Investigation of Maximum Monosyllabic Word Recognition as a Predictor of Speech Understanding with Cochlear Implant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Audiometry
2.3. Data Analysis
- (1)
- To investigate the impact of duration on hearing loss, we applied unpaired t-tests. Using the unpaired t-test, we compare the mean values of WRS65(CI) between the group with a hearing loss > 20 years and the group with a hearing loss ≤ 20 years.
- (2)
- The effect of etiology on WRS65(CI) was analysed using a Kruskal–Wallis test. To further investigate the impact of etiology on postoperative outcome, post hoc comparisons were made between the various causes of severe to profound hearing loss using Dunn’s test. To correct for multiple testing, a Holm adjustment was applied.
3. Results
3.1. Preoperative Results
- (1)
- Applying an unpaired t-test between subjects with a duration of hearing loss >20 years and those with ≤20 years, we found no significant difference in the WRS65(CI) (p > 0.05).
3.2. Postoperative Results
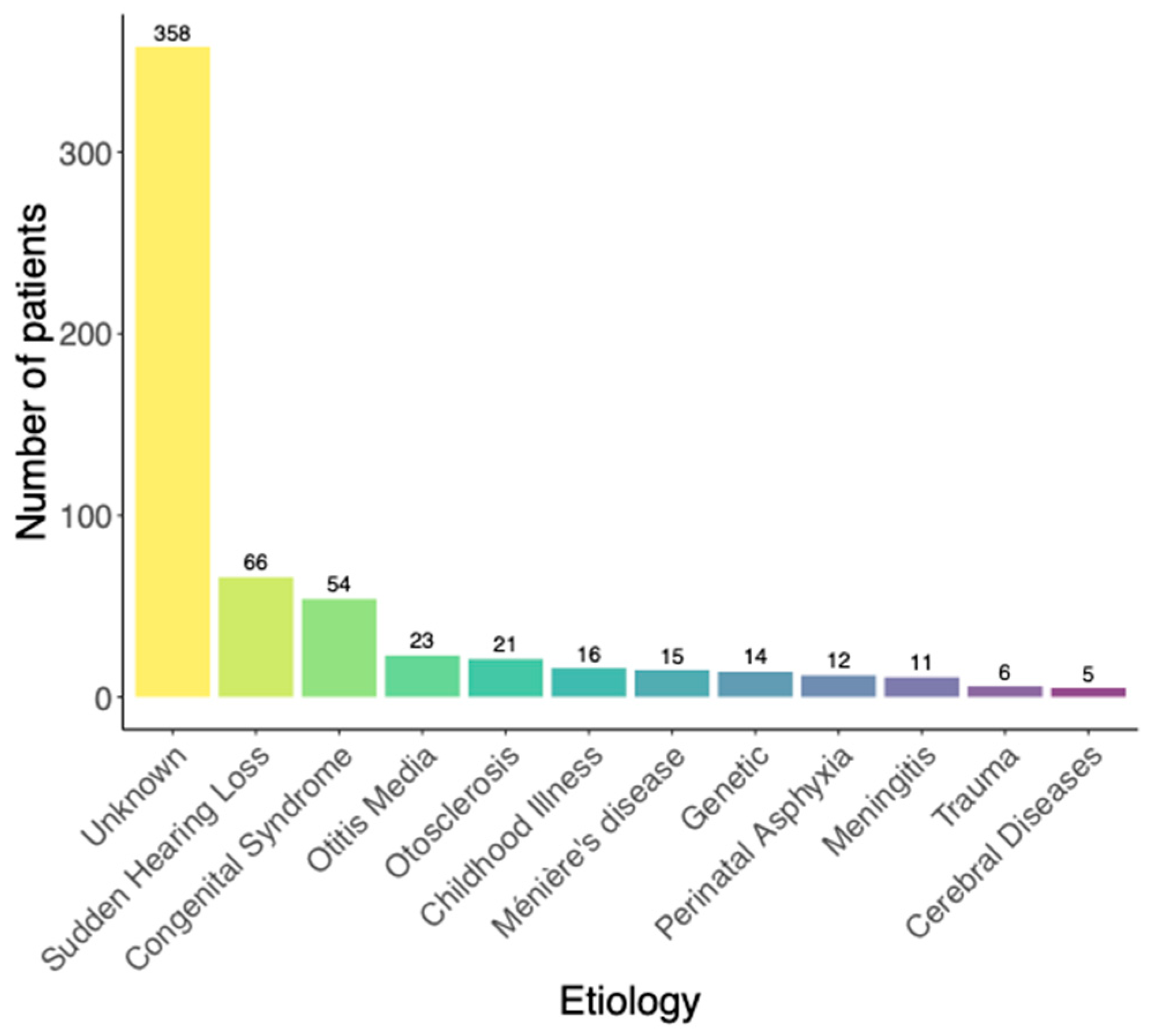
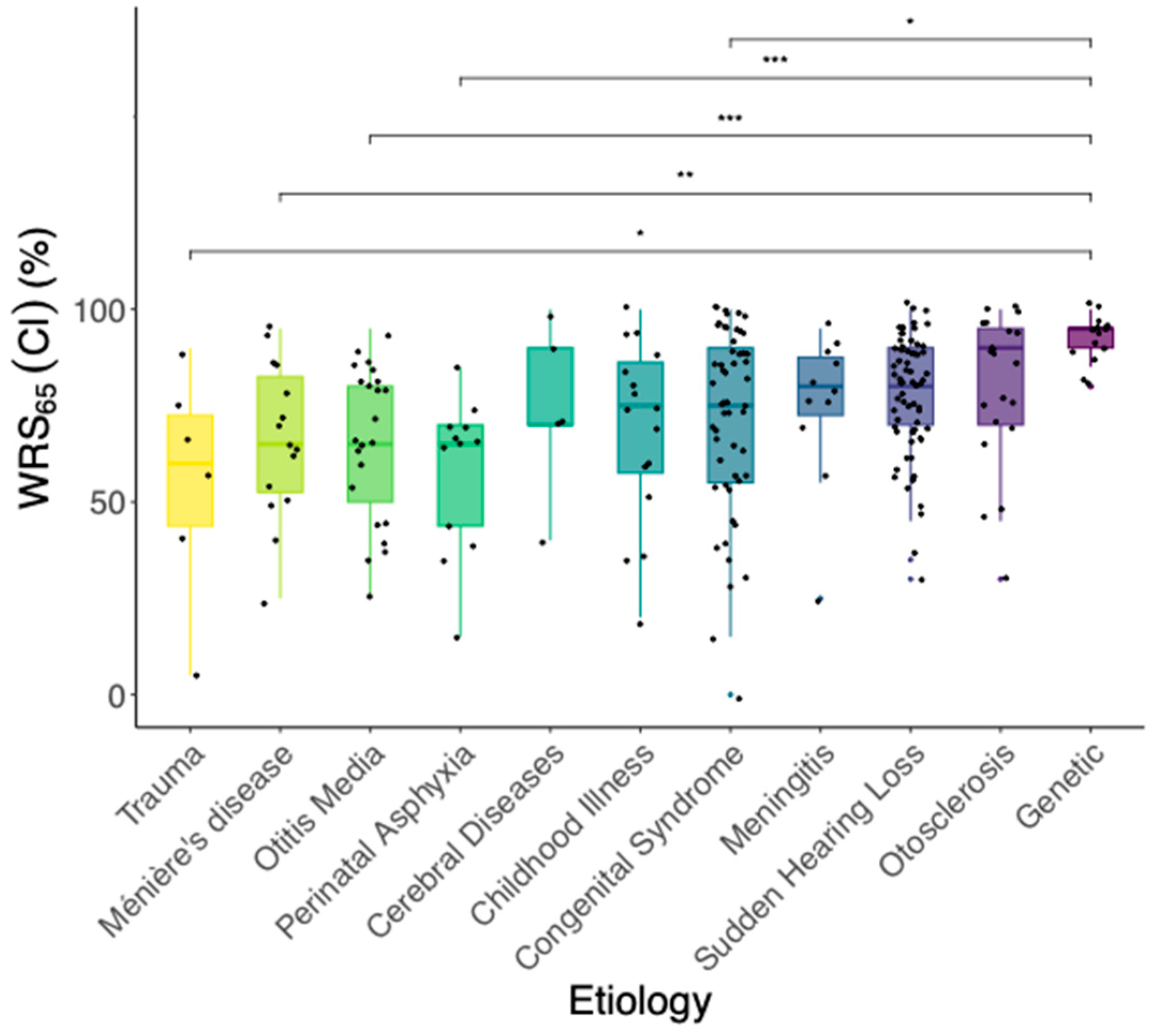
3.3. Effect of Etiology on Postoperative Speech Understanding
- (2)
- In the Kruskal–Wallis-Test, a statistically significant effect of the etiology of hearing loss on WRS65(CI) (χ2 = 36.75, p < 0.05) was found. Five out of the 55 pairwise comparisons of the etiologies (corrected with Holm) showed a significant difference in WRS65(CI). On the group median, cases with the etiology “Congenital”, “Trauma”, “Meniere’s disease”, “Otitis media” or “Perinatal asphyxia” revealed worse postoperative speech understanding compared to genetic hearing loss.
