato, P.; Reali, G.; Zoccolotti, P. Reaction and movement times in individuals with chronic traumatic brain injury with good motor recovery. Cortex 2004, 40, 111–115. [Google Scholar] [CrossRef]
- Han, K.; Chapman, S.B.; Krawczyk, D.C. Disrupted Intrinsic Connectivity among Default, Dorsal Attention, and Frontoparietal Control Networks in Individuals with Chronic Traumatic Brain Injury. J. Int. Neuropsychol. Soc. 2016, 22, 263–279. [Google Scholar] [CrossRef]
- Costa, A.; Riedel, M.; Pogarell, O.; Menzel-Zelnitschek, F.; Schwarz, M.; Reiser, M.; Möller, H.J.; Rubia, K.; Meindl, T.; Ettinger, U. Methylphenidate effects on neural activity during response inhibition in healthy humans. Cereb. Cortex 2013, 23, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Fallon, S.J.; van der Schaaf, M.E.; Ter Huurne, N.; Cools, R. The neurocognitive cost of enhancing cognition with methylphenidate: Improved distractor resistance but impaired updating. J. Cogn. Neurosci. 2017, 29, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.L.; Ridel, K.R.; Sallee, F.R.; Zhang, J.; Lipps, T.D.; Wassermann, E.M. Comparison of the inhibitory and excitatory effects of ADHD medications methylphenidate and atomoxetine on motor cortex. Neuropsychopharmacology 2006, 31, 442–449. [Google Scholar] [CrossRef]
- Marquand, A.F.; O’Daly, O.G.; De Simoni, S.; Alsop, D.C.; Maguire, R.P.; Williams, S.C.; Zelaya, F.O.; Mehta, M.A. Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: A multi-class pattern recognition approach. NeuroImage 2012, 60, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Donders, J.; Larsen, T. Clinical utility of the Tower of London—Drexel University, (TOLDX) after adolescent traumatic brain injury. Dev. Neuropsychol. 2012, 37, 333–342. [Google Scholar] [CrossRef]
- Brown, A.W.; Moessner, A.M.; Mandrekar, J.; Diehl, N.N.; Leibson, C.L.; Malec, J.F. A survey of very-long-term outcomes after traumatic brain injury among members of a population-based incident cohort. J. Neurotrauma 2011, 28, 167–176. [Google Scholar] [CrossRef]
- Berti, S.; Schröger, E. Working memory controls involuntary attention switching: Evidence from an auditory dis-traction paradigm. Eur. J. Neurosci. 2003, 17, 1119–1122. [Google Scholar] [CrossRef]
- de Fockert, J.W. Beyond perceptual load and dilution: A review of the role of working memory in selective attention. Front. Psychol. 2013, 4, 287. [Google Scholar] [CrossRef] [PubMed]
- Lavie, N.; Dalton, P. (Eds.) Load theory of attention and cognitive control. In The Oxford Handbook of Attention; Oxford Library of Psychology: Oxford, UK, 2014; pp. 56–75. [Google Scholar]
- Mayer, A.R.; Hanlon, F.M.; Dodd, A.B.; Ling, J.M.; Klimaj, S.D.; Meier, T.B. A functional magnetic resonance imaging study of cognitive control and neurosensory deficits in mild traumatic brain injury. Hum. Brain Mapp. 2015, 36, 4394–4406. [Google Scholar] [CrossRef]
- Sörqvist, P.; Dahlström, Ö.; Karlsson, T.; Rönnberg, J. Concentration: The neural underpinnings of how cognitive load shields against distraction. Front. Hum. Neurosci. 2016, 10, 221. [Google Scholar] [CrossRef]
- Scheid, R.; Walther, K.; Guthke, T.; Preul, C.; von Cramon, D.Y. Cognitive Sequelae of Diffuse Axonal Injury. Arch. Neurol. 2006, 63, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, A.R.; Levin, H.S. Cognitive Sequelae of Traumatic Brain Injury. Psychiatr. Clin. N. Am. 2014, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kozák, L.R.; van Graan, L.A.; Chaudhary, U.J.; Szabó, G.; Lemieux, L. ICN_Atlas: Automated description and quantification of functional MRI activation patterns in the framework of intrinsic connectivity networks. NeuroImage 2017, 163, 319–341. [Google Scholar] [CrossRef]
