Soluble PCSK9 Inhibition: Indications, Clinical Impact, New Molecular Insights and Practical Approach—Where Do We Stand?
Abstract
:1. Avoidable Cardiovascular Risk: Global Burden, Target of Therapy and New Frontiers
2. PCSK9 Inhibitors’ Pleiotropic Effects
- (a)
- Anti-atherosclerotic effect: PCSK9 inhibitors reduce the concentrations of pro-inflammatory cytokines and increase the levels of interleukin-10 (an anti-inflammatory interleukin), decreasing the expression of TNF-α and the C-C chemokine receptor type 2 (CCR2) and thereby inhibiting the mechanisms that promote atherosclerosis. [26]. Moreover, PCSK9 monoclonal antibodies reduce the expression of NADPH oxidase, involved in oxidative stress [27], and lower the expression of membrane-adhesive molecules (ICAM and VCAM) interfering with the TLR4/NF-kB cascade [28].
- (b)
- Stabilization of atherosclerotic plaque: PCSK9 inhibition reduces the necrotic core of atheroma, constituting one of the main players in plaque instabilization, induces autophagy of inflammatory cells, and favors elimination of necrotic cells. These mechanisms promote plaque stabilization [29]. However, it was also reported that PCSK9 has direct proinflammatory effects on vessels, possibly through effects on another member of the LDLR family, the LDLR-related protein 1, or LRP1, which exerts a strong regulatory effect on the inflammatory stance of plaque macrophages. Loss of LRP1 induces inflammation, and PCSK9 in the plaque reduces LRP1 levels [30,31]. Thus, therapeutic blockades of PCSK9 via monoclonal antibodies could have on coronary plaques a combination of positive and negative effects. The balance of these is difficult to assess in exclusively clinical studies, given the tremendously beneficial effect of plasma LDL-C reduction [32,33].
- (c)
- Anti-aggregation and anticoagulant effects: PCSK-9 is involved in platelet activation and aggregation through indirect and direct stimulation of CD36 (scavenger receptor) and low-density lipoprotein receptor-1 (LOX-1) on the surface of platelets. CD36 is activated directly by PCSK-9, but also by LDL and ox-LDL, while LOX-1 is activated by inflammatory stimuli, and its expression is increased by PCSK-9. Platelet aggregation is also favored by stimulation of TLR2 on the cell surface through lipid–peroxide-modified phospholipids transported by Lp(a). Inhibition of PCSK-9, therefore, has an antithrombotic effect for several reasons: reducing levels of LDL and ox-LDL, reducing Lp(a), and decreasing LOX-1 expression [34]. PCSK-9 is involved in coagulation through the downregulation of the low-density lipoprotein receptor-related protein (LRP-1). This one physiologically decreases the levels of tissue factor and factor VIII [35].
- (d)
- Antineoplastic effect: LDL-C and triglycerides have a negative impact on the risk of developing cancer. Mutations causing a decrease in PCSK-9 activity are associated with inhibition of the progression of colorectal and breast cancer, mainly, but not only, through a decrease in LDL-C concentration [36].
- (e)
- PCSK9-I and sepsis: the Toll-like receptor (TLR) plays a key role in the immune response and is activated by lipid molecules associated with the pathogen cell walls (lipopolysaccharides, lipoteichoic acid and phospholipomannan). PCSK9 reduces the elimination of lipids by downregulating LDL-R, thereby promoting the development of sepsis and septic shock. Use of PCSK-9 inhibitors may reverse this pathway [37].
3. PCSK9 Monoclonal Antibodies
Pharmacokinetics and Drug Interactions
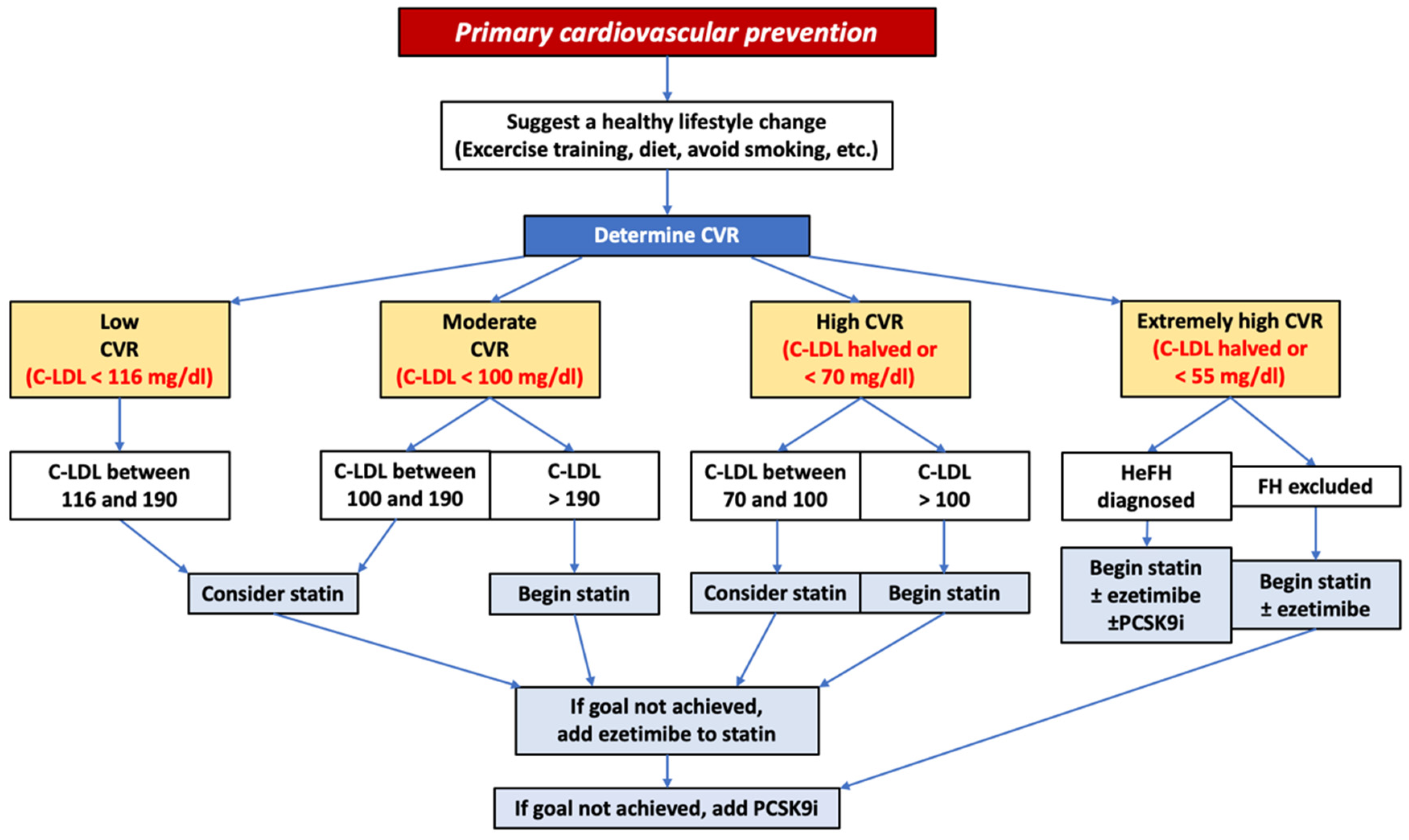
4. PCSK9 Monoclonal Antibodies’ Indications for Cardiovascular Primary Prevention
5. PCSK9 Monoclonal Antibodies’ Indications for Cardiovascular Secondary Prevention in Chronic Setting
6. Very Early Treatment of Acute Coronary Syndrome with Monoclonal Antibodies of Soluble PCSK9
7. Prescriptive Flow-Chart and Real-World Adoption
8. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality From Ischemic Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef] [PubMed]
