Alteration of the Functional Connectivity of the Cortical Areas Characterized by the Presence of Von Economo Neurons in Schizophrenia, a Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical and Cognitive Assessment
2.3. fMRI data Processing and Analysis
2.3.1. Image Acquisition
2.3.2. fMRI Data Pre-Processing
2.3.3. Definition of the Regions of Interest
2.3.4. Functional Connectivity Analysis
2.4. Clinical Association with Neuroimaging and Between-Group Comparison of Sociodemographic and Cognitive Variables
3. Results
3.1. Demographic, Clinical, and Cognitive Characteristics of the Sample
3.2. RsFC and Fuzzy Clustering Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von Economo, C.; Koskinas, G. Individuelle und Physiologische Bedeutung der Areale Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen; Springer: Berlin/Heidelberg, Germany, 1925. [Google Scholar]
- Allman, J.M.; Tetreault, N.A.; Hakeem, A.Y.; Manaye, K.F.; Semendeferi, K.; Erwin, J.M.; Park, S.; Goubert, V.; Hof, P.R. The von Economo neurons in the frontoinsular and anterior cingulate cortex. Ann. N. Y. Acad. Sci. 2011, 1225, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Nimchinsky, E.A.; Vogt, B.A.; Morrison, J.H.; Hof, P.R. Spindle neurons of the human anterior cingulate cortex. J. Comp. Neurol. 1995, 355, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Merkle, F.T.; Gaus, S.E.; Bud Craig, A.D.; Allman, J.M.; Hof, P.R. Distinctive neurons of the anterior cingulate and frontoinsular cortex: A historical perspective. Cereb. Cortex 2012, 22, 245–250. [Google Scholar] [CrossRef]
- Banovac, I.; Sedmak, D.; Judaš, M.; Petanjek, Z. Von Economo Neurons–Primate-Specific or Commonplace in the Mammalian Brain? Front. Neural Circuits 2021, 1, 714611. [Google Scholar] [CrossRef] [PubMed]
- Allman, J.; Hakeem, A.; Watson, K. Two phylogenetic specializations in the human brain. Neurosci. 2002, 8, 335–346. [Google Scholar] [CrossRef]
- Nimchinsky, E.A.; Gilissen, E.; Allman, J.M.; Perl, D.P.; Erwin, J.M.; Hof, P.R. A neuronal morphologic type unique to humans and great apes. Proc. Natl. Acad. Sci. USA 1999, 96, 5268–5273. [Google Scholar] [CrossRef]
- González-Acosta, C.A.; Ortiz-Muñoz, D.; Becerra-Hernández, L.V.; Casanova, M.F.; Buriticá, E. Von Economo neurons: Cellular specialization of human limbic cortices? J. Anat. 2022, 241, 20–32. [Google Scholar] [CrossRef]