- Friston, K.; Buechel, C.; Fink, G.; Morris, J.; Rolls, E.; Dolan, R.; Friston, K.; Buechel, C.; Fink, G.; Morris, J.; et al. Psychophysiological and Modulatory Interactions in Neuroimaging. NeuroImage 1997, 6, 218–229. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Zhong, J.; Blyth, B.; Zhu, T.; Kavcic, V.; Peterson, D. Diffusion tensor imaging detects clinically im-portant axonal damage after mild traumatic brain injury: A pilot study. J. Neurotrauma 2007, 24, 1447–1459. [Google Scholar] [CrossRef]
- Benson, R.R.; Meda, S.A.; Vasudevan, S.; Kou, Z.; Govindarajan, K.A.; Hanks, R.A.; Millis, S.R.; Makki, M.; Latif, Z.; Coplin, W.; et al. Global White Matter Analysis of Diffusion Tensor Images Is Predictive of Injury Severity in Traumatic Brain Injury. J. Neurotrauma 2007, 24, 446–459. [Google Scholar] [CrossRef]
- Dinkel, J.; Drier, A.; Khalilzadeh, O.; Perlbarg, V.; Czernecki, V.; Gupta, R.; Gomas, F.; Sanchez, P.; Dormont, D.; Galanaud, D.; et al. Long-Term White Matter Changes after Severe Traumatic Brain Injury: A 5-Year Prospective Cohort. Am. J. Neuroradiol. 2014, 35, 23–29. [Google Scholar] [CrossRef]
- Hayes, J.P.; Bigler, E.D.; Verfaellie, M. Traumatic Brain Injury as a Disorder of Brain Connectivity. J. Int. Neuropsychol. Soc. 2016, 22, 120–137. [Google Scholar] [CrossRef]
- Kinnunen, K.M.; Greenwood, R.; Powell, J.H.; Leech, R.; Hawkins, P.C.; Bonnelle, V.; Patel, M.C.; Counsell, S.J.; Sharp, D.J. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011, 134, 449–463. [Google Scholar] [CrossRef]
- Pischiutta, F.; Micotti, E.; Hay, J.R.; Marongiu, I.; Sammali, E.; Tolomeo, D.; Vegliante, G.; Stocchetti, N.; Forloni, G.; De Simoni, M.-G.; et al. Single severe traumatic brain injury produces progressive pathology with ongoing contralateral white matter damage one year after injury. Exp. Neurol. 2017, 300, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Sharp, D.J.; Beckmann, C.F.; Greenwood, R.; Kinnunen, K.M.; Bonnelle, V.; De Boissezon, X.; Powell, J.H.; Counsell, S.J.; Patel, M.C.; Leech, R. Default mode network functional and structural connectivity after traumatic brain injury. Brain 2011, 134, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yehudah, G.; Guediche, S.; Fiez, J.A. Cerebellar contributions to verbal working memory: Beyond cognitive theory. Cerebellum 2007, 6, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Dreher, J.; Grafman, J. The roles of the cerebellum and basal ganglia in timing and error prediction. Eur. J. Neurosci. 2002, 16, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Middleton, F.A.; Strick, P.L. Cerebellar output channels. Int. Rev. Neurobiol. 1997, 41, 61–82. [Google Scholar]
- Schmahmann, J.D.; Pandya, D.N. The cerebrocerebellar system. Int. Rev. Neurobiol. 1997, 41, 31–60. [Google Scholar] [PubMed]
- Schmahmann, J.D.; Pandya, D.N. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex 2008, 44, 1037–1066. [Google Scholar] [CrossRef] [PubMed]
- Perlstein, W.M.; Cole, M.A.; Demery, J.A.; Seignourel, P.J.; Dixit, N.K.; Larson, M.J.; Briggs, R.W. Parametric ma-nipulation of working memory load in traumatic brain injury: Behavioral and neural correlates. J. Int. Neuropsychol. Soc. 2004, 10, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.-W.; Kim, J.-M.; Shin, I.-S.; Yang, S.-J.; Yoon, J.-S. Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum. Psychopharmacol. Clin. Exp. 2005, 20, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.-T.; Ekstrom, A.D.; Ranganath, C. Neural Oscillations Associated with Item and Temporal Order Maintenance in Working Memory. J. Neurosci. 2011, 31, 10803–10810. [Google Scholar] [CrossRef] [PubMed]