- Silverio, A.; Di Maio, M.; Citro, R.; Esposito, L.; Iuliano, G.; Bellino, M.; Baldi, C.; De Luca, G.; Ciccarelli, M.; Vecchione, C.; et al. Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: Systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovasc. Disord. 2021, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Groenendyk, J.W.; Greenland, P.; Khan, S.S. Incremental Value of Polygenic Risk Scores in Primary Prevention of Coronary Heart Disease: A Review. JAMA Intern Med. 2022, 182, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Aimaretti, G.; Marino, P.; Sinigaglia, F.; Suryapranata, H.; De Luca, G.; Novara Atherosclerosis Study Group (NAS). Impact of diabetes on neutrophil-to-lymphocyte ratio and its relationship to coronary artery disease. Diabetes Metab. 2015, 41, 304–311. [Google Scholar] [CrossRef]
- De Luca, G.; Verdoia, M.; Cassetti, E.; Schaffer, A.; Cavallino, C.; Bolzani, V.; Marino, P.; Novara Atherosclerosis Study Group (NAS). High fibrinogen level is an independent predictor of presence and extent of coronary artery disease among Italian population. J. Thromb. Thrombolysis. 2011, 31, 458–463. [Google Scholar] [CrossRef]
- Barbieri, L.; Verdoia, M.; Schaffer, A.; Marino, P.; Suryapranata, H.; De Luca, G.; Novara Atherosclerosis Study Group (NAS). Impact of sex on uric acid levels and its relationship with the extent of coronary artery disease: A single-centre study. Atherosclerosis 2015, 241, 241–248. [Google Scholar] [CrossRef]
- Kedhi, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; Fabris, E.; Ijsselmuiden, A.J.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: The COMBINE OCT–FFR trial. Eur. Heart J. 2021, 42, 4671–4679. [Google Scholar] [CrossRef]
- Baldi, C.; Silverio, A.; Esposito, L.; Di Maio, M.; Tarantino, F.; De Angelis, E.; Fierro, G.; Attisano, T.; Di Muro, M.R.; Maione, A.; et al. Clinical outcome of patients with ST-elevation myocardial infarction and angiographic evidence of coronary artery ectasia. Catheter. Cardiovasc. Interv. 2022, 99, 340–347. [Google Scholar] [CrossRef]
- Esposito, L.; Di Maio, M.; Silverio, A.; Cancro, F.P.; Bellino, M.; Attisano, T.; Tarantino, F.F.; Esposito, G.; Vecchione, C.; Galasso, G.; et al. Treatment and Outcome of Patients with Coronary Artery Ectasia: Current Evidence and Novel Opportunities for an Old Dilemma. Front. Cardiovasc. Med. 2022, 8, 805727. [Google Scholar] [CrossRef]
- Silverio, A.; Buccheri, S.; Venetsanos, D.; Alfredsson, J.; Lagerqvist, B.; Persson, J.; Witt, N.; James, S.; Sarno, G. Percutaneous Treatment and Outcomes of Small Coronary Vessels: A SCAAR Report. JACC Cardiovasc. Interv. 2020, 13, 793–804. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wilson, P.W.; Kannel, W.B. Beyond established and novel risk factors: Lifestyle risk factors for cardiovascular disease. Circulation 2008, 117, 3031–3038. [Google Scholar] [CrossRef] [PubMed]
- Galasso, G.; De Angelis, E.; Silverio, A.; Di Maio, M.; Cancro, F.P.; Esposito, L.; Bellino, M.; Scudiero, F.; Damato, A.; Parodi, G.; et al. Predictors of Recurrent Ischemic Events in Patients with ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2021, 159, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, F.; Canonico, M.E.; Sanna, G.D.; Dossi, F.; Silverio, A.; Galasso, G.; Esposito, G.; Porto, I.; Parodi, G. Dual Antiplatelet Therapy with 3rd Generation P2Y12 Inhibitors in STEMI Patients: Impact of Body Mass Index on Loading Dose–Response. Cardiovasc. Drugs Ther. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomized trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Silverio, A.; Benvenga, R.M.; Piscione, F.; Gulizia, M.M.; Meessen, J.M.T.A.; Colivicchi, F.; Nardi, F.; Baldi, C.; Galasso, G.; Vecchione, C.; et al. Prevalence and Predictors of Out-of-Target LDL Cholesterol 1 to 3 Years After Myocardial Infarction. A Subanalysis From the EYESHOT Post-MI Registry. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 149–157. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Merz, C.N.B.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guideline. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Siontis, G.C.M.; Piccolo, R.; Mavridis, D.; Räber, L.; Mach, F.; Windecker, S. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: A meta-analysis of randomized trials. Eur. Heart J. 2018, 39, 1172–1180. [Google Scholar] [CrossRef]