- Banovac, I.; Sedmak, D.; Džaja, D.; Jalšovec, D.; Jovanov Milošević, N.; Rašin, M.R.; Petanjek, Z. Somato-dendritic morphology and axon origin site specify von Economo neurons as a subclass of modified pyramidal neurons in the human anterior cingulate cortex. J. Anat. 2019, 235, 651–669. [Google Scholar] [CrossRef]
- Fajardo, C.; Escobar, M.I.; Buriticá, E.; Arteaga, G.; Umbarila, J.; Casanova, M.F.; Pimienta, H. Von Economo neurons are present in the dorsolateral (dysgranular) prefrontal cortex of humans. Neurosci. Lett. 2008, 435, 215–218. [Google Scholar] [CrossRef]
- Hodge, R.D.; Miller, J.A.; Novotny, M.; Kalmbach, B.E.; Ting, J.T.; Bakken, T.E.; Aevermann, B.D.; Barkan, E.R.; Berkowitz-Cerasano, M.L.; Cobbs, C.; et al. Transcriptomic evidence that von Economo neurons are regionally specialized extratelencephalic-projecting excitatory neurons. Nat. Commun. 2020, 11, 1172. [Google Scholar] [CrossRef]
- Cobos, I.; Seeley, W.W. Human von Economo neurons express transcription factors associated with layer V subcerebral projection neurons. Cereb. Cortex 2015, 25, 213–220. [Google Scholar] [CrossRef]
- Watson, K.K. The von Economo Neurons: From Cells to Behavior. Ph.D. Dissertation, California Institute of Technology, Pasadena, CA, USA, 2006. [Google Scholar]
- Butti, C.; Santos, M.; Uppal, N.; Hof, P.R. Von Economo neurons: Clinical and evolutionary perspectives. Cortex 2013, 49, 312–326. [Google Scholar] [CrossRef]
- Cauda, F.; Geminiani, G.C.; Vercelli, A. Evolutionary appearance of von Economo’s neurons in the mammalian cerebral cortex. Front. Hum. Neurosci. 2014, 8, 104. [Google Scholar] [CrossRef]
- Allman, J.M.; Tetreault, N.A.; Hakeem, A.Y.; Manaye, K.F.; Semendeferi, K.; Erwin, J.M.; Park, S.; Goubert, V.; Hof, P.R. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct. Funct. 2010, 214, 495–517. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Yuan, J.; Sun, Y.; Dai, J.; Su, B. Transcriptomic Landscape of von Economo Neurons in Human Anterior Cingulate Cortex Revealed by Microdissected-Cell RNA Sequencing. Cereb. Cortex 2019, 29, 838–851. [Google Scholar] [CrossRef]
- Braak, H.; Tredici, K.D. Anterior Cingulate Cortex TDP-43 Pathology in Sporadic Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2018, 77, 74–83. [Google Scholar] [CrossRef]
- Gefen, T.; Papastefan, S.T.; Rezvanian, A.; Bigio, E.H.; Weintraub, S.; Rogalski, E.; Mesulam, M.; Geula, C. Von Economo neurons of the anterior cingulate across the lifespan and in Alzheimer’s disease. Cortex 2018, 99, 69–77. [Google Scholar] [CrossRef]