- Liakakis, G.; Nickel, J.; Seitz, R. Diversity of the inferior frontal gyrus—A meta-analysis of neuroimaging studies. Behav. Brain Res. 2011, 225, 341–347. [Google Scholar] [CrossRef]
- Wagner, A.D.; Paré-Blagoev, E.J.; Clark, J.; Poldrack, R.A. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron 2001, 31, 329–338. [Google Scholar] [CrossRef]
- D’antuono, G.; La Torre, F.R.; Marin, D.; Antonucci, G.; Piccardi, L.; Guariglia, C. Role of working memory, inhibition, and fluid intelligence in the performance of the Tower of London task. Appl. Neuropsychol. Adult 2017, 24, 548–558. [Google Scholar] [CrossRef]
- Gilhooly, K.; Wynn, V.; Phillips, L.; Logie, R.; Della Sala, S. Visuo-spatial and verbal working memory in the five-disc Tower of London task: An individual differences approach. Think. Reason. 2002, 8, 165–178. [Google Scholar] [CrossRef]
- Handley, S.J.; Capon, A.; Copp, C.; Harper, C. Conditional reasoning and the Tower of Hanoi: The role of spatial and verbal working memory. Br. J. Psychol. 2002, 93, 501–518. [Google Scholar] [CrossRef]
- Koppenol-Gonzalez, G.V.; Bouwmeester, S.; Boonstra, A.M. Understanding planning ability measured by the Tower of London: An evaluation of its internal structure by latent variable modeling. Psychol. Assess. 2010, 22, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.; Gilhooly, K.; Logie, R.; Della Sala, S.; Wynn, V. Age, working memory, and the Tower of London task. Eur. J. Cogn. Psychol. 2003, 15, 291–312. [Google Scholar] [CrossRef]
- Welsh, M.C.; Satterlee-Cartmell, T.; Stine, M. Towers of Hanoi and London: Contribution of Working Memory and Inhibition to Performance. Brain Cogn. 1999, 41, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Heuvel, O.A.v.D.; Groenewegen, H.J.; Barkhof, F.; Lazeron, R.H.; van Dyck, R.; Veltman, D.J. Frontostriatal system in planning complexity: A parametric functional magnetic resonance version of tower of london task. NeuroImage 2003, 18, 367–374. [Google Scholar] [CrossRef]
- Li, F.; Lu, L.; Chen, H.; Wang, P.; Zhang, H.; Chen, Y.-C.; Yin, X. Neuroanatomical and functional alterations of insula in mild traumatic brain injury patients at the acute stage. Brain Imaging Behav. 2020, 14, 907–916. [Google Scholar] [CrossRef]
- Spreng, R.N.; Stevens, W.D.; Chamberlain, J.P.; Gilmore, A.W.; Schacter, D.L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage 2010, 53, 303–317. [Google Scholar] [CrossRef]
- Trautwein, F.-M.; Singer, T.; Kanske, P. Stimulus-Driven Reorienting Impairs Executive Control of Attention: Evidence for a Common Bottleneck in Anterior Insula. Cereb. Cortex 2016, 26, 4136–4147. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Kinnison, J.; Pessoa, L.; Anderson, M.L. Beyond the tripartite cognition–emotion–interoception model of the human insular cortex. J. Cogn. Neurosci. 2014, 26, 16–27. [Google Scholar] [CrossRef]
- Augustine, J.R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Rev. 1996, 22, 229–244. [Google Scholar] [CrossRef]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The Role of the Dorsal Striatum in Reward and Decision-Making: Figure 1. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef]
- Grahn, J.A.; Parkinson, J.A.; Owen, A.M. The cognitive functions of the caudate nucleus. Prog. Neurobiol. 2008, 86, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Provost, J.-S.; Hanganu, A.; Monchi, O. Neuroimaging studies of the striatum in cognition Part I: Healthy individuals. Front. Syst. Neurosci. 2015, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.D.; Hiebert, N.M.; Seergobin, K.N.; Owen, A.M.; MacDonald, P.A. Dorsal striatum mediates cognitive control, not cognitive effort per se, in decision-making: An event-related fMRI study. NeuroImage 2015, 114, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Flynn, F.G. Anatomy of the insula functional and clinical correlates. Aphasiology 1999, 13, 55–78. [Google Scholar] [CrossRef]