- Silverio, A.; Cancro, F.P.; Di Maio, M.; Bellino, M.; Esposito, L.; Centore, M.; Carrizzo, A.; Di Pietro, P.; Borrelli, A.; De Luca, G.; et al. Lipoprotein(a) levels and risk of adverse events after myocardial infarction in patients with and without diabetes. J. Thromb. Thrombolysis 2022, 54, 382–392. [Google Scholar] [CrossRef]
- Cohen, J.; Pertsemlidis, A.; Kotowski, I.K.; Graham, R.; Garcia, C.K.; Hobbs, H.H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 2005, 37, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Coppinger, C.; Movahed, M.R.; Azemawah, V.; Peyton, L.; Gregory, J.; Hashemzadeh, M. A Comprehensive Review of PCSK9 Inhibitors. J. Cardiovasc. Pharmacol. Ther. 2022, 27, 10742484221100107. [Google Scholar] [CrossRef]
- Tavori, H.; Fan, D.; Blakemore, J.L.; Yancey, P.G.; Ding, L.; Linton, M.F.; Fazio, S. Serum Proprotein Convertase Subtilisin/Kexin Type 9 and Cell Surface Low-Density Lipoprotein Receptor: Evidence for a reciprocal regulation. Circulation 2013, 127, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-A.; Kosenko, T.; Lagace, T.A. Internalized PCSK9 dissociates from recycling LDL receptors in PCSK9-resistant SV-589 fibroblasts. J. Lipid Res. 2014, 55, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Nordestgaard, B.G.; Grande, P.; Schnohr, P.; Tybjærg-Hansen, A. PCSK9R46L, Low-Density Lipoprotein Cholesterol Levels, and Risk of Ischemic Heart Disease: 3 Independent Studies and Meta-Analyses. J. Am. Coll. Cardiol. 2010, 55, 2833–2842. [Google Scholar] [CrossRef]
- Basiak, M.; Kosowski, M.; Cyrnek, M.; Bułdak, Ł.; Maligłówka, M.; Machnik, G.; Okopień, B. Pleiotropic Effects of PCSK-9 Inhibitors. Int. J. Mol. Sci. 2021, 22, 3144. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Sun, C.; Wang, Y.; Mehta, J.L. Hemodynamic Shear Stress via ROS Modulates PCSK9 Expression in Human Vascular Endothelial and Smooth Muscle Cells and along the Mouse Aorta. Antioxidants Redox Signal. 2015, 22, 760–771. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Peng, J.; Ren, Z.; Yang, J.; Li, T.-T.; Li, T.-H.; Wang, Z.; Wei, D.-H.; Liu, L.-S.; Zheng, X.-L.; et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef]
- Omori, H.; Ota, H.; Hara, M.; Kawase, Y.; Tanigaki, T.; Hirata, T.; Sobue, Y.; Okubo, M.; Kamiya, H.; Matsuo, H. Effect of PCSK-9 Inhibitors on Lipid-Rich Vulnerable Coronary Plaque Assessed by Near-Infrared Spectroscopy. JACC Cardiovasc. Imaging 2020, 13, 1639–1641. [Google Scholar] [CrossRef]
- Zhu, L.; Giunzioni, I.; Tavori, H.; Covarrubias, R.; Ding, L.; Zhang, Y.; Ormseth, M.; Major, A.S.; Stafford, J.M.; Linton, M.F.; et al. Loss of Macrophage Low-Density Lipoprotein Receptor–Related Protein 1 Confers Resistance to the Antiatherogenic Effects of Tumor Necrosis Factor-α Inhibition. Arter. Thromb. Vasc. Biol. 2016, 36, 1483–1495. [Google Scholar] [CrossRef]
- Desai, N.R.; Giugliano, R.; Wasserman, S.M.; Gibbs, J.P.; Liu, T.; Scott, R.; Sabatine, M.S. Association between Circulating Baseline Proprotein Convertase Subtilisin Kexin Type 9 Levels and Efficacy of Evolocumab. JAMA Cardiol. 2017, 2, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, B.; Hu, M.; Zhang, Y.; Chan, P.; Liu, Z.M. Alirocumab for the treatment of hypercholesterolemia. Expert Opin. Biol. Ther. 2017, 17, 633–643. [Google Scholar] [CrossRef]
- Kasichayanula, S.; Grover, A.; Emery, M.G.; Gibbs, M.A.; Somaratne, R.; Wasserman, S.M.; Gibbs, J.P. Clinical Pharmacokinetics and Pharmacodynamics of Evolocumab, a PCSK9 Inhibitor. Clin. Pharmacokinet. 2018, 57, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Puccini, M.; Landmesser, U.; Rauch, U. Pleiotropic Effects of PCSK9: Focus on Thrombosis and Haemostasis. Metabolites 2022, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Canuel, M.; Sun, X.; Asselin, M.-C.; Paramithiotis, E.; Prat, A.; Seidah, N.G. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Can Mediate Degradation of the Low Density Lipoprotein Receptor-Related Protein 1 (LRP-1). PLoS ONE 2013, 8, e64145. [Google Scholar] [CrossRef]
- Liu, X.; Bao, X.; Hu, M.; Chang, H.; Jiao, M.; Cheng, J.; Xie, L.; Huang, Q.; Li, F.; Li, C.-Y. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature 2020, 588, 693–698. [Google Scholar] [CrossRef]