- Santillo, A.F.; Nilsson, C.; Englund, E. Von Economo neurones are selectively targeted in frontotemporal dementia. Neuropathol. Appl. Neurobiol. 2013, 39, 572–579. [Google Scholar] [CrossRef]
- Yang, Y.; Halliday, G.M.; Hodges, J.R.; Tan, R.H. Von Economo Neuron Density and Thalamus Volumes in Behavioral Deficits in Frontotemporal Dementia Cases with and without a C9ORF72 Repeat Expansion. J. Alzheimers. Dis. 2017, 58, 701–709. [Google Scholar] [CrossRef]
- Santos, M.; Uppal, N.; Butti, C.; Wicinski, B.; Schmeidler, J.; Giannakopoulos, P.; Heinsen, H.; Schmitz, C.; Hof, P.R. Von Economo neurons in autism: A stereologic study of the frontoinsular cortex in children. Brain Res. 2011, 1380, 206–217. [Google Scholar] [CrossRef]
- Uppal, N.; Wicinski, B.; Buxbaum, J.D.; Heinsen, H.; Schmitz, C.; Hof, P.R. Neuropathology of the anterior midcingulate cortex in young children with autism. J. Neuropathol. Exp. Neurol. 2014, 73, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Senatorov, V.V.; Damadzic, R.; Mann, C.L.; Schwandt, M.L.; George, D.T.; Hommer, D.W.; Heilig, M.; Momenan, R. Reduced anterior insula, enlarged amygdala in alcoholism and associated depleted von Economo neurons. Brain 2015, 138, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Brüne, M.; Schöbel, A.; Karau, R.; Benali, A.; Faustmann, P.M.; Juckel, G.; Petrasch-Parwez, E. Von Economo neuron density in the anterior cingulate cortex is reduced in early onset schizophrenia. Acta Neuropathol. 2010, 119, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Brüne, M.; Schöbel, A.; Karau, R.; Faustmann, P.M.; Dermietzel, R.; Juckel, G.; Petrasch-Parwez, E. Neuroanatomical correlates of suicide in psychosis: The possible role of von Economo neurons. PLoS ONE 2011, 6, e20936. [Google Scholar] [CrossRef]
- Krause, M.; Theiss, C.; Brüne, M. Ultrastructural Alterations of Von Economo Neurons in the Anterior Cingulate Cortex in Schizophrenia. Anat. Rec. 2017, 300, 2017–2024. [Google Scholar] [CrossRef]
- Rogalski, E.J.; Gefen, T.; Shi, J.; Samimi, M.; Bigio, E.; Weintraub, S.; Geula, C.; Mesulam, M.M. Youthful memory capacity in old brains: Anatomic and genetic clues from the Northwestern SuperAging Project. J. Cogn. Neurosci. 2013, 25, 29–36. [Google Scholar] [CrossRef]
- Cauda, F.; Torta, D.M.E.; Sacco, K.; D’Agata, F.; Geda, E.; Duca, S.; Geminiani, G.; Vercelli, A. Functional anatomy of cortical areas characterized by Von Economo neurons. Brain Struct. Funct. 2013, 218, 1–20. [Google Scholar] [CrossRef]