- Ghaziri, J.; Tucholka, A.; Girard, G.; Houde, J.-C.; Boucher, O.; Gilbert, G.; Descoteaux, M.; Lippé, S.; Rainville, P.; Nguyen, D.K. The Corticocortical Structural Connectivity of the Human Insula. Cereb. Cortex 2015, 27, 1216–1228. [Google Scholar] [CrossRef]
- Ghaziri, J.; Tucholka, A.; Girard, G.; Boucher, O.; Houde, J.-C.; Descoteaux, M.; Obaid, S.; Gilbert, G.; Rouleau, I.; Nguyen, D.K. Subcortical structural connectivity of insular subregions. Sci. Rep. 2018, 8, 8596. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.J.; Yarkoni, T.; Khaw, M.W.; Sanfey, A.G. Decoding the Role of the Insula in Human Cognition: Functional Parcellation and Large-Scale Reverse Inference. Cereb. Cortex 2013, 23, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Petrides, M. Lateral prefrontal cortex: Architectonic and functional organization. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 781–795. [Google Scholar] [CrossRef]
- Petrides, M.; Tomaiuolo, F.; Yeterian, E.H.; Pandya, D.N. The prefrontal cortex: Comparative architectonic organization in the human and the macaque monkey brains. Cortex 2012, 48, 46–57. [Google Scholar] [CrossRef]
- Koenigs, M.; Barbey, A.K.; Postle, B.R.; Grafman, J. Superior parietal cortex is critical for the manipulation of in-formation in working memory. J. Neurosci. 2009, 29, 14980–14986. [Google Scholar] [CrossRef]
- Watson, C.E.; Chatterjee, A. A bilateral frontoparietal network underlies visuospatial analogical reasoning. NeuroImage 2012, 59, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Jolly, E.A.; Scott, G.T.; Sharp, D.J.; Hampshire, A.H. Distinct patterns of structural damage underlie working memory and reasoning deficits after traumatic brain injury. Brain 2020, 143, 1158–1176. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Menon, D.K.; Salmond, C.H.; Outtrim, J.G.; Tavares, J.V.T.; Carpenter, T.A.; Pickard, J.D.; Sahakian, B.J.; Stamatakis, E.A. Traumatic brain injury alters the functional brain network mediating working memory. Brain Inj. 2011, 25, 1170–1187. [Google Scholar] [CrossRef] [PubMed]
- Lipton, M.L.; Gulko, E.; Zimmerman, M.E.; Friedman, B.W.; Kim, M.; Gellella, E.; Gold, T.; Shifteh, K.; Ardekani, B.A.; Branch, C.A. Diffusion-Tensor Imaging Implicates Prefrontal Axonal Injury in Executive Function Impairment Following Very Mild Traumatic Brain Injury. Radiology 2009, 252, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Little, D.M.; Kraus, M.F.; Joseph, J.; Geary, E.K.; Susmaras, T.; Zhou, X.J.; Pliskin, N.; Gorelick, P.B. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology 2010, 74, 558–564. [Google Scholar] [CrossRef]
- Palacios, E.; Sala-Llonch, R.; Junque, C.; Roig, T.; Tormos, J.; Bargallo, N.; Vendrell, P. White matter integrity related to functional working memory networks in traumatic brain injury. Neurology 2012, 78, 852–860. [Google Scholar] [CrossRef]
- Palacios, E.M.; Yuh, E.L.; Chang, Y.-S.; Yue, J.K.; Schnyer, D.M.; Okonkwo, D.O.; Valadka, A.B.; Gordon, W.A.; Maas, A.I.R.; Vassar, M.; et al. Resting-State Functional Connectivity Alterations Associated with Six-Month Outcomes in Mild Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1546–1557. [Google Scholar] [CrossRef]
- Shumskaya, E.; Andriessen, T.M.; Norris, D.G.; Vos, P.E. Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology 2012, 79, 175–182. [Google Scholar] [CrossRef]