- Walley, K.R.; Thain, K.R.; Russell, J.A.; Reilly, M.P.; Meyer, N.J.; Ferguson, J.F.; Christie, J.D.; Nakada, T.-A.; Fjell, C.D.; Thair, S.A.; et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci. Transl. Med. 2014, 6, 258ra143. [Google Scholar] [CrossRef]
- Gibbs, J.P.; Slatter, J.G.; Egbuna, O.; Geller, M.; Hamilton, L.; Dias, C.S.; Xu, R.Y.; Johnson, J.; Wasserman, S.M.; Emery, M.G. Evaluation of Evolocumab (AMG 145), a Fully Human Anti-PCSK9 IgG2 Monoclonal Antibody, in Subjects with Hepatic Impairment. J. Clin. Pharmacol. 2017, 57, 513–523. [Google Scholar] [CrossRef]
- Lee, E.; Gibbs, J.P.; Emery, M.G.; Block, G.; Wasserman, S.M.; Hamilton, L.; Kasichayanula, S.; Hanafin, P.; Somaratne, R.; Egbuna, O. Influence of Renal Function on Evolocumab Exposure, Pharmacodynamics, and Safety. Clin. Pharmacol. Drug Dev. 2019, 8, 281–289. [Google Scholar] [CrossRef]
- Dubuc, G.; Chamberland, A.; Wassef, H.; Davignon, J.; Seidah, N.G.; Bernier, L.; Prat, A. Statins Upregulate PCSK9, the Gene Encoding the Proprotein Convertase Neural Apoptosis-Regulated Convertase-1 Implicated in Familial Hypercholesterolemia. Arter. Thromb. Vasc. Biol. 2004, 24, 1454–1459. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.; Rocco, M.; Colquhoun, D.; Sullivan, D.; Rosenson, R.S.; Dent, R.; Xue, A.; Scott, R.; Wasserman, S.M.; Stroes, E. Clinical Profile of Statin Intolerance in the Phase 3 GAUSS-2 Study. Cardiovasc. Drugs Ther. 2016, 30, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, P.M.; Thompson, P.D.; Cannon, C.P.; Guyton, J.R.; Bergeron, J.; Zieve, F.J.; Bruckert, E.; Jacobson, T.A.; Kopecky, S.L.; Baccara-Dinet, M.T.; et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J. Clin. Lipidol. 2015, 9, 758–769. [Google Scholar] [CrossRef]
- Nissen, S.E.; Stroes, E.; Dent-Acosta, R.E.; Rosenson, R.S.; Lehman, S.J.; Sattar, N.; Preiss, D.; Bruckert, E.; Ceška, R.; Lepor, N.; et al. Efficacy and Tolerability of Evolocumab vs Ezetimibe in Patients With Muscle-Related Statin Intolerance: The GAUSS-3 Randomized Clinical Trial. JAMA 2016, 315, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Colhoun, H.M.; Szarek, M.; Baccara-Dinet, M.; Bhatt, D.L.; Bittner, A.V.; Budaj, A.J.; Diaz, R.; Goodman, S.G.; Hanotin, C.; et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: A prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.M.; McKenney, J.M. ODYSSEY MONO: Effect of alirocumab 75 mg subcutaneously every 2 weeks as monotherapy versus ezetimibe over 24 weeks. Futur. Cardiol. 2015, 11, 27–37. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Robinson, J.G.; Cannon, C.P.; Lorenzato, C.; Pordy, R.; Chaudhari, U.; Colhoun, H.M. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am. Heart J. 2015, 169, 906–915.e13. [Google Scholar] [CrossRef]
- Cannon, C.P.; Cariou, B.; Blom, D.; McKenney, J.M.; Lorenzato, C.; Pordy, R.; Chaudhari, U.; Colhoun, H.M. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: The ODYSSEY COMBO II randomized controlled trial. Eur. Heart J. 2015, 36, 1186–1194. [Google Scholar] [CrossRef]
- Bays, H.; Gaudet, D.; Weiss, R.; Ruiz, J.L.; Watts, G.; Gouni-Berthold, I.; Robinson, J.; Zhao, J.; Hanotin, C.; Donahue, S. Alirocumab as Add-On to Atorvastatin Versus Other Lipid Treatment Strategies: ODYSSEY OPTIONS I Randomized Trial. J. Clin. Endocrinol. Metab. 2015, 100, 3140–3148. [Google Scholar] [CrossRef]
- Farnier, M.; Jones, P.; Severance, R.; Averna, M.; Steinhagen-Thiessen, E.; Colhoun, H.M.; Du, Y.; Hanotin, C.; Donahue, S. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: The ODYSSEY OPTIONS II randomized trial. Atherosclerosis 2016, 244, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Rader, D.J.; Raal, F.J.; Guyton, J.R.; Baccara-Dinet, M.T.; Lorenzato, C.; Pordy, R.; Stroes, E. Efficacy and Safety of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia and LDL-C of 160 mg/dL or Higher. Cardiovasc. Drugs Ther. 2016, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.P.; Robinson, J.G.; Farnier, M.; Krempf, M.; Langslet, G.; Lorenzato, C.; Gipe, D.A.; Baccara-Dinet, M.T. Efficacy and Safety of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia not Adequately Controlled with Current Lipid-Lowering Therapy: Design and Rationale of the ODYSSEY FH Studies. Cardiovasc. Drugs Ther. 2014, 28, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Stroes, E.; Guyton, J.R.; Lepor, N.; Civeira, F.; Gaudet, D.; Watts, G.F.; Baccara-Dinet, M.T.; Lecorps, G.; Manvelian, G.; Farnier, M.; et al. Efficacy and Safety of Alirocumab 150 mg Every 4 Weeks in Patients With Hypercholesterolemia Not on Statin Therapy: The ODYSSEY CHOICE II Study. J. Am. Heart Assoc. 