Advanced Paternal Age: A New Indicator for the Use of Microfluidic Devices for Sperm DNA Fragmentation Selection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ovarian Stimulation
2.3. Oocyte Retrieval
2.4. Semen Preparation
2.5. ICSI and Embryo Culture
2.6. Embryo Transfer and Clinical Outcomes
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halvaei, I.; Litzky, J.; Esfandiari, N. Advanced paternal age: Effects on sperm parameters, assisted reproduction outcomes and offspring health. Reprod. Biol. Endocrinol. 2020, 18, 110. [Google Scholar] [CrossRef]
- Dviri, M.; Madjunkova, S.; Koziarz, A.; Antes, R.; Abramov, R.; Mashiach, J.; Moskovtsev, S.; Kuznyetsova, I.; Librach, C. Is there a correlation between paternal age and aneuploidy rate? An analysis of 3118 embryos derived from young egg donors. Fertil. Steril. 2020, 114, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Dviri, M.; Madjunkova, S.; Koziarz, A.; Madjunkov, M.; Mashiach, J.; Nekolaichuk, E.; Trivodaliev, K.; Al-Asmar, N.; Moskovtsev, S.I.; Librach, C. Is there an association between paternal age and aneuploidy? Evidence from young donor oocyte-derived embryos: A systematic review and individual patient data meta-analysis. Hum. Reprod. Update 2021, 27, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Fonseka, K.G.; Griffin, D.K. Is there a paternal age effect for aneuploidy? Cytogenet. Genome Res. 2011, 133, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Belloc, S.; Benkhalifa, M.; Cohen-Bacrie, M.; Dalleac, A.; Amar, E.; Zini, A. Sperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motility. Fertil. Steril. 2014, 101, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.C.; Ory, J.; Blachman-Braun, R.; Nackeeran, S.; Best, J.C.; Ramasamy, R. Advanced paternal age and sperm DNA fragmentation: A systematic review. World J. Mens Health 2022, 40, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Pecora, G.; Pallotti, F.; Faja, F.; Pelloni, M.; Lenzi, A.; Lombardo, F. Cytological and molecular aspects of the ageing sperm. Hum. Reprod. 2019, 34, 218–227. [Google Scholar] [CrossRef]
- Petersen, C.G.; Mauri, A.L.; Vagnini, L.D.; Renzi, A.; Petersen, B.; Mattila, M.; Comar, V.; Ricci, J.; Dieamant, F.; Oliveira, J.B.A.; et al. The effects of male age on sperm DNA damage: An evaluation of 2178 semen samples. JBRA Assist. Reprod. 2018, 22, 323–330. [Google Scholar] [CrossRef]
- Akmal, M.; Aulanni’am, A.; Widodo, M.; Sumitro, S.; Purnomo, B.; Widodo. The important role of protamine in spermatogenesis and quality of sperm: A mini review. Asian Pac. J. Reprod. 2016, 5, 357–360. [Google Scholar] [CrossRef]
- Pacey, A. Is sperm DNA fragmentation a useful test that identifies a treatable cause of male infertility? Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 53, 11–19. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Yeste, M.; Becerra-Tomás, N.; Aston, K.I.; James, E.R.; Salas-Huetos, A. Clinical implications of sperm DNA damage in IVF and ICSI: Updated systematic review and meta-analysis. Biol. Rev. Camb. Philos. Soc. 2021, 96, 1284–1300. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Benet, J. Single and double strand sperm DNA damage: Different reproductive effects on male fertility. Genes 2019, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.S. Function of sperm chromatin structural elements in fertilization and development. Mol. Hum. Reprod. 2010, 16, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wykes, S.M.; Krawetz, S.A. The structural organization of sperm chromatin. J. Biol. Chem. 2003, 278, 29471–29477. [Google Scholar] [CrossRef] [PubMed]
- Lara-Cerrillo, S.; Ribas-Maynou, J.; Rosado-Iglesias, C.; Lacruz-Ruiz, T.; Benet, J.; García-Peiró, A. Sperm selection during ICSI treatments reduces single- but not double-strand DNA break values compared to the semen sample. J. Assist. Reprod. Genet. 2021, 38, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, A.; Ribas-Maynou, J.; Lara-Cerrillo, S.; Jimenez-Macedo, A.R.; Hortal, O.; Benet, J.; Carrera, J.; García-Peiró, A. Double-stranded sperm DNA damage is a cause of delay in embryo development and can impair implantation rates. Fertil. Steril. 2019, 111, 699–707. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; García-Peiró, A.; Fernandez-Encinas, A.; Amengual, M.J.; Prada, E.; Cortés, P.; Navarro, J.; Benet, J. Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female Factor. PLoS ONE 2012, 7, e44679. [Google Scholar] [CrossRef]
- Setti, A.S.; Braga, D.P.A.F.; Provenza, R.R.; Iaconelli, A.; Borges, E. Oocyte ability to repair sperm DNA fragmentation: The impact of maternal age on intracytoplasmic sperm injection outcomes. Fertil. Steril. 2021, 116, 123–129. [Google Scholar] [CrossRef]
- Simon, L.; Emery, B.; Carrell, D.T. Sperm DNA fragmentation: Consequences for reproduction. Adv. Exp. Med. Biol. 2019, 1166, 87–105. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2010; Available online: https://apps.who.int/iris/handle/10665/343208 (accessed on 15 January 2023).