- Venkatesan, U.M.; Dennis, N.A.; Hillary, F.G. Chronology and chronicity of altered resting-state functional connectivity after traumatic brain injury. J. Neurotrauma 2015, 32, 252–264. [Google Scholar] [CrossRef]
- Xiong, K.; Zhang, J.; Zhang, Y.; Chen, H.; Qiu, M. Brain functional connectivity and cognition in mild traumatic brain injury. Neuroradiology 2016, 58, 733–739. [Google Scholar] [CrossRef]
- Dagher, A.; Owen, A.M.; Boecker, H.; Brooks, D.J. Mapping the network for planning: A correlational PET activation study with the Tower of London task. Brain 1999, 122, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.; Dagher, A.; Aston, J.; Doyon, J. Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. NeuroImage 2003, 20, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Keren-Happuch, E.; Chen, S.A.; Ho, M.R.; Desmond, J.E. A Meta-analysis of Cerebellar Contributions to Higher Cognition from PET and FMRI Studies. Hum. Brain Mapp 2014, 35, 593–615. [Google Scholar]
- Behrmann, M.; Geng, J.J.; Shomstein, S. Parietal cortex and attention. Curr. Opin. Neurobiol. 2004, 14, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef]
- Hester, R.; Foxe, J.J.; Molholm, S.; Shpaner, M.; Garavan, H. Neural mechanisms involved in error processing: A comparison of errors made with and without awareness. NeuroImage 2005, 27, 602–608. [Google Scholar] [CrossRef]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Rosenberg, M.D.; Finn, E.S.; Scheinost, D.; Papademetris, X.; Shen, X.; Constable, R.T.; Chun, M.M. A neuromarker of sustained attention from whole-brain functional connectivity. Nat. Neurosci. 2016, 19, 165–171. [Google Scholar] [CrossRef]
- Rosenberg, M.D.; Zhang, S.; Hsu, W.-T.; Scheinost, D.; Finn, E.S.; Shen, X.; Constable, R.T.; Li, C.-S.R.; Chun, M.M. Methylphenidate Modulates Functional Network Connectivity to Enhance Attention. J. Neurosci. 2016, 36, 9547–9557. [Google Scholar] [CrossRef]
- Farr, O.M.; Hu, S.; Matuskey, D.; Zhang, S.; Abdelghany, O.; Li, C.-S.R. The effects of methylphenidate on cerebral activations to salient stimuli in healthy adults. Exp. Clin. Psychopharmacol. 2014, 22, 154–165. [Google Scholar] [CrossRef] [PubMed]
- de Wit, S.; Watson, P.; Harsay, H.A.; Cohen, M.X.; van de Vijver, I.; Ridderinkhof, K.R. Corticostriatal Connectivity Underlies Individual Differences in the Balance between Habitual and Goal-Directed Action Control. J. Neurosci. 2012, 32, 12066–12075. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: Lessons from dopamine-deficient mice. Ann. N. Y. Acad. Sci. 2008, 1129, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Asl, M.M.; Vahabie, A.-H.; Valizadeh, A. Dopaminergic Modulation of Synaptic Plasticity, Its Role in Neuropsychiatric Disorders, and Its Computational Modeling. Basic Clin. Neurosci. 2019, 10, 1–12. [Google Scholar]
- Fettes, P.; Schulze, L.; Downar, J. Cortico-striatal-thalamic loop circuits of the orbitofrontal cortex: Promising therapeutic targets in psychiatric illness. Front. Syst. Neurosci. 2017, 11, 25. [Google Scholar] [CrossRef]
- Peters, S.K.; Dunlop, K.; Downar, J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front. Syst. Neurosci. 2016, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 2014, 282, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016, 18, 7–21. [Google Scholar] [CrossRef]
- Nakano, K.; Kayahara, T.; Tsutsumi, T.; Ushiro, H. Neural circuits and functional organization of the striatum. J. Neurol. 2000, 247, V1–V15. [Google Scholar] [CrossRef]
- Parent, A.; Hazrati, L.-N. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Rev. 1995, 20, 91–127. [Google Scholar] [CrossRef]