- American Psychiatric Association (DSM-5®). Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Colombo, L.; Sartori, G.; Brivio, C. Stima del quoziente intellettivo tramite l’applicazione del TIB (test breve di Intelligenza). G. Ital. Di Psicol. 2002, 29, 613–638. [Google Scholar]
- Nelson, H.E. National Adult Reading Test (NART): For the Assessment of Premorbid Intelligence in Patients with Dementia: Test Manual, 1st ed.; NFER-NELSON Publishing Company Ltd.: Slough, UK, 1982. [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Wallwork, R.S.; Fortgang, R.; Hashimoto, R.; Weinberger, D.R.; Dickinson, D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr. Res. 2012, 137, 246–250. [Google Scholar] [CrossRef]
- Mucci, A.; Galderisi, S.; Merlotti, E.; Rossi, A.; Rocca, P.; Bucci, P.; Piegari, G.; Chieffi, M.; Vignapiano, A.; Maj, M. The Brief Negative Symptom Scale (BNSS): Independent validation in a large sample of Italian patients with schizophrenia. Eur. Psychiatry 2015, 30, 641–647. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Strauss, G.P.; Nguyen, L.; Fischer, B.A.; Daniel, D.G.; Cienfuegos, A.; Marder, S.R. The brief negative symptom scale: Psychometric properties. Schizophr. Bull. 2011, 37, 300–305. [Google Scholar] [CrossRef]
- Strauss, G.P.; Hong, L.E.; Gold, J.M.; Buchanan, R.W.; McMahon, R.P.; Keller, W.R.; Fischer, B.A.; Catalano, L.T.; Culbreth, A.J.; Carpenter, W.T.; et al. Factor structure of the Brief Negative Symptom Scale. Schizophr. Res. 2012, 142, 96–98. [Google Scholar] [CrossRef]
- Addington, D.; Addington, J.; Maticka-Tyndale, E. Assessing depression in schizophrenia: The Calgary Depression Scale. Br. J. Psychiatry 1993, 163, 39–44. [Google Scholar] [CrossRef]
- Morosini, P.L.; Magliano, L.; Brambilla, L.; Ugolini, S.; Pioli, R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr. Scand. 2000, 101, 323–329. [Google Scholar] [CrossRef]
- Leucht, S.; Samara, M.; Heres, S.; Davis, J.M. Dose Equivalents for Antipsychotic Drugs: The DDD Method. Schizophr. Bull. 2016, 42, S90–S94. [Google Scholar] [CrossRef]
- Simpson, G.M.; Angus, J.W. A rating scale for extrapyramidal side effects. Acta Psychiatr. Scand. Suppl. 1970, 212, 11–19. [Google Scholar] [CrossRef]
- Keefe, R.S.; Goldberg, T.E.; Harvey, P.D.; Gold, J.M.; Poe, M.P.; Coughenour, L. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004, 68, 283–297. [Google Scholar] [CrossRef]