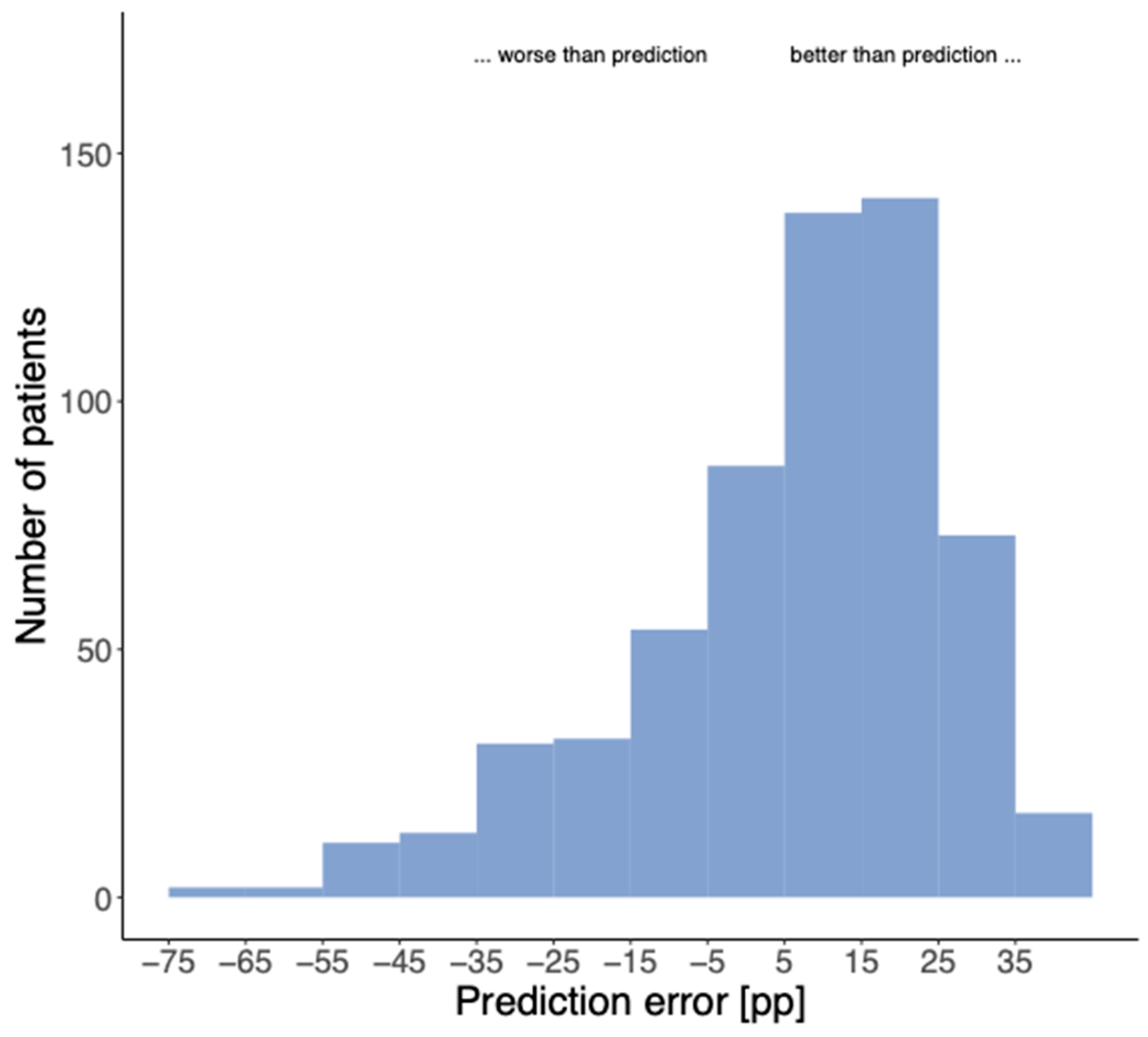
3.4. Validation of the Prediction Model
4. Discussion
4.1. Etiology and Modeling
4.2. Limits of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institute for Health and Care Excellence. Cochlear Implants for Children and Adults with Severe to Profound Deafness. 2019. Available online: https://www.nice.org.uk/guidance/ta566 (accessed on 23 October 2023).
- AWMF. Leitlinien: Cochlea-Implantat Versorgung und Zentral-Auditorische Implantate. 2020. Available online: https://www.awmf.org/uploads/tx_szleitlinien/017-071l_S2k_Cochlea-Implantat-Versorgung-zentral-auditorische-Implantate_2020-12.pdf (accessed on 23 October 2023).
- Van der Straaten, T.F.K.; Briaire, J.J.; Vickers, D.; Boermans, P.; Frijns, J.H.M. Selection Criteria for Cochlear Implantation in the United Kingdom and Flanders: Toward a Less Restrictive Standard. Ear Hear. 2020, 42, 68. [Google Scholar] [CrossRef] [PubMed]
- Buchman, C.A.; Gifford, R.H.; Haynes, D.S.; Lenarz, T.; O’Donoghue, G.; Adunka, O.; Biever, A.; Briggs, R.J.; Carlson, M.L.; Dai, P.; et al. Unilateral Cochlear Implants for Severe, Profound, or Moderate Sloping to Profound Bilateral Sensorineural Hearing Loss: A Systematic Review and Consensus Statements. JAMA Otolaryngol.-Head Neck Surg. 2020, 146, 942–953. [Google Scholar] [CrossRef] [PubMed]
- DGHNO-KHC. Weißbuch Cochlea-Implantat(CI)-Versorgung, 2nd Edition. 2021. Available online: https://cdn.hno.org/media/2021/ci-weissbuch-20-inkl-anlagen-datenblocke-und-zeitpunkte-datenerhebung-mit-logo-05-05-21.pdf (accessed on 23 October 2023).