- Avendaño, C.; Franchi, A.; Taylor, S.; Morshedi, M.; Bocca, S.; Oehninger, S. Fragmentation of DNA in morphologically normal human spermatozoa. Fertil. Steril. 2009, 91, 1077–1084. [Google Scholar] [CrossRef]
- Celik-Ozenci, C.; Jakab, A.; Kovacs, T.; Catalanotti, J.; Demir, R.; Bray-Ward, P.; Ward, D.; Huszar, G. Sperm selection for ICSI: Shape properties do not predict the absence or presence of numerical chromosomal aberrations. Hum. Reprod. 2004, 19, 2052–2059. [Google Scholar] [CrossRef]
- Alikani, M.; Cohen, J.; Tomkin, G.; Garrisi, G.J.; Mack, C.; Scott, R.T. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil. Steril. 1999, 71, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Schoolcraft, W.B. In vitro culture of human blastocysts. In Towards Reproductive Certainty; Parthenon Publishing Group Ltd.: Sydney, Australia, 1999; pp. 378–388. [Google Scholar]
- Alpha Scientists in Reproductive Medicine; ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Racowsky, C.; Vernon, M.; Mayer, J.; Ball, G.D.; Behr, B.; Pomeroy, K.O.; Wininger, D.; Gibbons, W.; Conaghan, J.; Stern, J.E. Standardization of grading embryo morphology. Fertil. Steril. 2010, 94, 1152–1153. [Google Scholar] [CrossRef] [PubMed]
- Pregl Breznik, B.; Kovačič, B.; Vlaisavljević, V. Are sperm DNA fragmentation, hyperactivation, and hyaluronan-binding ability predictive for fertilization and embryo development in in vitro fertilization and intracytoplasmic sperm injection? Fertil. Steril. 2013, 99, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Brunborg, G.; Stevenson, M.; Lutton, D.; McManus, J.; Lewis, S.E. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum. Reprod. 2010, 25, 1594–1608. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Zini, A.; Dyachenko, A.; Ciampi, A.; Carrell, D.T. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J. Androl. 2017, 19, 80–90. [Google Scholar] [CrossRef]