- Haber, S.N.; Calzavara, R. The cortico-basal ganglia integrative network: The role of the thalamus. Brain Res. Bull. 2009, 78, 69–74. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, X.; Ji, W. The Mechanism of Cortico-Striato-Thalamo-Cortical Neurocircuitry in Response Inhibition and Emotional Responding in Attention Deficit Hyperactivity Disorder with Comorbid Disruptive Behavior Disorder. Neurosci. Bull. 2018, 34, 566–572. [Google Scholar] [CrossRef]
- Diwadkar, V.A.; Carpenter, P.A.; Just, M.A. Collaborative Activity between Parietal and Dorso-Lateral Prefrontal Cortex in Dynamic Spatial Working Memory Revealed by fMRI. NeuroImage 2000, 12, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.; Owen, A.; Johnsrude, I.; Passingham, R. Imaging the mental components of a planning task. Neuropsychologia 2001, 39, 315–327. [Google Scholar] [CrossRef]
- Brissenden, A.J.; Somers, D.C. Cortico–cerebellar networks for visual attention and working memory. Curr. Opin. Psychol. 2019, 29, 239–247. [Google Scholar] [CrossRef]
- Stoodley, C.J. The Cerebellum and Cognition: Evidence from Functional Imaging Studies. Cerebellum 2011, 11, 352–365. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Valera, E.M.; Schmahmann, J.D. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. NeuroImage 2012, 59, 1560–1570. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage 2009, 44, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Glickstein, M. Subcortical projections of the parietal lobes. Adv. Neurol. 2003, 93, 43–55. [Google Scholar] [PubMed]
- Rüsch, N.; Spoletini, I.; Wilke, M.; Bria, P.; Di Paola, M.; Di Iulio, F.; Martinotti, G.; Caltagirone, C.; Spalletta, G. Prefrontal–thalamic–cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr. Res. 2007, 93, 79–89. [Google Scholar] [CrossRef]
- Fama, R.; Sullivan, E.V. Thalamic structures and associated cognitive functions: Relations with age and aging. Neurosci. Biobehav. Rev. 2015, 54, 29–37. [Google Scholar] [CrossRef]
- Pelzer, E.A.; Melzer, C.; Timmermann, L.; von Cramon, D.Y.; Tittgemeyer, M. Basal ganglia and cerebellar inter-connectivity within the human thalamus. Brain Struct. Funct. 2017, 222, 381–392. [Google Scholar] [CrossRef]
- Bush, G.; Spencer, T.J.; Holmes, J.; Shin, L.M.; Valera, E.M.; Seidman, L.J.; Makris, N.; Surman, C.; Aleardi, M.; Mick, E.; et al. Functional Magnetic Resonance Imaging of Methylphenidate and Placebo in Attention-Deficit/Hyperactivity Disorder During the Multi-Source Interference Task. Arch. Gen. Psychiatry 2008, 65, 102–114. [Google Scholar] [CrossRef]
- Jenkins, P.O.; Mehta, M.A.; Sharp, D.J. Catecholamines and cognition after traumatic brain injury. Brain 2016, 139, 2345–2371. [Google Scholar] [CrossRef]
- Allen, G.; McColl, R.; Barnard, H.; Ringe, W.K.; Fleckenstein, J.; Cullum, C.M. Magnetic resonance imaging of cer-ebellar–prefrontal and cerebellar–parietal functional connectivity. NeuroImage 2005, 28, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Englander, J.; Bushnik, T.; Oggins, J.; Katznelson, L. Fatigue after traumatic brain injury: Association with neuro-endocrine, sleep, depression and other factors. Brain Inj. 2010, 24, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Ponsford, J.L.; Downing, M.G.; Olver, J.; Ponsford, M.; Acher, R.; Carty, M.; Spitz, G. Longitudinal Follow-Up of Patients with Traumatic Brain Injury: Outcome at Two, Five, and Ten Years Post-Injury. J. Neurotrauma 2014, 31, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, S.; Andelic, N.; Roe, C.; Schanke, A.-K. Cognitive recovery and predictors of functional outcome 1 year after traumatic brain injury. J. Int. Neuropsychol. Soc. 2009, 15, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]