- Gifford, R.H.; Dorman, M.F.; Shallop, J.K.; Sydlowski, S.A. Evidence for the expansion of adult cochlear implant candidacy. Ear Hear. 2010, 31, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.K.; Metzner, T.; Aschendorff, A.; Arndt, S.; Speck, I.; Laszig, R.; Beck, R.L. Speech processor upgrade increases speech comprehension in patients with cochlear implants. HNO 2019, 67, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Wesarg, T.; Voss, B.; Hassepass, F.; Beck, R.; Aschendorff, A.; Laszig, R.; Arndt, S. Speech Perception in Quiet and Noise With an Off the Ear CI Processor Enabling Adaptive Microphone Directionality. Otol. Neurotol. 2018, 39, e240–e249. [Google Scholar] [CrossRef] [PubMed]
- Aschendorff, A.; Briggs, R.; Brademann, G.; Helbig, S.; Hornung, J.; Lenarz, T.; Marx, M.; Ramos, A.; Stöver, T.; Escudé, B.; et al. Clinical investigation of the Nucleus Slim Modiolar Electrode. Audiol. Neurotol. 2017, 22, 169–179. [Google Scholar] [CrossRef]
- Aschendorff, A.; Klenzner, T.; Arndt, S.; Beck, R.; Schild, C.; Röddiger, L.; Maier, W.; Laszig, R. Insertion results for Contour and Contour Advance electrodes: Are there individual learning curves? HNO 2011, 59, 448–452. [Google Scholar] [CrossRef]
- Hey, M.; Böhnke, B.; Mewes, A.; Munder, P.; Mauger, S.J.; Hocke, T. Speech comprehension across multiple CI processor generations: Scene dependent signal processing. Laryngoscope Investig. Otolaryngol. 2021, 6, 807–815. [Google Scholar] [CrossRef]
- Hoppe, U.; Hocke, T.; Hast AIro, H. Maximum preimplantation monosyllabic score as predictor of cochlear implant outcome. HNO 2019, 67, 62–68. [Google Scholar] [CrossRef]
- Thangavelu, K.; Nitzge, M.; Weiß, R.M.; Mueller-Mazzotta, J.; Stuck, B.A.; Reimann, K. Role of cochlear reserve in adults with cochlear implants following post-lingual hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2022, 280, 1063–1071. [Google Scholar] [CrossRef]
- Rieck, J.H.; Beyer, A.; Mewes, A.; Caliebe, A.; Hey, M. Extended Preoperative Audiometry for Outcome Prediction and Risk Analysis in Patients Receiving Cochlear Implants. J. Clin. Med. 2023, 12, 3262. [Google Scholar] [CrossRef] [PubMed]
- Blamey, P.; Artieres, F.; Başkent, D.; Bergeron, F.; Beynon, A.; Burke, E.; Dillier, N.; Dowell, R.; Fraysse, B.; Gallégo, S.; et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiol. Neuro-Otol. 2013, 18, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Holden, L.K.; Finley, C.C.; Firszt, J.B.; Holden, T.A.; Brenner, C.; Potts, L.G.; Gotter, B.D.; Vanderhoof, S.S.; Mispagel, K.; Heydebrand, G.; et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013, 34, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Hocke, T.; Hast, A.; Iro, H. Cochlear Implantation in Candidates With Moderate-to-Severe Hearing Loss and Poor Speech Perception. Laryngoscope 2021, 131, E940–E945. [Google Scholar] [CrossRef] [PubMed]