- Happé, F.G. An advanced test of theory of mind: Understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J. Autism. Dev. Disord. 1994, 24, 129–154. [Google Scholar] [CrossRef]
- Soddu, A.; Vanhaudenhuyse, A.; Bahri, M.A.; Bruno, M.-A.; Boly, M.; Demertzi, A.; Tshibanda, J.-F.; Phillips, C.; Stanziano, M.; Ovadia-Caro, S.; et al. Identifying the default-mode component in spatial IC analyses of patients with disorders of consciousness. Hum. Brain Mapp. 2012, 33, 778–796. [Google Scholar] [CrossRef]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014, 84, 320–341. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.J.; Castañón, A.N.; Ongür, D.; Whitfield-Gabrieli, S. Anticorrelations in resting state networks without global signal regression. Neuroimage 2012, 59, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Birn, R.M.; Handwerker, D.A.; Jones, T.B.; Bandettini, P.A. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage 2019, 44, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Cordes, D.; Haughton, V.M.; Arfanakis, K.; Carew, J.D.; Turski, P.A.; Moritz, C.H.; Quigley, M.A.; Meyerand, M.E. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. Am. J. Neuroradiol. 2001, 22, 1326–1333. [Google Scholar]
- Fan, L.; Li, H.; Zhuo, J.; Zhang, Y.; Wang, J.; Chen, L.; Yang, Z.; Chu, C.; Xie, S.; Laird, A.R.; et al. The human brainnetome atlas: A new brain atlas based on connectional architecture. Cereb. Cortex 2016, 26, 3508–3526. [Google Scholar] [CrossRef]
- Morel, A.; Gallay, M.M.; Baechler, A.; Wyss, M.; Gallay, D.S. The human insula: Architectonic organization and postmortem MRI registration. Neuroscience 2013, 236, 117–135. [Google Scholar] [CrossRef]
- Vogt, B.A.; Hof, P.R.; Zilles, K.; Vogt, L.J.; Herold, C.; Palomero-Gallagher, N. Cingulate area 32 homologies in mouse, rat, macaque and human: Cytoarchitecture and receptor architecture. J. Comp. Neurol. 2013, 521, 4189–4204. [Google Scholar] [CrossRef]
- Smolders, A.; De Martino, F.; Staeren, N.; Scheunders, P.; Sijbers, J.; Goebel, R.; Formisano, E. Dissecting cognitive stages with time-resolved fMRI data: A comparison of fuzzy clustering and independent component analysis. Magn. Reson. Imaging 2007, 25, 860–868. [Google Scholar] [CrossRef]
- Forman, S.D.; Cohen, J.D.; Fitzgerald, M.; Eddy, W.F.; Mintun, M.A.; Noll, D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn. Reson. Med. 1995, 33, 636–647. [Google Scholar] [CrossRef]