- Jayaprakasan, K.; Chan, Y.Y.; Sur, S.; Deb, S.; Clewes, J.S.; Raine-Fenning, N.J. Prevalence of uterine anomalies and their impact on early pregnancy in women conceiving after assisted reproduction treatment. Ultrasound Obstet. Gynecol. 2011, 37, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zheng, L.; Zhao, D.; Xu, Y.; Wang, Y. The role of immune cells in recurrent spontaneous abortion. Reprod. Sci. 2021, 28, 3303–3315. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Benchaib, M.; Braun, V.; Lornage, J.; Hadj, S.; Salle, B.; Lejeune, H.; Guérin, J.F. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum. Reprod. 2003, 18, 1023–1028. [Google Scholar] [CrossRef]
- Xue, L.T.; Wang, R.X.; He, B.; Mo, W.Y.; Huang, L.; Wang, S.K.; Mao, X.-B.; Cheng, J.-P.; Huang, Y.-Y.; Liu, R. Effect of sperm DNA fragmentation on clinical outcomes for Chinese couples undergoing in vitro fertilization or intracytoplasmic sperm injection. J. Int. Med. Res. 2016, 44, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Yetkinel, S.; Kilicdag, E.B.; Aytac, P.C.; Haydardedeoglu, B.; Simsek, E.; Cok, T. Effects of the microfluidic chip technique in sperm selection for intracytoplasmic sperm injection for unexplained infertility: A prospective, randomized controlled trial. J. Assist. Reprod. Genet. 2019, 36, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Colaco, S.; Sakkas, D. Paternal factors contributing to embryo quality. J. Assist. Reprod. Genet. 2018, 35, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Sertyel, S. Sperm DNA Damage and Oocyte Repair Capability; Springer: Cham, Switzerland, 2018; pp. 321–346. [Google Scholar]
- Green, K.A.; Patounakis, G.; Dougherty, M.P.; Werner, M.D.; Scott, R.T.; Franasiak, J.M. Sperm DNA fragmentation on the day of fertilization is not associated with embryologic or clinical outcomes after IVF/ICSI. J. Assist. Reprod. Genet. 2020, 37, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Anbari, F.; Khalili, M.A.; Sultan Ahamed, A.M.; Mangoli, E.; Nabi, A.; Dehghanpour, F.; Sabour, M. Microfluidic sperm selection yields higher sperm quality compared to conventional method in ICSI program: A pilot study. Syst. Biol. Reprod. Med. 2021, 67, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Parrella, A.; Keating, D.; Cheung, S.; Xie, P.; Stewart, J.D.; Rosenwaks, Z.; Palermo, G.D. A treatment approach for couples with disrupted sperm DNA integrity and recurrent ART failure. J. Assist. Reprod. Genet. 2019, 36, 2057–2066. [Google Scholar] [CrossRef]