- Shafieibavani, E.; Goudey, B.; Kiral, I.; Zhong, P.; Jimeno-Yepes, A.; Swan, A.; Gambhir, M.; Buechner, A.; Kludt, E.; Eikelboom, R.H.; et al. Predictive models for cochlear implant outcomes: Performance, generalizability, and the impact of cohort size. Trends Hear. 2021, 25, 23312165211066174. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Hast, A.; Hocke, T. Validation of a predictive model for speech discrimination after cochlear impIant provision. HNO 2023, 71, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Holube, I. Test-retest reliability of the Freiburg monosyllabic speech test. HNO 2016, 64, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Kanona, H.; Forde, C.; Van Rooyen, A.M.; Keating, P.; Bradley, J.; Pendolino, A.L.; Mehta, N.; Manjaly, J.G.; Khalil, S.; Lavy, J.; et al. Cochlear implant outcomes in patients with Meniere’s disease: A large case series. Cochlear Implant. Int. 2022, 23, 339–346. [Google Scholar] [CrossRef]
- Hast, A.; Meßbacher, M.E.; Liebscher, T.; Hornung, J.; Hoppe, U. Fluctuation in electrical hearing in a Morbus Meniere’s patient. Clin. Case Rep. 2021, 9, e04411. [Google Scholar] [CrossRef]
- Pfeiffer, C.J.; Gehl, H.B.; Scholtz, L.U.; Goon, P.; Sudhoff, H.; Todt, I. Endolymphatic Hydrops Magnet Resonance Imaging in Ménière’s Disease Patients after Cochlea Implantation. Brain Sci. 2023, 13, 853. [Google Scholar] [CrossRef]
- Wrobel, C.; Bevis, N.F.; Klinge-Strahl, A.; Strenzke, N.; Beutner, D. Performance and self-perceived hearing impairment after cochlear implantation in Menière’s disease. Laryngoscope Investig. Otolaryngol. 2022, 7, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Samy, R.N.; Houston, L.; Scott, M.; Choo, D.I.; Meinzen-Derr, J. Cochlear implantation in patients with Meniere’s disease. Cochlear Implant. Int. 2016, 16, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, O.M.; Özkan, B.; Bajin, D.; Sennaroğlu, G.; Sennaroğlu, L. Long-Term Outcome of Cochlear Implantation in Post-meningitic Deafness. J. Int. Adv. Otol. 2021, 17, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Hocke, T.; Iro, H. Age-Related Decline of Speech Perception. Front. Aging Neurosci. 2022, 14, 891202. [Google Scholar] [CrossRef] [PubMed]
- Riggs, W.J.; Roche, J.P.; Giardina, C.K.; Harris, M.S.; Bastian, Z.J.; Fontenot, T.E.; Buchman, C.A.; Brown, K.D.; Adunka, O.F.; Fitzpatrick, D.C. Intraoperative Electrocochleographic Characteristics of Auditory Neuropathy Spectrum Disorder in Cochlear Implant Subjects. Front. Neurosci. 2017, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Walger, M.; Foerst, A.; Beutner, D.; Streicher, B.; Stürmer, K.; Lang-Roth, R. Auditory synaptopathy/neuropathy: Clinical findings and diagnosis. HNO 2011, 59, 414–424. [Google Scholar] [CrossRef]
- Moberly, A.C.; Bates, C.; Harris, M.S.; Pisoni, D.B. The Enigma of Poor Performance by Adults With Cochlear Implants. Otol. Neurotol. 2016, 37, 1522–1528. [Google Scholar] [CrossRef]
- Aschendorff, A.; Arndt, S.; Kröger, S.; Wesarg, T.; Ketterer, M.C.; Kirchem, P.; Pixner, S.; Hassepaß, F.; Beck, R. Quality of cochlear implant rehabilitation under COVID-19 conditions. HNO 2021, 69, 1–6. [Google Scholar] [CrossRef]
- Ma, C.; Fried, J.; Nguyen, S.A.; Schvartz-Leyzac, K.C.; Camposeo, E.L.; Meyer, T.A.; Dubno, J.R.; McRackan, T.R. Longitudinal Speech Recognition Changes After Cochlear Implant: Systematic Review and Meta-analysis. Laryngoscope 2023, 133, 1014–1024. [Google Scholar] [CrossRef]