- Giordano, G.M.; Stanziano, M.; Papa, M.; Mucci, A.; Prinster, A.; Soricelli, A.; Galderisi, S. Functional connectivity of the ventral tegmental area and avolition in subjects with schizophrenia: A resting state functional MRI study. Eur. Neuropsychopharmacol. 2018, 28, 589–602. [Google Scholar] [CrossRef]
- Lee, M.H.; Hacker, C.D.; Snyder, A.Z.; Corbetta, M.; Zhang, D.; Leuthardt, E.C.; Shimony, J.S. Clustering of resting state networks. PLoS ONE 2012, 7, e40370. [Google Scholar] [CrossRef]
- Litt, A.; Plassmann, H.; Shiv, B.; Rangel, A. Dissociating valuation and saliency signals during decision-making. Cereb. Cortex 2011, 21, 95–102. [Google Scholar] [CrossRef]
- Laird, A.R.; Fox, P.M.; Eickhoff, S.B.; Turner, J.A.; Ray, K.L.; McKay, D.R.; Glahn, D.C.; Beckmann, C.F.; Smith, S.M.; Fox, P.T. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011, 23, 4022–4037. [Google Scholar] [CrossRef]
- Picó-Pérez, M.; Vieira, R.; Fernández-Rodríguez, M.; De Barros, M.A.P.; Radua, J.; Morgado, P. Multimodal meta-analysis of structural gray matter, neurocognitive and social cognitive fMRI findings in schizophrenia patients. Psychol. Med. 2022, 52, 614–624. [Google Scholar] [CrossRef]
- Miyata, J. Toward integrated understanding of salience in psychosis. Neurobiol. Dis. 2019, 131, 104414. [Google Scholar] [CrossRef] [PubMed]
- Liloia, D.; Brasso, C.; Cauda, F.; Mancuso, L.; Nani, A.; Manuello, J.; Costa, T.; Duca, S.; Rocca, P. Updating and characterizing neuroanatomical markers in high-risk subjects, recently diagnosed and chronic patients with schizophrenia: A revised coordinate-based meta-analysis. Neurosci. Biobehav. Rev. 2021, 123, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Brandl, F.; Avram, M.; Weise, B.; Shang, J.; Simões, B.; Bertram, T.; Ayala, D.H.; Penzel, N.; Gürsel, D.A.; Bäuml, J.; et al. Specific Substantial Dysconnectivity in Schizophrenia: A Transdiagnostic Multimodal Meta-analysis of Resting-State Functional and Structural Magnetic Resonance Imaging Studies. Biol. Psychiatry 2019, 85, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Wang, Y.; Chang, X.; Luo, C.; Yao, D. Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophr. Bull. 2018, 44, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Supekar, K.; Cai, W.; Krishnadas, R.; Palaniyappan, L.; Menon, V. Dysregulated Brain Dynamics in a Triple-Network Saliency Model of Schizophrenia and Its Relation to Psychosis. Biol. Psychiatry 2019, 85, 60–69. [Google Scholar] [CrossRef]
- Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef]