2016, 5, e003421. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.-J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Pedersen, T.R.; Park, J.-G.; De Ferrari, G.M.; Gaciong, A.Z.; Ceska, R.; Toth, K.; Gouni-Berthold, I.; Lopez-Miranda, J.; Schiele, F.; et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: A prespecified secondary analysis of the FOURIER trial. Lancet 2017, 390, 1962–1971. [Google Scholar] [CrossRef]
- Blom, D.J.; Hala, T.; Bolognese, M.; Lillestol, M.J.; Toth, P.D.; Burgess, L.; Ceska, R.; Roth, E.; Koren, M.J.; Ballantyne, C.M.; et al. A 52-Week Placebo-Controlled Trial of Evolocumab in Hyperlipidemia. N. Engl. J. Med. 2014, 370, 1809–1819. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.P.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Stroes, E.; Colquhoun, D.; Sullivan, D.; Civeira, F.; Rosenson, R.S.; Watts, G.F.; Bruckert, E.; Cho, L.; Dent, R.; Knusel, B.; et al. Anti-PCSK9 Antibody Effectively Lowers Cholesterol in Patients with Statin Intolerance: The GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J. Am. Coll. Cardiol. 2014, 63, 2541–2548. [Google Scholar] [CrossRef]
- Koren, M.J.; Lundqvist, P.; Bolognese, M.; Neutel, J.M.; Monsalvo, M.L.; Yang, J.; Kim, J.B.; Scott, R.; Wasserman, S.M.; Bays, H. Anti-PCSK9 Monotherapy for Hypercholesterolemia: The MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J. Am. Coll. Cardiol. 2014, 63, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Stein, A.E.; Dufour, R.; Turner, T.; Civeira, F.; Burgess, L.; Langslet, G.; Scott, R.; Olsson, A.G.; Sullivan, D.; et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 385, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Nedergaard, B.S.; Rogers, W.J.; Fialkow, J.; Neutel, J.M.; Ramstad, D.; Somaratne, R.; Legg, J.C.; Nelson, P.; Scott, R.; et al. Effect of Evolocumab or Ezetimibe Added to Moderate- or High-Intensity Statin Therapy on LDL-C Lowering in Patients with Hypercholesterolemia: The LAPLACE-2 randomized clinical trial. JAMA 2014, 311, 1870–1883. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Honarpour, N.; Blom, D.J.; Hovingh, G.K.; Xu, F.; Scott, R.; Wasserman, S.M.; Stein, A.E. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 385, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Nissen, S.E.; Prati, F.; Windecker, S.; Kataoka, Y.; Puri, R.; Hucko, T.; Kassahun, H.; Liao, J.; Somaratne, R.; et al. Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: Rationale and design of the randomized, placebo-controlled HUYGENS study. Cardiovasc. Diagn. Ther. 2021, 11, 120–129. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Windecker, S.; Pedrazzini, G.; Mueller, C.; Cook, S.; Matter, C.M.; Muller, O.; Häner, J.; Gencer, B.; Crljenica, C.; et al. Evolocumab for Early Reduction of LDL Cholesterol Levels in Patients With Acute Coronary Syndromes (EVOPACS). J. Am. Coll. Cardiol. 2019, 74, 2452–2462. [Google Scholar] [CrossRef]
- Leucker, T.M.; Blaha, M.J.; Jones, S.R.; Vavuranakis, M.A.; Williams, M.S.; Lai, H.; Schindler, T.H.; Latina, J.; Schulman, S.P.; Gerstenblith, G. Effect of Evolocumab on Atherogenic Lipoproteins During the Peri- and Early Postinfarction Period: A Placebo-Controlled, Randomized Trial. Circulation 2020, 142, 419–421. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Robertson, M.; Catapano, A.L.; Watts, G.F.; Kastelein, J.J.; Packard, C.J.; Ford, I.; Ray, K.K. Low-Density Lipoprotein Cholesterol Lowering for the Primary Prevention of Cardiovascular Disease among Men With Primary Elevations of Low-Density Lipoprotein Cholesterol Levels of 190 mg/dL or above: Analyses From the WOSCOPS (West of Scotland Coronary Prevention Study) 5-Year Randomized Trial and 20-Year Observational Follow-Up. Circulation 2017, 136, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Palacio, C.H.; Harring, T.R.; Nguyen, N.T.T.; Goss, J.A.; O’Mahony, C.A. Homozygous Familial Hypercholesterolemia: Case Series and Review of the Literature. Case Rep. Transplant. 2011, 2011, 154908. [Google Scholar] [CrossRef] [PubMed]