- Dziemba, O.C.; Merz, S.; Hocke, T. Evaluative audiometry after cochlear implant provision. German Version. HNO 2023, 72, 56–62. [Google Scholar] [CrossRef]
- Patro, A.; Lindquist, N.R.; Holder, J.T.; Tawfik, K.O.; O’Malley, M.R.; Bennett, M.L.; Haynes, D.S.; Gifford, R.; Perkins, E.L. Further Evidence for Individual Ear Consideration in Cochlear Implant Candidacy Evaluation. Otol. Neurotol. 2022, 43, 1033–1040. [Google Scholar] [CrossRef] [PubMed]



| Male Ears | Female Ears | ||
|---|---|---|---|
| Sex | 271 | 330 | |
| Mean age at cochlear implantation [years] | 56.2 | 51.4 | |
| Mean value | Standard deviation | ||
| Duration of hearing loss [years] | 26.3 | 16.9 | |
| Presumed duration of hearing aid fitting [years] | 20.4 | 14.1 | |
| Implant side | Right | Left | |
| 290 | 311 | ||
| Yes | No | Unknown | |
| Tinnitus | 363 | 214 | 24 |
| Vestibulopathies | 98 | 477 | 26 |
| Preoperative | Minimum | Maximum | Mean | Standard Deviation |
|---|---|---|---|---|
| 4PTA (dB HL) | 49.8 | 120.0 | 91.0 | 14.2 |
| mWRS (%) | 5.0 | 100.0 | 33.2 | 22.6 |
| WRS65(HA) (%) | 0.0 | 60.0 | 10.4 | 14.2 |
| Absolute | Relative to Model | ||||||
|---|---|---|---|---|---|---|---|
| Etiology | Number of Cases | Mean WRS65(CI) | Difference to Median WRS65(CI) | Median Error [pp] | Adjusted Median Error [pp] | Interquartile Range of Error | Number of Cases Where Prediction Is Missed by More than 20 pp |
| Genetic hearing loss | 14 | 95 | 15 | 15. 5 | 5.5 | 17.3 | 0 (0%) |
| Sudden hearing loss | 66 | 80 | 0 | 11.0 | 1.0 | 21.2 | 3 (5%) |
| Childhood illness | 16 | 75 | −5 | 10.8 | 0.9 | 26.5 | 3 (20%) |
| Congenital syndrome | 54 | 75 | −5 | 9.5 | −0.5 | 31.9 | 10 (19%) |
| Meningitis | 11 | 80 | 0 | 15.0 | 5.1 | 14.6 | 2 (18%) |
| Ménière’s disease | 15 | 65 | −15 | −1.8 | −11.7 | 33.7 | 2 (13%) |
| Otitis media | 23 | 65 | −15 | 2.2 | −7.7 | 35.7 | 5 (22%) |
| Otosclerosis | 21 | 90 | 10 | 16.3 | 6.3 | 22.7 | 1 (5%) |
| Perinatal asphyxia | 12 | 65 | −15 | −6.8 | −16.8 | 29.7 | 4 (33%) |
| Trauma | 6 | 60 | −20 | −6.2 | −16.1 | 34.0 | 1 (17%) |
| Unknown | 358 | 80 | 0 | 10.7 | 0.8 | 23.1 | 45 (13%) |
| Cerebral diseases | 5 | 70 | −10 | 6.2 | −3.7 | 35.8 | 1 (20%) |
| Total | 601 | 80 | 0 | 9.9 | 0 | 17.3 | 77 (13%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czurda, R.; Wesarg, T.; Aschendorff, A.; Beck, R.L.; Hocke, T.; Ketterer, M.C.; Arndt, S. Investigation of Maximum Monosyllabic Word Recognition as a Predictor of Speech Understanding with Cochlear Implant. J. Clin. Med. 2024, 13, 646. https://doi.org/10.3390/jcm13030646
Czurda R, Wesarg T, Aschendorff A, Beck RL, Hocke T, Ketterer MC, Arndt S. Investigation of Maximum Monosyllabic Word Recognition as a Predictor of Speech Understanding with Cochlear Implant. Journal of Clinical Medicine. 2024; 13(3):646. https://doi.org/10.3390/jcm13030646
Chicago/Turabian StyleCzurda, Ronja, Thomas Wesarg, Antje Aschendorff, Rainer Linus Beck, Thomas Hocke, Manuel Christoph Ketterer, and Susan Arndt. 2024. "Investigation of Maximum Monosyllabic Word Recognition as a Predictor of Speech Understanding with Cochlear Implant" Journal of Clinical Medicine 13, no. 3: 646. https://doi.org/10.3390/jcm13030646