- Bègue, I.; Kaiser, S.; Kirschner, M. Pathophysiology of negative symptom dimensions of schizophrenia—Current developments and implications for treatment. Neurosci. Biobehav. Rev. 2020, 116, 74–88. [Google Scholar] [CrossRef]
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatry 2018, 5, 664–677. [Google Scholar] [CrossRef]
- Shukla, D.K.; Chiappelli, J.J.; Sampath, H.; Kochunov, P.; Hare, S.M.; Wisner, K.; Rowland, L.M.; Hong, L.E. Aberrant Frontostriatal Connectivity in Negative Symptoms of Schizophrenia. Schizophr. Bull. 2019, 45, 1051–1059. [Google Scholar] [CrossRef]
- Ventura, R.; Morrone, C.; Puglisi-Allegra, S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc. Natl. Acad. Sci. USA 2007, 104, 5181–5186. [Google Scholar] [CrossRef]
- Manoliu, A.; Riedl, V.; Doll, A.; Bäuml, J.G.; Mühlau, M.; Schwerthöffer, D.; Scherr, M.; Zimmer, C.; Förstl, H.; Bäuml, J.; et al. Insular Dysfunction Reflects Altered Between-Network Connectivity and Severity of Negative Symptoms in Schizophrenia during Psychotic Remission. Front. Hum. Neurosci. 2013, 7, 216. [Google Scholar] [CrossRef]
- Tian, Y.; Zalesky, A.; Bousman, C.; Everall, I.; Pantelis, C. Insula Functional Connectivity in Schizophrenia: Subregions, Gradients, and Symptoms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 399–408. [Google Scholar] [CrossRef]

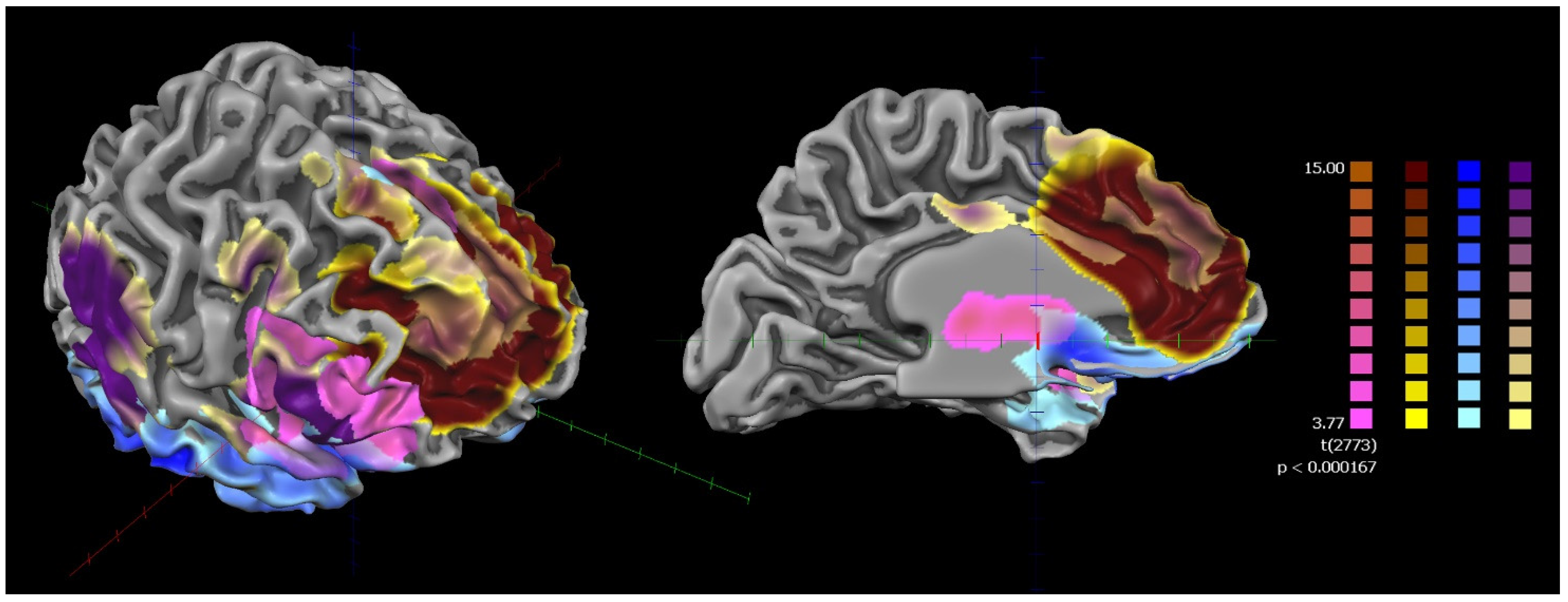
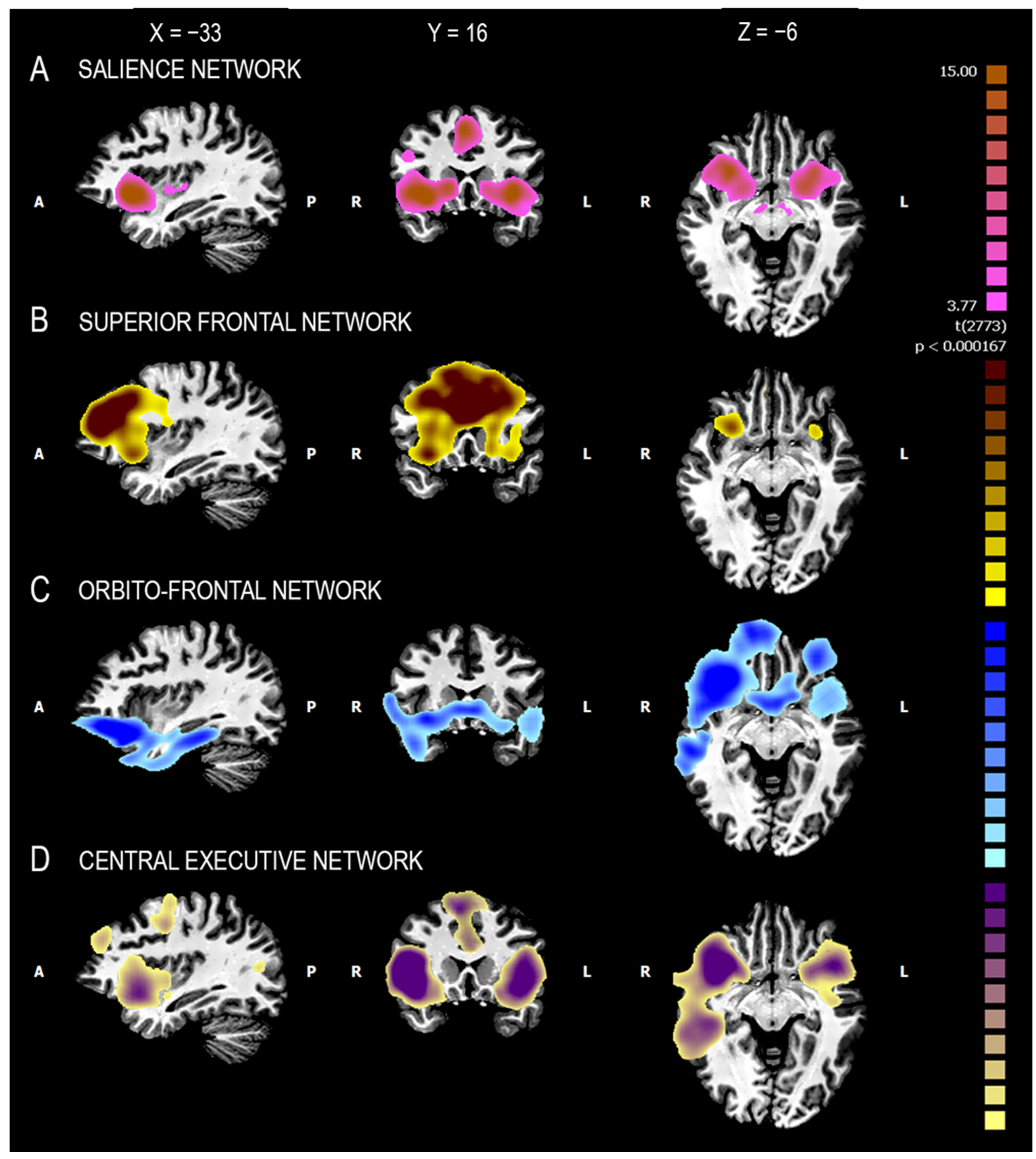
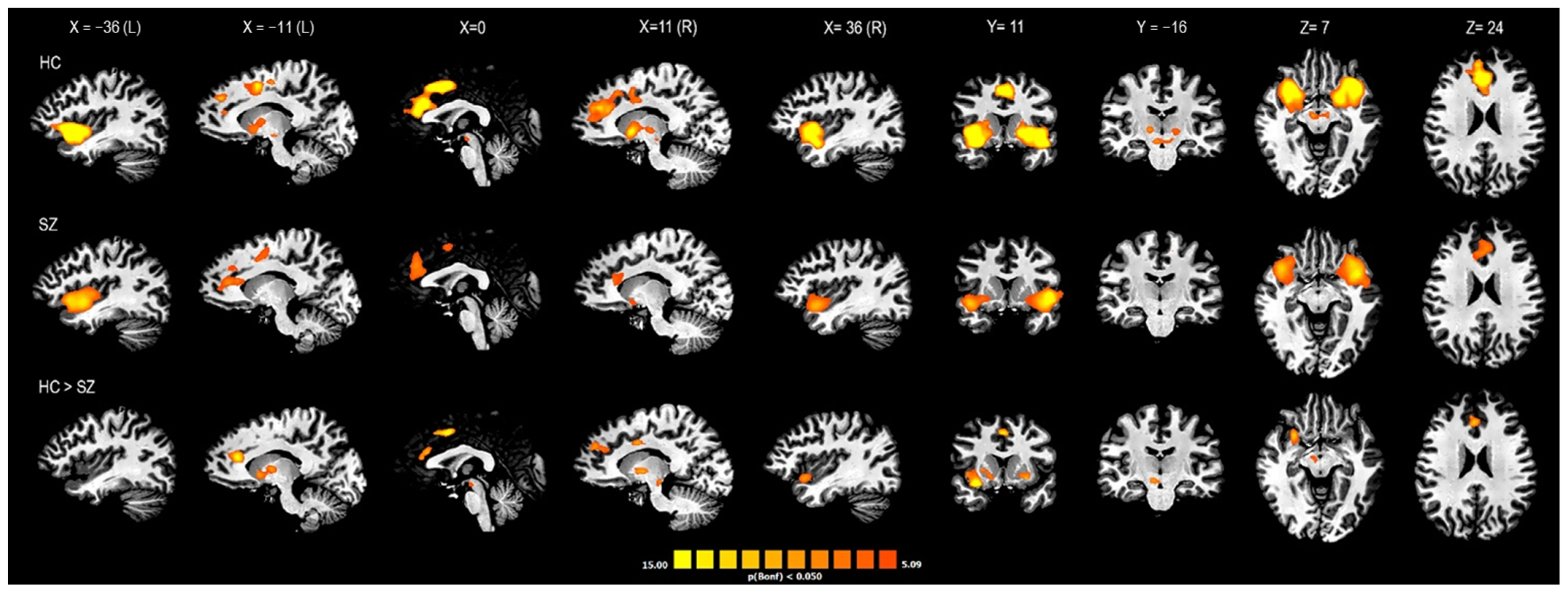
| SZ Group | HC Group | Statistic F/χ2 | p Value | |
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age, Years | 41.5 (11.3) | 42.1 (10.8) | 0.003 | 0.954 |
| Gender, M/F | 13/7 | 13/7 | 0.000 | 1 |
| Education, Years | 13.7 (4.3) | 13.85 (4.2) | 0.012 | 0.912 |
| Clinical variables | ||||
| Duration of Illness, Years | 14.60 (9.81) | |||
| PANSS–Positive, Score | 7.45 (1.82) | |||
| PANSS–Disorganization, Score | 6.25 (2.63) | |||
| BNSS avolition | 21.85 (9.22) | |||
| BNSS expressive deficit | 11.65 (7.40) | |||
| CDSS, Total score | 4.10 (5.08) | |||
| PSP, Score | 61.60 (13.05) | |||
| CPZ Equivalent, mg/d | 371.80 (144.87) | |||
| Concomitant SSRI and SM, N {%} | 7 {35} | |||
| Concomitant BDZ and NBDZ, N {%} | 5 {25} | |||
| SAS, Total score | 0.75 (2.15) | |||
| Cognitive Performance | ||||
| BACS, Verbal memory | 38.56 (9.94) | 47.11 (7.78) | 9.171 | 0.004 |
| BACS, Verbal fluency | 33.05 (11.74) | 46.81 (11.10) | 14.499 | <0.001 |
| BACS, Digit sequencing | 15.49 (4.59) | 20.96 (4.17) | 15.564 | <0.001 |