- O’donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.C.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; López, J.A.G.; et al. Long-Term Evolocumab in Patients With Established Atherosclerotic Cardiovascular Disease. Circulation 2022, 146, 1109–1119. [Google Scholar] [CrossRef]
- De Luca, G.; Navarese, E.P.; Suryapranata, H. A meta-analytic overview of thrombectomy during primary angioplasty. Int. J. Cardiol. 2013, 166, 606–612. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Schaffer, A.; Wirianta, J.; Suryapranata, H. Comprehensive meta-analysis of radial vs femoral approach in primary angioplasty for STEMI. Int. J. Cardiol. 2013, 168, 2070–2081. [Google Scholar] [CrossRef]
- De Luca, G.; Suryapranata, H.; Stone, G.W.; Antoniucci, D.; Tcheng, J.E.; Neumann, F.J.; Bonizzoni, E.; Topol, E.J.; Chiariello, M. Relationship between patient’s risk profile and benefits in mortality from adjunctive abciximab to mechanical revascularization for ST-segment elevation myocardial infarction: A meta-regression analysis of randomized trials. J. Am. Coll. Cardiol. 2006, 47, 685–686. [Google Scholar] [CrossRef]
- De Luca, G.; Navarese, E.P.; Cassetti, E.; Verdoia, M.; Suryapranata, H. Meta-analysis of randomized trials of glycoprotein IIb/IIIa inhibitors in high-risk acute coronary syndromes patients undergoing invasive strategy. Am. J. Cardiol. 2011, 107, 198–203. [Google Scholar] [CrossRef]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Cassetti, E.; Piccolo, R.; Galasso, G.; Marino, P.; Sinigaglia, F.; De Luca, G. Benefits from new ADP antagonists as compared with clopidogrel in patients with stable angina or acute coronary syndrome undergoing invasive management: A meta-analysis of randomized trials. J. Cardiovasc. Pharmacol. 2014, 63, 339–350. [Google Scholar] [CrossRef]
- Costa, F.; Montalto, C.; Branca, M.; Hong, S.J.; Watanabe, H.; Franzone, A.; Vranckx, P.; Hahn, J.Y.; Gwon, H.C.; Feres, F.; et al. Dual antiplatelet therapy duration after percutaneous coronary intervention in high bleeding risk: A meta-analysis of randomized trials. Eur. Heart J. 2023, 44, 954–968. [Google Scholar] [CrossRef]
- Secco, G.G.; Ghione, M.; Mattesini, A.; Dall’ara, G.; Ghilencea, L.; Kilickesmez, K.; De Luca, G.; Fattori, R.; Parisi, R.; Marino, P.N.; et al. Very high-pressure dilatation for undilatable coronary lesions: Indications and results with a new dedicated balloon. Eurointervention 2016, 12, 359–365. [Google Scholar] [CrossRef]
- De Luca, G.; Smits, P.; Hofma, S.H.; Di Lorenzo, E.; Vlachojannis, G.; Van’t Hof, A.W.J.; van Boven, A.J.; Kedhi, E.; Stone, G.W.; Suryapranata, H. Drug-Eluting Stent in Primary Angioplasty (DESERT 3) cooperation. Everolimus eluting stent vs first generation drug-eluting stent in primary angioplasty: A pooled patient-level meta-analysis of randomized trials. Int. J. Cardiol. 2017, 244, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cimaglia, P.; Fortini, F.; Vieceli Dalla Sega, F.; Cardelli, L.S.; Massafra, R.F.; Morelli, C.; Trichilo, M.; Ferrari, R.; Rizzo, P.; Campo, G. Relationship between PCSK9 and endothelial function in patients with acute myocardial infarction. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Ruscica, M.; Lupo, M.G.; Vicenzi, M.; Sirtori, C.R.; Corsini, A. Pharmacological rationale for the very early treatment of acute coronary syndrome with monoclonal antibodies anti-PCSK9. Pharmacol. Res. 2022, 184, 106439. [Google Scholar] [CrossRef]
- Sinnaeve, P.R.; Schwartz, G.G.; Wojdyla, D.M.; Alings, M.; Bhatt, D.L.; Bittner, A.V.; Chiang, C.-E.; Flores, R.M.C.; Diaz, R.; Dorobantu, M.; et al. Effect of alirocumab on cardiovascular outcomes after acute coronary syndromes according to age: An ODYSSEY OUTCOMES trial analysis. Eur. Heart J. 2019, 41, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, J.-Y.; Lu, P.-J.; Xiao, J.-Y.; Gao, M.-D.; Li, C.-P.; Cui, Z.; Liu, Y. Effects of Evolocumab Added to Moderate-Intensity Statin Therapy in Chinese Patients With Acute Coronary Syndrome: The EMSIACS Trial Study Protocol. Front. Physiol. 2021, 12, 750872. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Andreotti, F.; Raggi, P.; Kołodziejczak, M.; Buffon, A.; Bliden, K.; Tantry, U.; Kubica, J.; Sardella, G.; Lauten, A.; et al. Baseline low-density lipoprotein cholesterol to predict the extent of cardiovascular benefit from lipid-lowering therapies: A review. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 47–54. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Nault, P.; Giugliano, R.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Low-Density Lipoprotein Cholesterol Lowering with Evolocumab and Outcomes in Patients with Peripheral Artery Disease: Insights from the FOURIER Trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk). Circulation 2018, 137, 338–350. [Google Scholar] [CrossRef]
- Raal, F.; Scott, R.; Somaratne, R.; Bridges, I.; Li, G.; Wasserman, S.M.; Stein, E.A. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: The Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation 2012, 126, 2408–2417. [Google Scholar] [CrossRef]
- März, W.; Dippel, F.-W.; Theobald, K.; Gorcyca, K.; Iorga, R.; Ansell, D. Utilization of lipid-modifying therapy and low-density lipoprotein cholesterol goal attainment in patients at high and very-high cardiovascular risk: Real-world evidence from Germany. Atherosclerosis 2018, 268, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Niman, S.; Pereira, E.; Lewis, T.; Reid, J.; Choksi, R.; Goldfaden, R.F. A Critical Review of the Efficacy and Safety of Inclisiran. Am. J. Cardiovasc. Drugs 2021, 21, 629–642. [Google Scholar] [CrossRef]
- Ray, K.K.; Reeskamp, L.F.; Laufs, U.; Banach, M.; Mach, F.; Tokgözoğlu, L.S.; Connolly, D.L.; Gerrits, A.J.; Stroes, E.S.G.; Masana, L.; et al. Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur. Heart J. 2022, 43, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Hegele, R.A.; Fazio, S.; Cannon, C.P. The Evolving Future of PCSK9 Inhibitors. J. Am. Coll. Cardiol. 2018, 72, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, A.D.; Liu, M.; Toth, P.P.; Zhao, S.; Agrawal, D.K.; Libby, P.; Chatzizisis, Y.S. Pleiotropic Anti-atherosclerotic Effects of PCSK9 Inhibitors From Molecular Biology to Clinical Translation. Curr. Atheroscler. Rep. 2018, 20, 20. [Google Scholar] [CrossRef]
- Katzmann, J.L.; Cupido, A.J.; Laufs, U. Gene Therapy Targeting PCSK9. Metabolites 2022, 12, 70. [Google Scholar] [CrossRef]
- Mitchell, T.; Chao, G.; Sitkoff, D.; Lo, F.; Monshizadegan, H.; Meyers, D.; Low, S.; Russo, K.; DiBella, R.; Denhez, F.; et al. Pharmacologic Profile of the Adnectin BMS-962476, a Small Protein Biologic Alternative to PCSK9 Antibodies for Low-Density Lipoprotein Lowering. J. Pharmacol. Exp. Ther. 2014, 350, 412–424. [Google Scholar] [CrossRef]
- McNutt, M.C.; Kwon, H.J.; Chen, C.; Chen, J.R.; Horton, J.D.; Lagace, T.A. Antagonism of Secreted PCSK9 Increases Low Density Lipoprotein Receptor Expression in HepG2 Cells. J. Biol. Chem. 2009, 284, 10561–10570. [Google Scholar] [CrossRef]
- Hapiro, M.D.; Tavori, H.; Fazio, S. PCSK9: From Basic Science Discoveries to Clinical Trials. Circ. Res. 2018, 122, 1420–1438. [Google Scholar] [CrossRef]
- Paciullo, F.; Momi, S.; Gresele, P. PCSK9 in Haemostasis and Thrombosis: Possible Pleiotropic Effects of PCSK9 Inhibitors in Cardiovascular Prevention. Thromb. Haemost. 2019, 119, 359–367. [Google Scholar] [CrossRef]

| Trial Name | Patients’ Population | Patients | Treatments | LDL Reduction |
|---|---|---|---|---|
| ALIROCUMAB | ||||
| ODYSSEY OUTCOMES [46] | Prior ACS (1–12 months earlier) and LDL > 70 mg/dL on statin therapy | 18924 | Alirocumab 75 mg Q2W vs. placebo | 55% |
| ODYSSEY MONO [47] | Hypercholesterolemia (with or without statin) | 103 | Alirocumab 75/150 mg and oral placebo vs. Ezetimibe and subcutaneous placebo | 47.2% |
| ODYSSEY ALTERNATIVE [44] | Moderate (or higher) CV risk with statin intolerance | 251 | Alirocumab 75/150 mg plus oral placebo vs. ezetimibe plus SC placebo vs. atorvastatin 20 mg plus SC placebo | 52.2% |
| ODYSSEY COMBO I [48] | High CV risk in uncontrolled hypercholesterolemia on maximally tolerated statin therapy +/− other LLT | 316 | Alirocumab 75/150 mg Q2W vs. placebo | 48.2% |
| ODYSSEY COMBO II [49] | High CV risk in uncontrolled hypercholesterolemia on maximally tolerated statin therapy +/− other LLT | 720 | Alirocumab 75 mg Q2W (plus oral placebo) vs. ezetimibe (plus SC placebo) | 50.6% |