| Patient Number | Time since Injury (Months) | GSC Score | GSC Level | GOSE Score | Age at Scan | Sex | Scan Result (Acute) |
|---|---|---|---|---|---|---|---|
| 2001 | 25 | 7 | Severe | 4 | 27 | Male | Evidence of haemorrhage in both frontal lobes at gray/white matter interfaces and corpus callosum as well as the superior cerebellar cistern. No mass lesions. |
| 2002 | 17 | 12 | Moderate | 3 | 55 | Female | Subarachnoid haemorrhage in the sulci of the left frontoparietal convexity. |
| 2003 | 14 | 5 | Severe | 4 | 29 | Male | Multiple haemorrhagic contusions left temporal lobe. Haemorrhage left basal ganglia. Right thalamus. Right subcortical diffuse axonal injury. |
| 2004 | 36 | 5 | Severe | 4 | 49 | Female | Small, scattered petechial haemorrhages in both cerebral hemispheres. A slightly larger hemorrhage in the left temporal lobe (superior to the petrous ridge). |
| 2006 | 32 | 7 | Severe | 4 | 19 | Male | Subarachnoid haemorrhage in the left interpeduncular fossa and foramen magnum. |
| 2007 | 9 | 14 | Mild | 5 | 58 | Male | Haemorrhagic contusions in orbital frontal cortex. Subarachnoid blood in both cerebral convexities. |
| 2008 | 10 | 3 | Severe | 3 | 23 | Male | Subarachnoid haemorrhage in convexity sulci bilaterally and interpeduncular fossa. |
| 2009 | 39 | 3 | Severe | 3 | 21 | Male | N/A |
| 2010 | 7 | 8 | Severe | 5 | 19 | Male | R frontoparietal epidural haematoma. Small haemorrhagic contusions left inferior frontal, lateral orbitofrontal gyri and anterior aspect of left temporal lobe. |
| 2011 | 27 | 8 | Severe | 4 | 49 | Male | Haemorrhagic contusion left lentiform nucleus. Small focal lesion pons. Bilateral subcortical area frontal lobes. Signal change in corpus callosum. |
| 2012 | 11 | 6 | Severe | 4 | 36 | Male | Right temporal epidural haematoma, haemorrhagic contusions anterior aspect left temporal lobe, posterior inferior right frontal lobe. Scattered areas traumatic subarachnoid haemorrhage in interpeduncular fossa and some of the posterior convexity sulci of both hemispheres. |
| 2013 | 25 | 7 | Severe | 4 | 26 | Male | Intraventricular haemorrhage. |
| 2015 | 41 | N/A | N/A | 5 | 34 | Female | Right temporal/parietal contusion. |
| 2016 | 7 | 10 | Moderate | 5 | 43 | Male | Right subarachnoid haemorrhage and subdural haemorrhage. |
| 2018 | 8 | 8 | Severe | 5 | 19 | Female | Left temporal lobe contusion and left tentorial subdural haemorrhage. |
| 2019 | 32 | 14 | Mild | 4 | 53 | Male | Right subarachnoid haemorrhage and subdural haemorrhage. Haemorrhagic contusion right posterior temporal lobes. Multiple areas contusion superior frontal lobes and right cerebellar hemisphere, right temporal and inferior frontal lobes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peattie, A.R.D.; Manktelow, A.E.; Sahakian, B.J.; Menon, D.K.; Stamatakis, E.A. Methylphenidate Ameliorates Behavioural and Neurobiological Deficits in Executive Function for Patients with Chronic Traumatic Brain Injury. J. Clin. Med. 2024, 13, 771. https://doi.org/10.3390/jcm13030771
Peattie ARD, Manktelow AE, Sahakian BJ, Menon DK, Stamatakis EA. Methylphenidate Ameliorates Behavioural and Neurobiological Deficits in Executive Function for Patients with Chronic Traumatic Brain Injury. Journal of Clinical Medicine. 2024; 13(3):771. https://doi.org/10.3390/jcm13030771
Chicago/Turabian StylePeattie, Alexander R. D., Anne E. Manktelow, Barbara J. Sahakian, David K. Menon, and Emmanuel A. Stamatakis. 2024. "Methylphenidate Ameliorates Behavioural and Neurobiological Deficits in Executive Function for Patients with Chronic Traumatic Brain Injury" Journal of Clinical Medicine 13, no. 3: 771. https://doi.org/10.3390/jcm13030771