| BACS, Symbol coding | 39.01 (11.12) | 54.88 (10.16) | 22.216 | <0.001 |
| BACS, Token motor | 66.96 (17.28) | 91.55 (8.09) | 33.226 | <0.001 |
| BACS, Tower of London | 17.26 (5.09) | 19.50 (3.98) | 2.402 | 0.129 |
| SST, Correct answers | 3.85 (1.90) | 5.45 (0.69) | 12.552 | 0.001 |
| Clinical/Cognitive Variables | R-vSr | R/L-dACC/ pre-SMA | R-VTA | R-FIC |
|---|---|---|---|---|
| PANSS–Positive, Score | −0.088 | −0.028 | −0.086 | 0.138 |
| PANSS–Disorganization, Score | 0.485 | 0.484 | 0.228 | 0.420 |
| BNSS avolition | −0.353 | −0.649 | −0.869 ** | −0.778 ** |
| BNSS poor emotional expression | −0.213 | −0.344 | −0.379 | −0.523 |
| CDSS, Total score | −0.021 | −0.285 | −0.457 | −0.380 |
| PSP, Score | 0.189 | 0.499 | 0.731 * | 0.694 * |
| BACS, Verbal memory | −0.080 | 0.317 | 0.274 | 0.458 |
| BACS, Verbal fluency | 0.149 | 0.118 | 0.165 | 0.356 |
| BACS, Digit sequencing | −0.220 | 0.139 | 0.121 | 0.066 |
| BACS, Symbol coding | 0.092 | 0.324 | 0.402 | 0.334 |
| BACS, Token motor | −0.046 | −0.146 | 0.015 | −0.013 |
| SST, correct answers | −0.516 | −0.381 | −0.266 | −0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brasso, C.; Stanziano, M.; Bosco, F.M.; Morese, R.; Valentini, M.C.; Vercelli, A.; Rocca, P. Alteration of the Functional Connectivity of the Cortical Areas Characterized by the Presence of Von Economo Neurons in Schizophrenia, a Pilot Study. J. Clin. Med. 2023, 12, 1377. https://doi.org/10.3390/jcm12041377
Brasso C, Stanziano M, Bosco FM, Morese R, Valentini MC, Vercelli A, Rocca P. Alteration of the Functional Connectivity of the Cortical Areas Characterized by the Presence of Von Economo Neurons in Schizophrenia, a Pilot Study. Journal of Clinical Medicine. 2023; 12(4):1377. https://doi.org/10.3390/jcm12041377
Chicago/Turabian StyleBrasso, Claudio, Mario Stanziano, Francesca Marina Bosco, Rosalba Morese, Maria Consuelo Valentini, Alessandro Vercelli, and Paola Rocca. 2023. "Alteration of the Functional Connectivity of the Cortical Areas Characterized by the Presence of Von Economo Neurons in Schizophrenia, a Pilot Study" Journal of Clinical Medicine 12, no. 4: 1377. https://doi.org/10.3390/jcm12041377
APA StyleBrasso, C., Stanziano, M., Bosco, F. M., Morese, R., Valentini, M. C., Vercelli, A., & Rocca, P. (2023). Alteration of the Functional Connectivity of the Cortical Areas Characterized by the Presence of Von Economo Neurons in Schizophrenia, a Pilot Study. Journal of Clinical Medicine, 12(4), 1377. https://doi.org/10.3390/jcm12041377