| Variables | Non-MSS | MSS | p-Value | ||
|---|---|---|---|---|---|
| n | M ± SD | n | M ± SD | ||
| Receptor age | 87 | 42.7 ± 4.0 | 102 | 41.7 ± 3.3 | NS |
| Paternal age | 87 | 42.7 ± 6.4 | 102 | 41.7 ± 5.3 | NS |
| n | % | n | % | ||
| FR (M ± SD) | 87 | 71.7 ± 16 | 102 | 75.0 ± 18 | NS |
| BR (M ± SD) | 63 | 57.5 ± 24 | 100 | 61.0 ± 22 | NS |
| IR | 179 | 42.5 | 182 | 57.7 | 0.004 ** |
| LBR | 178 | 30.3 | 177 | 42.9 | 0.014 * |
| MR | 78 | 34.6 | 103 | 33 | NS |
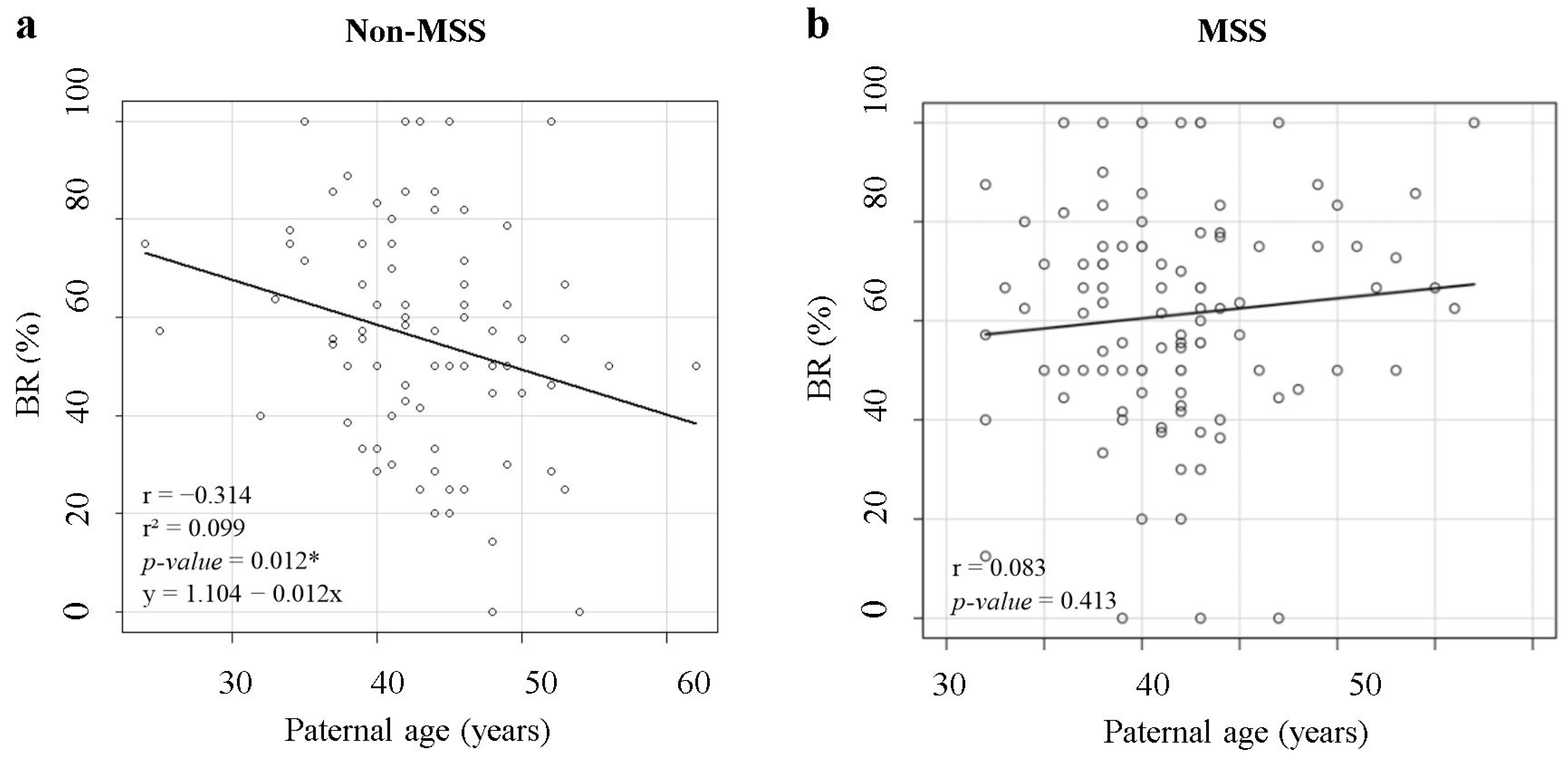
| Variables | n | Correlation | p-Value | |
|---|---|---|---|---|
| Non-MSS | FR | 87 | −0.006 | NS |
| BR | 63 | −0.314 | 0.012 * | |
| MSS | FR | 102 | −0.118 | NS |
| BR | 100 | 0.083 | NS |
| Variables | Non-MSS | MSS | p-Value | |||
|---|---|---|---|---|---|---|
| Paternal Age | n | % | n | % | ||
| <45 years | FR (M ± SD) | 51 | 71.7 ± 15 | 81 | 76.5 ± 18 | NS |
| BR (M ± SD) | 38 | 62.4 ± 22 | 79 | 60.2 ± 22 | NS | |
| IR | 103 | 50.5 | 149 | 59.1 | NS | |
| LBR | 102 | 35.3 | 144 | 43.1 | NS | |
| MR | 50 | 34 | 87 | 33.3 | NS | |
| ≥45 years | FR (M ± SD) | 36 | 71.7 ± 18 | 21 | 69.3 ± 15 | NS |
| BR (M ± SD) | 25 | 50.1 ± 25 | 21 | 64.4 ± 23 | 0.034 * | |
| IR | 76 | 31.6 | 33 | 51.5 | 0.048 * | |
| LBR | 76 | 23.7 | 33 | 42.4 | 0.048 * | |
| MR | 28 | 35.7 | 16 | 31.2 | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudé-Logares, L.; Serrano-Novillo, C.; Uroz, L.; Galindo, A.; Márquez, C. Advanced Paternal Age: A New Indicator for the Use of Microfluidic Devices for Sperm DNA Fragmentation Selection. J. Clin. Med. 2024, 13, 457. https://doi.org/10.3390/jcm13020457
Escudé-Logares L, Serrano-Novillo C, Uroz L, Galindo A, Márquez C. Advanced Paternal Age: A New Indicator for the Use of Microfluidic Devices for Sperm DNA Fragmentation Selection. Journal of Clinical Medicine. 2024; 13(2):457. https://doi.org/10.3390/jcm13020457
Chicago/Turabian StyleEscudé-Logares, Laura, Clara Serrano-Novillo, Laia Uroz, Anna Galindo, and Carmen Márquez. 2024. "Advanced Paternal Age: A New Indicator for the Use of Microfluidic Devices for Sperm DNA Fragmentation Selection" Journal of Clinical Medicine 13, no. 2: 457. https://doi.org/10.3390/jcm13020457