| ODYSSEY OPTIONS I [50] | Very high CV risk and LDL > 70 mg/dl or high CV risk and LDL > 100 mg/dl on atorvastatin 20/40 mg | 355 | Alirocumab 75 mg Q2W vs. ezetimibe vs. double-dose atorvastatin vs. switch to rosuvastatin 40 mg | 44.1/54% |
| ODYSSEY OPTIONS II [51] | Very high CV risk and LDL > 70 mg/dl or high risk and LDL > 100 mg/dL on rosuvastatin 10/20 mg | 305 | Alirocumab 75 mg Q2W vs. ezetimibe vs. double-dose rosuvastatin | 50.6/36.3% |
| ODYSSEY HIGH FH [52] | HeFH and LDL > 160 mg/dL on maximally tolerated statin +/− other LLT | 107 | Alirocumab 150 mg Q2W vs. placebo | 46% |
| ODYSSEY LONG TERM [53] | High CV risk and LDL > 70 mg/dL on maximally tolerated statin +/− other LLT | 2341 | Alirocumab 150 mg Q2W vs. placebo | 62% |
| ODYSSEY FH I and II [54] | HeFH and uncontrolled LDL on maximally tolerated statin +/− other LLT | 486/249 | Alirocumab 75/150 mg Q2W vs. placebo | 57.9/51.4% |
| ODYSSEY CHOICE II [55] | Statin intolerance and hypercholesterolemia | 233 | Alirocumab 150 mg Q4W or 75 mg Q2W vs. placebo | 51.7/53.5% |
| PACMAN-AMI [56] | Intracoronary imaging of non-IRAs in early ACS on rosuvastatin 20 mg | 300 | Alirocumab 150 mg Q2W vs. placebo | −54.7 mg/dL |
| EVOLOCUMAB | ||||
| FOURIER [57] | Prior CVD and LDL > 70 mg/dL Background therapy of statin +/− ezetimibe | 27564 | Evolocumab 140 mg Q2W or 420 mg monthly vs. placebo | 59% |
| DESCARTES [58] | LDL > 75 mg/dL Background therapy of statin +/− ezetimibe | 901 | Evolocumab 420 mg monthly vs. placebo | 55% |
| GLAGOV [59] | Angiographic coronary disease and LDL > 80 mg/dL or 60-80 mg/dL plus any risk factor | 968 | Evolocumab 420 mg monthly vs. placebo | −56.5 mg/dL |
| GAUSS-2 [60] | Hypercholesterolemia with statin intolerance | 307 | 1. Evolocumab 140 mg Q2W or 420 mg monthly2. Ezetimibe3. Oral and subcutaneous placebo | 54% |
| MENDEL-2 [61] | Hypercholesterolemia (LDL > 100 mg/dL) and CV risk < 10% | 614 | 1. Evolocumab 140 mg Q2W or 420 mg monthly2. Ezetimibe3. Oral and subcutaneous placebo | 58% |
| RUTHERFORD-2 [62] | HeFH and LDL > 100% Background therapy of statin +/− ezetimibe | 331 | Evolocumab 140 mg Q2W or 420 mg monthly vs. placebo | 56% |
| LAPLACE-2 [63] | Hypercholesterolemia Background therapy of statin +/− ezetimibe | 2067 | 1. Moderate-intensity vs. high-intensity statin2. Evolocumab 140 mg Q2W or 420 mg monthly3. Ezetimibe4. Oral and subcutaneous placebo | 70% |
| TESLA Part B [64] | HoFH and LDL > 130 mg/dL on maximal therapy | 50 | Evolocumab 420 mg monthly vs. placebo | 23,1% |
| OSLER-1 and OSLER-2 (open label) [65] | Hypercolesterolemia on standard therapy | 4465 | Evolocumab 420 mg monthly or 140 mg Q2W plus standard therapy vs. standard therapy alone | 61% |
| HUYGENS [66] | Intracoronary imaging of non-IRAs in early ACS on maximally tolerated statin therapy | 150 | Evolocumab 420 mg monthly vs. placebo | NK |
| EVOPACS [67] | In-hospital phase of ACS on atorvastatin 40 mg | 308 | Evolocumab 420 mg monthly vs. placebo | 40,7% |
| EVACS [68] | In-hospital phase of ACS on maximally tolerated statin therapy | 60 | Evolocumab 420 mg vs. placebo | −27 mg/dL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellino, M.; Galasso, G.; Silverio, A.; Tedeschi, M.; Formisano, C.; Romei, S.; Esposito, L.; Cancro, F.P.; Vassallo, M.G.; Accarino, G.; et al. Soluble PCSK9 Inhibition: Indications, Clinical Impact, New Molecular Insights and Practical Approach—Where Do We Stand? J. Clin. Med. 2023, 12, 2922. https://doi.org/10.3390/jcm12082922
Bellino M, Galasso G, Silverio A, Tedeschi M, Formisano C, Romei S, Esposito L, Cancro FP, Vassallo MG, Accarino G, et al. Soluble PCSK9 Inhibition: Indications, Clinical Impact, New Molecular Insights and Practical Approach—Where Do We Stand? Journal of Clinical Medicine. 2023; 12(8):2922. https://doi.org/10.3390/jcm12082922
Chicago/Turabian StyleBellino, Michele, Gennaro Galasso, Angelo Silverio, Michele Tedeschi, Ciro Formisano, Stefano Romei, Luca Esposito, Francesco Paolo Cancro, Maria Giovanna Vassallo, Giulio Accarino, and et al. 2023. "Soluble PCSK9 Inhibition: Indications, Clinical Impact, New Molecular Insights and Practical Approach—Where Do We Stand?" Journal of Clinical Medicine 12, no. 8: 2922. https://doi.org/10.3390/jcm12082922





